Abstract
Flavonoids are the secondary metabolites synthesized by microalgae and have diverse applications in various fields. The present study compares among two microalgal strains, Chlorella vulgaris (CV) and Chlorella pyrenoidosa (CP), for the production of flavonoids and lipids, which were cultivated in mixotrophic as well as autotrophic modes. This study also focuses on the role of l-phenylalanine as a supplement to enhance flavonoids. The current work establishes the relation between nitrates, external carbon, and l-phenylalanine for enhanced production of flavonoids and lipids. The extracted flavonoids were found to be maximum in CP-autotrophic mode followed by CV-mixotrophic mode with 138 μg/ml and 118 μg/ml, respectively. The common flavonoids observed with both the microalgal strains were quercetin, catechin, and p-coumaric acid. In comparison, the production of the maximum lipid of 23.7% was reported with mixotrophic operation in CV, followed by CP (19.4%). The scavenging activity of the extracted flavonoids was determined using a hydrogen peroxide assay and was found to be in the range of 63–73% at all experimental conditions. CP was found to produce more flavonoids in autotrophic mode, whereas mixotrophic mode showed maximum production of lipids and flavonoids in the CV. The simultaneous production of high value-added products, viz., flavonoids and lipids, not only paves a pathway for the biorefinery approach but also elevates the commercial potential during scale-up.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, an urge to discover novel and useful drugs against resistant pathogenic strains has increased. Natural products such as flavonoids are gaining significance in pharmaceuticals, neutraceutical, and cosmetic industries [1,2,3]. Flavonoids are the secondary metabolites that belong to the polyphenols class, which act as modulators of intracellular communications. Moreover, they also alter the phosphorylation state of the target molecules and can function as enzyme activity modulators [4]. To understand and improve the stress responses, researchers have focused on the signaling perception, transcriptional regulation, and expression of functional proteins in the stress response mechanisms [5]. Flavonoids have been known for their therapeutic properties like anti-oxidative, anti-mutagenic, and anti-carcinogenic that can avoid premature aging of the cells [6]. It is a belief that flavonoids are present mainly in plants, but recent studies show that microalgae have the enzyme pool required for flavonoid biosynthesis [7]. Algae is a known source for several classes of bioactive compounds that protect against oxidative degeneration [8]. Several secondary metabolites like phenols, flavones, flavonols, pigments (β-carotene), sulfated polysaccharides, and vitamins are effectively explored in feed and pharmaceuticals, as well as nutraceutical industries [9].
Algae are recognized as the world’s oldest plants. Their earliest presence is from around 1.5 billion years old [10]. They are facilitating a potential bioeconomy that involves photosynthetic microalgae as the leading player and requires investigations for finding a path toward biofuel and bioproduct generation. The engineering and commercial obstacles to deploying algae-derived biofuels and bioproducts on the market are of particular concern [11]. One way to lower biofuel prices is always to reduce the expense of growing biomass. The latest techno-economic current research reported the need for reduced costs, thereby facilitating the economic feasibility of biofuels [12]. A few of these microalgal species can be quickly grown on an industrial scale to retrieve bio-active molecules for its use in the pharmaceutical sector, human and animal consumption, beauty products, and biofuel industries. Flavonoids extracted from red, green, and brown algae have a uniqueness in its molecular framework and structures that lead to significant antioxidant activity [13].
In general, the flavonoids are synthesized along the phenylpropanoid pathway [14], and in the presence of l-phenylalanine, flavonoid production is enhanced [15]. In the past few years, microalgae have gained a great interest in biodiesel production due to the ease in its cultivation, high photosynthetic efficiency, and high amount of triglyceride production. However, the relative amount of lipid accumulation was lower, which resulted in expensive biodiesel production [16, 17]. One of the possible solutions for economic feasibility in the production of biodiesel by using microalgae is the simultaneous production of value-added products such as flavonoids along with lipids. This can be achieved only in the stress condition. Under nutrient stress conditions, the growth is inhibited, and the fixed carbon of algal biomass is predominantly converted to secondary metabolite in most cases [18]. Nitrate stress leads to the accumulation of small molecules with antioxidative activity like flavonoids. It has often been discussed with respect to their role in mitigating the accumulation of secondary matabolites induced by stresses [19].
The main focus of this study is to enhance the synthesis of flavonoids by supplementing l-phenylalanine and simultaneous lipid extraction along with flavonoids. The experiments were performed to comparatively evaluate the efficiency of 2 different microalgal strains, viz., Chlorella vulgaris (CV) and Chlorella pyrenoidosa (CP), for simultaneous production of both lipid and flavonoids under mixotrophic and autotrophic modes of operation. The work presented in this study, i.e., the simultaneous production of flavonoids along with lipid extraction for biodiesel production, is first of its kind.
2 Materials and methods
2.1 Inoculum preparation
Mother cultures of both CV (collected from JNTU, Hyderabad) [11] and CP (NCIM no. 2738) were maintained at 28 °C for 20 days in sterile BG-11 media and under illumination 55 μmol m−2 s−1 (16:8 photoperiod). For the present study, the subcultures of CV and CP were cultivated in autotrophic and mixotrophic modes, as mentioned in Table 1.
Also, to enhance the production of flavonoids, l-phenylalanine was added at the end of the growth phase (10th day) in both modes of cultivation of CV and CP. The end of the growth phase was determined by the exhaust of nitrogen source supplemented through media along with external carbon in mixotrophic mode. From each cultivation conditions, viz., CV-autotrophic, CV-mixotrophic, CP-autotrophic, and CP-mixotrophic, 100 ml of microalgal culture was taken and dried in a hot air oven for 48 h. For the extraction of flavonoids, cells were disrupted using a pestle and mortar with methanol as a solvent. The resultant mixture was heated at 70 °C for half an hour, followed by centrifugation at 10,000 rpm for a period of 10–15 min. The supernatant and the pellet were further subjected to flavonoid estimation and lipid extraction [20].
2.2 Estimation of flavonoids
To study the presence of flavonoids, a qualitative estimation of flavonoids was performed [21]. Briefly, a diluted ammonia solution of 2.5 ml was added to aliquot methanolic extracts (1 ml). The algal extracts’ total flavonoid concentration was found using an adapted Dowd method, as explained by Arvouet-Grand et al. [22]. Briefly, 200 μl of the extract was mixed with the same volume of 10% AlCl3 made in methanol. Furthermore, 0.2 ml of potassium acetate and 1.8 ml of distilled water were added to this mixture and incubated for 30 min at room temperature [23]. The total flavonoid was determined by measuring the sample absorbance at 415 nm. The standard calibration curve for the total flavonoid content was expressed in equivalents of quercetin.
2.3 Thin-layer chromatography and high-performance liquid chromatography
Thin-layer chromatographic (TLC) analysis of microalgal methanol extracts was performed using a stationary phase comprising a plate with aluminum silica gel 60F254 (Merck) with a size of 10 × 10 cm [24]. All four samples were loaded onto silica plates using capillary tubes. The mobile phase employed was petroleum ether: ethyl acetate (2:1 (v/v)) for screening flavonoids for all samples. The retention factor (Rf) value of samples was calculated using Eq. 1
The methanolic extract of the algal sample was dissolved in acetonitrile and methanolic extract to analyze flavonoids by reverse-phase HPLC (RP-HPLC, Waters 2489 Empower) [25]. The mobile phase was a combination of acetonitrile: acetic acid (in 1:9 ratio), and the sample was eluted through a 5-μm spherical C18 column (Sun fire, 4.6 × 250 mm) at a flow rate of 1 ml/min. The injection volume and retention time were 50 μl and 40 min, respectively. The absorbance was monitored at 470 nm with the help of a PDA detector, and the sample solutions were filtered using 0.2-μm syringe filters.
2.4 Biochemical analysis
2.4.1 Estimation of dry cell weight
The obtained biomass (Thermo Science Sorvall ST16R) was centrifuged at 5000 rpm for 5 min at 28 °C after the growth process. The dry weight measurement was obtained by pipetting 2 ml of algal sample on to a pre-dried Whatman no.1 filter paper (FP) by gravimetrical analysis. The dry weight was analyzed for every 24 h and was considered as the variance of filter paperweights with and without sample. The dry cell weight of the samples was calculated using Eq. 2.
2.4.2 Estimation of nitrates and chlorophyll
The exhaustion of the growth phase was estimated by analyzing the nitrate concentration. The utilization of nitrates during the growth phase of microalgae was observed as complete depletion of nitrogen source leading to the stress phase. It was analyzed according to the protocols given in standard methods, which were widely accepted [11, 13]. Estimation of nitrates was carried out for every 2 days. In short, 10 ml of culture from all the experimental conditions has been obtained, and samples were centrifuged to extract the supernatant. Later, nitrates were analyzed by adding 1 ml of concentrated hydrochloric acid, and the concentration was determined at 220 nm.
For the estimation of chlorophyll in the samples, 10 ml of microalgal culture was centrifuged at 10,000 rpm for 10 min. The pellet was resuspended and incubated in 10-ml ethanolic KOH for 10 min. The extract was centrifuged at 5000 rpm for 3 min. Optical density measurements were performed with the aid of a UV-visible spectrophotometer (ELICO 210) at 647 nm and 664 nm to estimate both chlorophyll A and B as specified in the Eqs. 4 and 5 [26].
2.4.3 Estimation of lipids and transesterification
The total lipid extraction was performed by following the Bligh and Dyer method, which is a well-known procedure for lipid extraction [27]. Samples were subjected to (2:1) chloroform: methanol as solvents. The extract was taken into a pre-weighed lipid vial and dried at 70 °C. The lipid percentage of the sample was quantified gravimetrically using Eq. 7.
Transesterification was performed by refluxing lipids extracted previously by adding 10 ml of methanol with 2% sulfuric acid to the sample tubes. The tubes were placed on a hot plate at 65–70 °C for 4 h. Ethyl acetate and water were added to the residue in the ratio (1:1). Using sodium sulfate (anhydrous), the organic phase comprising fatty acid methyl esters (FAME), isolated from the aqueous phase, was segregated. With the solvent recovered, the organic phase was evaporated, and the FAME mix was collected in a tube.
Previously collected FAME mix was subjected to gas chromatographic analysis (Agilent 7890 B), which was fitted with a FI detector via the capillary column DB-225 (30 m × 0.25 mm i.d × 0.25 um film thick). Inert nitrogen was used as the carrier gas (1.5 ml/min) at an oven temperature of 160 °C for 2 min until analysis (at 300 and 325 °C of injector and detector temperatures, respectively). The temperature was subsequently raised to 3000 °C at a 50 °C/min ramp and maintained for 20 min. Comparison of the FAME formulation with standard FAME mix C4–C24 (18919-1AMP SUPELCO) [28] was made.
2.4.4 Scavenging activity by hydrogen peroxide assay
Methanolic extracts of microalgal biomass were analyzed for scavenging activity [29]. A 2 mM H2O2 solution was prepared in 50 mM phosphate buffer at a pH of 7.4. And 0.1 ml of the sample was made up of 0.4 ml using phosphate buffer. 0.6 ml of the H2O2 solution was added to the samples. The samples were then incubated for 10 min. The absorbance of samples and control (H2O2) was measured at 560 nm against the blank.
3 Results and discussion
3.1 Estimation of biomass, chlorophyll, internal carbohydrates, and nitrates
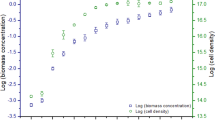
Dry cell weights of both microalgal biomass under 2 different operational conditions, viz., autotrophic and mixotrophic modes, showed linear increment up to 16th day, after which a gradual decrease was observed until the 20th day (end of stress phase) (Fig. 1). The maximum dry cell weight of 13.7 g/l was observed with CV-mixotrophic followed by CP-mixotrophic (12.9 g/l), CV-autotrophic (12.15 g/l), and CP-autotrophic (11.2 g/l). Maximum biomass was obtained in a mixotrophic mode, which can be attributed to the provision of light and external carbon sources [30].
In plastids, the existence of chalcones synthase gives a benefit of the doubt that chlorophyll content and flavonoid synthesis might be related. The possibility of a multi-branching system of flavonoid-producing enzymes has been reported [31]. Hence, chlorophyll estimation has been performed in the current study at regular intervals. A gradual increase in chlorophyll content was observed during the study (Fig. 2). As indicated in Fig. 2, the highest chlorophyll content of 14.818 μg/g was observed with CP-mixotrophic followed by CV-mixotrophic (12.779 μg/g), CP-autotrophic (10.868 μg/g), and CV-autotrophic (6.4579 μg/g). This indicated that CP had higher chlorophyll content in comparison with CV. However, in the current study, there was no significant correlation between flavonoids and chlorophyll content observed as anticipated. In support of this, it can be stated that the chloroplast might not have a role in the biosynthesis of flavonoids in microalgae [32].
Carbohydrates in microalgae are generally attributed to biofuel production. In order to compare two different products, biosynthesis and its correlation with internal carbohydrates were assessed. In all four experimental variations, the internal carbohydrate accumulation was observed in the present study. As indicated in Fig. 3, it was indicated that more internal carbohydrates were accumulated in mixotrophic conditions in both the Chlorella strains used in this study. Also, among the four strains, CV-mixotrophic accumulated the maximum amounts of carbohydrates. This might be attributed to the conditions maintained in mixotrophic mode.
Nitrates play a pivotal role in both the microalgal cultures, either in enhancing the biomass production or the depletion of nitrogen source eventually leading to the accumulation of secondary metabolites: this can be achieved by hindering biomass growth at low nitrate level. Hence, the determination of nitrate concentration can vary with respect to growth and stress phases of microalgal growth operation and also paves a way to identify the accumulation of secondary metabolites. It has been described that low nitrogen tends to accumulate secondary metabolites like lipids, phenylpropanoid, and flavonoids [33]. The maximum removal efficiency of up to 90% was observed in mixotrophic conditions while 80% in autotrophic mode. The correlation between biomass growth and nitrate depletion was established from (Fig. 1) and (Fig. 4).
3.2 Qualitative and quantitative estimation of flavonoids
A preliminary analysis of the entire four samples was conducted. All the samples changed from green to yellow color on the addition of sulfuric acid. The change in yellow color indicated the presence of flavonoids.
The quantity of flavonoids was determined by total flavonoid content estimation. The highest amounts of flavonoids were observed in the case of CP-autotrophic (138 mg/L) at day 18, followed by CP-mixotrophic (120 μg/ml; day 16), CV-mixotrophic (118 μg/ml; day 17), and CV-autotrophic (113 μg/ml; day 17) (Fig. 5). This indicates that maximum flavonoids were observed in C. pyrenoidosa among the two species as the nitrate consumption led to the stress phase. Hence, the correlation was observed with nitrate removal efficiency and flavonoid synthesis. Among the four experimental variations, CP-autotrophic was observed with the maximum amount of flavonoids with 138 μg/ml since it is subject to nitrogen stress [34]. The Rf values of obtained compounds were depicted in Table 2, thereby indicating the similarity of compounds in all the experimental conditions. Some of the compounds with Rf (0.6) and Rf (0.8) are assumed to be quercetin and a hydroxy flavone, respectively [35].
The presence of different flavonoid compounds was further confirmed by HPLC analysis. Compounds like quercetin, catechins, and caffeic acid were found in the four experimental variations, i.e., CV-A, CV-M, CP-A, and CP-M. Quercetin, catechin, and kaempferol are some more essential flavonoids traced in the chromatograms of the algal samples.
3.3 Scavenging activity and FAME analysis
Scavenging activity was performed for the samples, which had the highest flavonoid content. The flavonoids synthesized by microalgae possess scavenging activity indicating their antioxidant property. In a similar study, it was reported that C.vulgaris had shown antioxidant activity in autotrophic mode [36]. In the present study, it was observed that CV-A showed the highest scavenging activity of 77.42% (Table 3). Probably, the compounds which show antioxidant activity are produced in higher quantity in CV-auto.
Lipid percentages at the end of growth were reported to be 9% and 8.5% in autotrophic modes of CV and CP, respectively, whereas, in mixotrophic modes, it was 13.1% and 12.6%. In nitrogen-limited conditions, there was a steep increase in lipids under mixotrophic mode with the highest lipid percentages of 23.7% and 19.4% in both CV and CP, respectively (Fig. 6). In the present study, CV was observed to accumulate a higher amount of lipids when compared with CP. The transesterified samples were subjected to gas chromatography for lipid profiling. Linoleic acid—C18:2, oleic acid—C18:1, elaidic acid—C18:1, and γ-linolenic acid—C18:3 compounds were observed in all the four samples (Table 4).
3.4 Role of l-phenylalanine and synergic effect of nitrates and carbon on biomass, lipids, and total flavonoid content
3.4.1 Role of l-phenylalanine in flavonoid synthesis
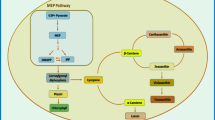
l-Phenylalanine (Phe) is a vital biochemical module in photosynthesis, which plays a critical role in the connectivity among primary and secondary metabolism. Phe serves as a building block for protein synthesis and also an essential precursor in the photosynthesis process of several microorganisms that are critical to reproduction, growth, development, and defensive performance toward various environmental stresses. Phe’s metabolism plays a crucial role in directing the carbon from photosynthesis to phenylpropanoid synthesis, which contains a side chain of a C3 phenyl ring [37]. The term “phenylpropanoids” also covers metabolites, such as flavonoids, stilbenes, coumarins, salicylate, and gallate, sourced from these compounds, and Phe synthesis intermediates. Hence the external supplementation of Phe helps in the enhancing production of flavonoids. When l-phenylalanine was supplemented directly, it enters to the phenylpropanoid pathway bypassing the shikimate pathway; this Phe converts to flavonoids directly by forming cinnamyl-coA or by forming cinnamic and β-coumaric acids, which finally results in the production of flavonoids; this was clearly represented in (Fig. 1).
3.4.2 Role of nitrates in enhancing lipids and total flavonoid content
In the case of total flavonoid content, maximum upregulation was noted in autotrophic modes of both microalgal strains (Fig. 6), since it has depletion nitrogen during the stress phase. Enhancement in flavonoid content synthesis can be observed after the end of the growth phase (Fig. 6). As nitrates play a vital role in biomass growth in the growth phase, its depletion leads to the stress condition, upregulation of phenolic flavonoids antioxidant property products, and storage molecules like lipids occur in the stress phase [38]. Hence, nitrates are considered as major rate-limiting molecules for the synthesis of high-value compounds in the stress phase.
3.4.3 Role of carbohydrates in enhancing lipids and total flavonoid content
External supplementation of carbon in the stress phase with nitrogen deficiency leads to the overproduction of acetyl-CoA. Algae produce polyketides through the condensation of acetyl-CoA as a starter unit and malonyl-CoA for chain elongation, leading to flavones and flavonols [39]. This implementation of the stress phase intrinsically paves to the lipid accumulation and flavonoid production. This is because the common molecule for these two products is malonyl CoA represented in the pathway (Fig. 1). When external carbon source was supplemented in mixotrophic conditions, especially both in the growth and stress phase, the growth phase leads to increased biomass and carbohydrate accumulation, and in the stress phase, secondary metabolites along with lipids are produced. Supplemented carbon, in turn, increases malonyl-CoA production, which is another key flavonoid precursor molecule derived from the citrate of the TCA cycle. Acetyl-CoA, a key molecule for the TCA cycle, diverts its pathway and converts to malonyl-CoA. As mentioned previously (Fig. 1), flavonoids are produced by the metabolic pathway of phenylpropanoid, in which the Phe is used to generate 4-coumaroyl-CoA. This can be coupled with malonyl-CoA to produce the actual flavonoid backbone [40,41,42]. In the present study by external carbon source and l-phenylalanine supplementation in mixotrophic conditions, the simultaneous production of flavonoids and lipid content increased with respect to control (autotrophic mode).
3.4.4 Synergic effect of nitrates, carbohydrates, and l-phenylalanine in lipids and total flavonoid content
High flavonoid content with low lipid content in autotrophic is by only nitrate stress whereas mixotrophic mode that yielded high flavonoid along with high lipid content is attributed to nitrate stress with supplementation of external carbon source. Moreover, the impact of l-phenylalanine in flavonoid synthesis can be taken into consideration as it is the precursor molecule that triggers the flavonoid pathway. Firstly, excess carbon in the mixotrophic stress phase yields a common molecule malonyl Co A for both lipids and flavonoids (Fig. 7); in addition to this, l-phenylalanine directly enters to flavonoid synthesis pathway. The upregulation of both lipid and flavonoid pathways must not be possible without the nitrogen stress or nitrogen depletion stage. Hence, carbon nitrates and l-phenylalanine have a synergic effect on lipid and flavonoid syntheses. These two syntheses can be simultaneously achieved maximum by mixotrophic mode.
4 Conclusions
It is concluded from this study that microalgal strains, viz., Chlorella pyrenoidosa and Chlorella vulgaris, are potential candidates for flavonoid production. Although CP demonstrated high potential to flavonoid synthesis in comparison with both the Chlorella cultures, CV-mixotrophic has reported a simultanious high amount of lipid and flavonoid. This upregulation was observed in mixotrophic mode by a cascade of reactions parallelly in lipid and flavonoid pathways by external supplementation of carbon, leading to the excess synthesis of malonyl CoA, a common moiety for both pathways. These pathways can only be triggered to produce a high amount of lipid and flavonoid only in nitrogen stress added with l-phenylalanine, a precursor for flavonoid synthesis. This study elucidated the link between external carbon and l-phenylalanine supplementation in the mixotrophic mode under nitrogen stress. Thus, CV-mixotrophic reported a high lipid percentage of 23.7% and flavonoid content of 118 μg/ml, whereas CP-autotrophic yielded high flavonoid content of 138 μg/ml. It was demonstrated that malonyl CoA, a vital moiety of flavonoids and lipid synthesis, allows the simultaneous production of these value-added products. This exciting result of the simultaneous production of flavonoids and lipids paves the way for a biorefinery approach while scaling up the process in future studies.
References
Salvador N, Garreta AG, Lavelli L, Ribera M (2007) Antimicrobial activity of Iberian macro algae. Sci Mar 71:101–113
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375
Zakaria NA, Ibrahim D, Sulaiman FS, Supardy NA (2011) Assessment of antioxidant activity, total phenolic content and in- vitro toxicity of Malaysian red seaweed, Acanthophoraspicifera. J Chem Pharm Res 3:182–119
Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C (2009) Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des 15:1072–1084
Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V (2015) Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci 16:26378–26394
Ann M, Zigang D (2013) Signal transduction and molecular targets of selected flavonoids. Antioxid Redox Signal 19:163–180
Goiris K, Muylaert K, Voorspoels S, Noten B, De Paepe D, GJ EB, De Cooman L (2014) Detection of flavonoids in microalgae from different evolutionary lineages. J Phycol 50:483–492
Kumar KS, Ganessan K, Rao PVS (2007) Antioxidant potential of solvent extract of Kappaphycus alverezii (Doty). Doty—edible seaweed. Food Chem 107:289–295
Borowitzka MA, Borowitzka LJ (1988) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 96–100
Martin W, Rotte C, Hoffmeister M, Theissen U, Gelius-Dietrich G, Ahr S, Henze K (2003) Early cell evolution, and a tree of genomes revisited. 55:193–204
Swaroopa Rani A, Goutham RVN, Rao H, Bharath Kumar A, Shruthi M Eco-friendly approach for treating dairy effluent and lipid estimation using microalgae. Biotechnol J Int 7(1, 2015):33–39
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36
Alghazeer R et al (2013) In vitro antibacterial activity of alkaloid extracts from green, red and brown macroalgae from western coast of Libya. Afr J Biotechnol 12(51):7086–7091
Fujino N, Tenma N, Waki T, Ito K, Komatsuzaki Y, Sugiyama K, Yamazaki T, Yoshida S, Hatayama M, Yamashita S, Tanaka Y, Motohashi R, Denessiouk K, Takahashi S, Nakayama T (2018) Physical interactions among flavonoid enzymes in snapdragon and torenia reveal the diversity in the flavonoid metabolon organization of different plant species. Plant J 94:372–392
UdoMargna EM, VainjÄrv T (1989) Influence of nitrogen nutrition on the utilization of l-phenylalanine for building flavonoids in buckwheat seedling tissues. J Plant Physiol 134:697–702
Akazawa K, Okamoto K (1980) Biosynthesis of sucrose. Biochem Plants 3:199–218
Jantasee W, Yongmanitchai W, Chonudomkul D (2015) Optimization of lipid accumulation by starchless mutant Chlorella sorokiniana for biodiesel production. Kasetsart J (Nat Sci) 49:54–66
Seigler DS (1998) Plant secondary metabolism. Chapman and Hall (Kluwer Academic Publishers), Boston, p 711
Nalewajko C, Thomas PM (2001) Effects of temperature, and availability of nitrogen and phosphorus on the abundance of Anabaena and Microcystis in Lake Biwa, Japan: an experimental approach. Limno 2:45–48
Panga K, Krishna SV, KavitaVerma KP, Vurimindi H (2018) Bio oil production from microalgae via hydrothermal liquefaction technology under subcritical water conditions. J Microbiol Methods 153:108–117
Hanaa H, Abd B, Farouk K, Gamal S (2009) Production of phenoliccompounds from Spirulinamaxima microalgae and its protective effects invitro toward hepatotoxicity model. Afr J Pharm Pharmacol 3:133–139
Yadavalli R, Peasari JR, Mamindla P, Kumar P, Mounikaa S, Ganugapati J (2018) Phytochemical screening and in silico studies of flavonoids from Chlorella pyrenoidosa. Inform Med Unlocked 10:89–99
Farasat M, Ramazan-Ali K-N, Mohammad S, Nabavi B, Namjooyanc F (2014) Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf Iran. J Pharm Res 13:163–170
Yessuf AM, Khan MA (2015) Phytochemical investigation and characterization on the fruit peel of Solanum marginatum (methanol extract). AARJMD 1:184–195
Seal T (2006) Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in SSleaves, Sonchusarvensis and Oenanthelinearisof North eastern region in India. J Appl Sci 6:152–166
Jeffrey SW, Humfrey GF New spectrometric equations for determining chlorophyll a,b,c,in higher plants ,algae and natural phytoplankton. Biochemie und Physiologie der Pflazen 165(1975):191–194
Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Rathnapuram HP, Vutukuru SS, Yadavalli R (2018) Mixotrophic transition induced lipid productivity in Chlorella pyrenoidosa under stress conditions for biodiesel production. Heliyon 4:e00496
J.W. Baviskar, S.R. Khandelwal, Extraction, detection and identification of flavonoids from microalgae: an emerging secondary metabolite, Int J Curr Microbiol App Sci (2015) Special Issue-2: 110–117
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang JW Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155(2014):330–333
Wang HL, Wang W, Ping Z, Pan QH, JiCheng Z, WeiDong H (2010) Gene transcript accumulation, tissue and subcellular localization of anthocyanidin synthase (ANS) in developing grape berries. Plant Sci 179:103–113
Thresh K, Ibrahim RK (1985) Are spinach chloroplasts involved in flavonoid O-methylation. Z Naturforsch 40:331–335
Vander Werf A, Vannuenen M, Visser AJ, Lambers H (1993) Effects of N-supply on the rates of photosynthesis and shoot and root respiration of inherently fast- and slow-growing monocotyledonous species. Physiol Plant 89:563–569
Coronado C, Zuanazzi JAS, Sallaud C, Quirion JC, Esnault R, Husson HP, Kondorosi A, Ratet P (1995) Alfalfa root flavonoid production is nitrogen regulated. Plant Physiol 108:533–542
Medic-Saric M, Jasprica I, Smolcic-Bubalo A, Mornar A (2004) Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croat Chem Acta 77:361–366
Jayshree A, Jayashree S, Thangaraju N (2016) Chlorella vulgaris and Chlamydomonasreinhardtii: effective antioxidant, antibacterial and anticancer mediators. Indian J Pharm Sci 78:575–581
Heldt HW (2005) Plant Biochemistry. Elsevier, San Diego, pp 165–192
Mukherjee P, Gorain PC, Paul I, Bose R, Bhadoria PBS, Pal R (2020) Investigation on the effects of nitrate and salinity stress on the antioxidant properties of green algae with special reference to the use of processed biomass as potent fish feed ingredient. Aquac Int 28:211–234
Hertweck C (2009) The biosynthetic logic of polyketide diversity. Angew Chem Int Ed 48:4688–4716
Ohlrogge JB, Jaworski JG (1997) Annu Rev. Regulation of fatty acid synthesis. Plant Physiol Plant Mol Biol 48:109–136
Enamala MK, Dixit R, Tangellapally A, Singh M, Dinakarrao SMP, Chavali M, Pamanji SR, Ashokkumar V, Kadier A, Chandrasekhar K (2020) Photosynthetic microorganisms (Algae) mediated bioelectricity generation in microbial fuel cell: concise review. Environ Technol Innov 19:100959
Enamala MK, Enamala S, Chavali M, Donepudi J, Yadavalli R, Kolapalli B, Aradhyula TV, Velpuri J, Kuppam C (2018) Production of biofuels from microalgae - a review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sust Energ Rev 94:49–68
Funding
This work was funded by the Department of Biotechnology, Government of India, under the research grant # BT/PR13125/PBD/26/448/2015. YR and CNR thank the Management and Principal of CBIT for constant support and encouragement in carrying out this work. The CK would like to acknowledge the financial assistance from Ton Duc Thang University, Ho Chi Minh City, Vietnam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chlorella vulgaris in mixotrophic mode has reported simultaneous production of both flavonoids and lipid content
• Chlorella pyrenoidosa showed maximum flavonoid production of 138 μg/ml in autotrophic mode.
• Supplementation of carbon and l-phenylalanine in mixotrophic mode yielded maximum lipids and flavonoids, favoring the biorefinery approach.
• The scavenging activity of the extracted flavonoids was found to be in the range of 63–73%.
Rights and permissions
About this article
Cite this article
Yadavalli, R., Ratnapuram, H., Motamarry, S. et al. Simultaneous production of flavonoids and lipids from Chlorella vulgaris and Chlorella pyrenoidosa. Biomass Conv. Bioref. 12, 683–691 (2022). https://doi.org/10.1007/s13399-020-01044-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01044-x