Abstract
The production of high-valuable chemicals, such as furans, from biomass is a hot topic in the past decade. It used to be abstracted from fossil resources. This paper presents a study on the production of furan compounds via catalytic pyrolysis of cellulose on a two-staged fixed-bed reactor. Four solid acidic catalysts including Nb2O5, γ-Al2O3, ZSM-5, and TS-1, were used for the catalytic cracking. The selectivity of furans is 9.6% in a blank test and increased to 43.9% and 20.7% over TS-1 and Nb2O5, respectively. Nevertheless, ZSM-5 and γ-Al2O3 lowered the furan selectivity to 6.8% and 8.8%, respectively. The relationship between the physicochemical properties of different catalysts and their catalytic selectivity towards furan compounds were discussed based on characterization results of N2 physisorption, XRD, NH3-TPD, Py-IR, and TG/DTG.

Furan compounds were studied in catalytic pyrolysis of cellulose, and high selectivity of furans was achieved over TS-1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy plays a significant role in economic development and promotes the progress of human society [1]. Petroleum, natural gas, and coal are categorized fossil resources and have the main effect in production of fuels and chemicals since the industrial revolution [2]. However, people gradually became aware of that fossil resources are limited. The climate change and global warming are triggered by slather fossil resources [3]. Large amounts of greenhouse gases (GHGs) were released into atmosphere when fossil fuels were burnt, and these gases will cause planet to warm. Beyond that, poisonous gas such as sulfur dioxide is produced in the process of burning fossil fuels [4]. It has been widely accepted that global warming is the greatest threat and challenge to prevent economy from moving forward and raise the living standard of mankind [5].
For these reasons, much attention has been drawn attention to renewable energy such as biomass. Biomass energy is one of humanity’s earliest sources of energy particularly in rural areas. And it is often the only accessible and affordable source of energy in poor areas [6]. Physical, chemical, and biological conversion processes can be used to convert biomass into valuable fuels and chemicals [7]. Biomass is highly beneficial to save fossil fuels and reduce CO2 emission for the reason that biomass consumes the same amount of CO2 from the atmosphere during growth as is released during combustion [8, 9].
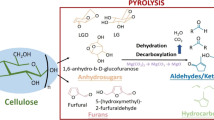
Furan compounds (furan, furfural, 5-hydroxymethylfurfural (HMF), 5-methylfurfural, etc.), as bridges connecting biomass raw materials to the biorefinery industry [10], can be used in the synthesis of renewable diesel and jet fuel [11]. Moreover, furans have been widely used as organic and reagent for the production of medicines, resins, food additives, fuel additives, and other special chemicals [12]. The research of biomass conversion and preparation of furans has significant meaning to reduce the environment impact of carbon dioxide and mitigate the greenhouse effect.
Some studies have been published work regarding synthesis and preparation of furans. The tradition method for furan production involves the use of catalyst in organic solvent, and the solvent was usually poisonous [13]. Here in this work, an environment-friendly method for furan production via catalytic pyrolysis of cellulose is proposed. Fixed bed reactor was used, and the volatiles from pyrolysis of cellulose were passed through a catalyst bed for the production of the target furan compounds. Cellulose is the most abundant form of biomass, with an annual large production and is a linear polymer made of glucose. It is recognized as a promising alternative for fossil-based fuels and chemicals. [14]
Xu et al. reported the catalytic pyrolysis of cellulose to produce furans. They used a physically mixed mixture of cellulose and catalyst with masses of 0.3 mg and 2.4 mg, respectively [15]. With the increase of temperature, cellulose started to crack; however, the catalysts had not been activated yet in the low temperature. The reaction temperature was not optimized for the use of catalyst. In addition, the mass ratio of catalyst and cellulose was as high as 8:1, which is costly. In this work, two-staged packed-bed reactor was used to improve the catalytic pyrolysis of cellulose for the production of furans. The mass ratio of catalyst and cellulose was reduced to 1:1.
There are two approaches to convert pyrolysis volatiles into furans: glucose dehydration and oxygen-containing molecules epoxidation. Detailed reaction process of how furans produce is shown in Scheme 1.
It is reported that Wang et al. prepared HMF from fructose over Nb2O5 in DMSO at 120 °C. HMF was obtained from fructose dehydration, the conversion of fructose was 100%, and the yield of HMF was 86.2% [16]. Zhou et al. reported that the water yield from rice husks during pyrolysis was 6.45% at 350 °C without catalyst, and the presence of NiO/γ-Al2O3 increased the water yield to 15.95% [17]. Tang et al. obtained a result that Co-ZSM-5 achieved 88.4–92.4 mol% conversion with the overall selectivity of 94.6–96.6% for the epoxidation of styrene and 92.8–90.6 mol% conversion with the selectivity of 86.1–88.3% for the epoxidation of α-pinene [18]. Au/TS-1 was used to synthesis propylene oxide by means of propene epoxidation with H2 and O2 by Song et al. [19]. Therefore, these four commercial catalysts including Nb2O5, TS-1, ZSM-5, and γ-Al2O3 were used for catalytic cracking of cellulose pyrolysis volatiles. Nb2O5 and γ-Al2O3 have been used for dehydration reactions, so these two solid acidic catalysts can be used to produce furans by dehydration. ZSM-5 and TS-1 were selected for their catalytic activity of epoxidation. The catalytic performance towards furan production was detailed investigated. The physicochemical properties of these catalysts were characterized by several methods. In this study, the dehydration and epoxidation measures of various catalysts on the basis of the same pyrolysis conditions were identified to see if selective dehydration and epoxidation are suitable methods to improve selectivity of furans.
In this work, the selectivity of furans is 9.6% in a blank test and increased to 43.9% and 20.7% over TS-1 and Nb2O5, respectively. Nevertheless, ZSM-5 and γ-Al2O3 lowered the furan selectivity to 6.8% and 8.8%, respectively. TS-1 was often applied for alkene and alkane epoxidation, cyclohexanone ammoxidation with NH3 and phenol hydroxylation [20]. In this research, TS-1 was used for catalytic conversion of cellulose to furans and has achieved significant results.
1.1 Materials
Cellulose, Nb2O5, TS-1, γ-Al2O3, and ZSM-5 were purchased from Macklin. Argon (99.99%) was purchased from Linde Industrial Gases.
1.2 Catalyst characterization
The surface areas, pore volumes, and pore size distribution of Nb2O5, γ-Al2O3, ZSM-5, and TS-1 were determined on a volumetric adsorption unit (Micromeritics ASAP 2020). Catalyst samples were degassed at 300 °C for 3 h under vacuum before experiments and then were carried out at a temperature of − 190 °C.
X-ray diffraction patterns (XRD) were recorded on a Rigaku Ultima IV apparatus using Ni-filtered Cu Kα radiation (λ = 0.15406 nm), and Bragg’s angles between 10 and 90° were scanned at a rate of 5°/min.
NH3 temperature programmed desorption (NH3-TPD) is carried out using Autosorb-IQ-C chemisorption analyzer Quantachrome, USA. About 100 mg was pretreated in a He stream at 450 °C for 1 h with a flow rate of 50 mL/min to clean the catalyst surface. After cooling to 120 °C, NH3 adsorption and He purge were performed on the samples. The samples were then heated to 800 °C at a temperature ramp rate of 10 °C/min, and the TPD signal was recorded simultaneously using a thermal conductivity detector (TCD).
Pyridine-thermal desorption-infrared analysis (Py-IR) was carried out using Nicolet380. Prior to analysis, about 20 mg of the catalyst was activated in situ cell at 450 °C for 1 h in helium at a flow rate of 50 mL/min and at 10−3 Pa. Following which, the samples were exposed to pyridine vapor until the saturation of adsorption capacity after cooling to room temperature. Then, the cell was then heated to 150 °C at a rate of 10 °C/min, remained at this temperature for 1 h to remove the physically adsorbed pyridine, and desorption curve was obtained. The mass of Nb2O5, TS-1, ZSM-5 and γ-Al2O3 were 11.3 mg, 13.9 mg, 10.4 mg and 16.3 mg, respectively.
The carbon deposited on used catalysts was examined by temperature-programmed oxidation (TPO), which was conducted on a Netsch STA449F5 thermal. Typically, about 10 mg of sample was heated from 50 to 800 °C at a rate of 10 °C/min with 100 mL/min air. The weight change as a function of temperature was recorded.
1.3 Catalytic activity evaluation
As is shown in Fig. 1, the structure of the reactor is divided into two parts: the 1st reactor is for pyrolytic reaction and 2nd reactor is for catalytic reaction. So, the catalyst and cellulose were not mixed. The temperature control systems of the two parts were independent of each other. For each experiment, cellulose was put in the 1st reactor and the catalyst was put in the 2nd reactor, respectively. The internal diameter of the quartz tube was 8 mm.
In this work, catalysts, raw material, and product were placed apart, and it is convenient for the product collection and reuse of the catalysts. This approach is an efficient and environmental method to produce furans. The catalytic cracking of pyrolysis vapors over different catalysts was evaluated in a tubular quartz packed-bed reactor.
The weight loss change as a function of temperature was investigated under N2 atmosphere, as shown in Fig. 2. Cellulose pyrolysis was mainly at the temperature range of 300–350 °C with the maximum weight loss rate at 343 °C. When temperature was higher than 350 °C, almost all cellulose was pyrolyzed with a very low solid residue left [21]. For the reason above, the final pyrolysis temperature was determined at 350 °C.
Before the reaction, the cold trap had already started work; argon with flow rate of 60 mL/min was introduced into the reactor for 10 min to eliminate the air. The catalyst was heated to 500 °C at a rate of 30 °C/min, held for 5 min to activate catalyst firstly, and then heat the cellulose to 350 °C at a rate of 30 °C/min held for 5 min to collect products. The liquid products were condensed with at − 20 °C. The mass of raw material was 0.5 g, and catalyst-to-cellulose ratio was 1:1.
1.4 Product analysis
The collected liquid product was dissolved in methanol, after removing water with anhydrous magnesium sulfate the product analyzed by GC-QP2010 gas chromatograph equipped with a capillary column Rtx-5 MS (30 m × 0.25 mm × 0.25 μm). For data processing, MassHunter Qualitative analysis software from OPGU Technologies was used. The injector was operated at 240 °C. The oven temperature was raised from 50 °C to 260 °C at a heating rate 5 °C/min and hold for 2 min. He was used as carrier gas with 1 mL/min. Injection volume was 0.4 μL and injected at a split ratio of 80. The selectivity (SEL) of furans or other compounds is calculated by the peak area of chromatographs.
2 Results and discussion
2.1 Physicochemical properties of the catalysts
Figure 3a shows the N2 adsorption/desorption isotherms. For four catalysts, the presence of intra-crystalline voids accessible only via entrances smaller than 1.6 nm, 4.5 nm, 1 nm, and 5 nm are evident from abrupt closure at P/P0 = 0.16, 0.5, 0.05, and 0.42 on the desorption branch [22]. The Nb2O5 exhibit type VI with H3 hysteresis loop and TS-1 shows IV isotherm with type H4 hysteresis loop. ZSM-5 presents type I isotherm with H4 hysteresis loop, and γ-Al2O3 shows type V with H3 hysteresis loop [23]. TS-1 exhibits a sharp uptake at low relative pressure, suggesting the presence of microporous structure in TS-1 [24]. The abundant pore structure of TS-1 is beneficial for more gas molecule diffusion, including glucose molecule and other smaller organic molecules.
The textural properties of catalysts are shown in Table 1. It can be seen that TS-1 has the maximum specific surface area of 443 m2/g with pore volume and average pore diameter of 0.30 cm3/g and 2.7 nm, respectively. The specific surface area of ZSM-5 and γ-Al2O3 is 337 m2/g and 273 m2/g, respectively. Nb2O5 has the minimum specific surface area of 4.3 m2/g. The specific surface area of the catalyst was decreased in the order of TS-1 > ZSM-5 > γ-Al2O3 > Nb2O5. TS-1 presents excellent catalytic activity in the catalytic reforming reaction due to its high specific surface area.
From Fig. 3b, it shows the pore size distribution of four samples. The peak of TS-1 and γ-Al2O3 both appears at 3.9 nm.
The X-ray diffraction patterns are shown in Fig. 4. For Nb2O5, clear diffractions at 22.4°, 28.36°, 28.96°, and 36.56° can be detected, which is matched with the pseudohexagonal Nb2O5 crystals (TT phase) [25]. XRD pattern of TS-1 shows several diffraction peaks at 14.74°, 23.04°, 23.24°, 23.66°, and 23.88°, indicating that TS-1 is MFI structure with orthorhombic symmetry and highly crystalline [26]. The sharp single diffraction peaks at 2θ = 24.36° suggests the presence of Ti in the framework, and the presence of orthorhombic unit cell structure is proved by the absence of splitting peak at 2θ of 25.34° [27]. The XRD pattern of ZSM-5 shows five resolved peaks at 14.74°, 23.04°, 23.28°, 23.7°, and 23.92°, which are associated with the hexagonal symmetry [28]. Two broad peaks at 14.42° and 28.36° can be observed for the γ-Al2O3, but peak intensity is weak, suggesting a low degree of crystallinity.
Average particle sizes of Nb2O5, TS-1, ZSM-5, and γ-Al2O3 calculated by the Debye-Scherrer equation [29] are 71.6 nm, 42.1 nm, 73.4 nm, and 65.4 nm, respectively.
The acidic properties of the catalysts are studied by NH3-TPD, and the results are shown in Fig. 5. The TS-1 and ZSM-5 mainly contain a weak acid center (< 300 °C), appear separately at 101 °C and 110 °C, but ZSM-5 also contains a strong acid (> 300 °C) at 726 °C. γ-Al2O3 shows two desorption peaks at both the weak acid center and the strong acid center (> 300 °C) at 108 °C and 443 °C, and the peak of strong acid center is much high than weak acid center. The curve of Nb2O5 is almost a straight line without any peak, which illustrates that its acidity is extremely weak [30].
Figure 6 shows Py-IR spectra recorded after pyridine adsorption/desorption at 200 °C. The concentrations of Brønsted and Lewis acid sites were commonly detected in the frequency range of 1400–1650/cm [31]. As shown in Fig. 6, the Brønsted acid sites on Nb2O5, TS-1, ZSM-5, and γ-Al2O3 are corresponded to 1548/cm, 1542/cm, 1540/cm, and 1540/cm, respectively, and Lewis acid sites are assigned to 1456/cm, 1446/cm, 1444/cm, and 1446/cm, respectively. Table 2 shows the concentration of Brønsted and Lewis acid sites. The quantitative evaluation of the acidity shows that Nb2O5 has little acidic, which agrees well with results of NH3-TPD. The acidity of γ-Al2O3 is stronger than Nb2O5 but is much weaker than TS-1 and ZSM-5.
2.2 Catalytic activity
During catalytic pyrolysis process, an appropriate catalyst plays an important role in furan production. Nb2O5, γ-Al2O3, ZSM-5, and TS-1 were used. The product distribution details of in catalytic pyrolysis of cellulose with various catalysts are shown in Fig. 7. It can be seen that the products from catalytic pyrolysis of cellulose over different catalysts were glucose, furan, hydrocarbons, and so on. Without the addition of catalysts, selectivity of furans and glucose is 9.6% and 49.7%; the selectivity of furans and glucose is 43.9% and 20.0% over TS-1. Although TS-1 and ZSM-5 both have weak acidity, only TS-1 shows high selectivity to furans. The catalytic dehydration of γ-Al2O3 is too strong so that plenty of oxygen containing compounds including glucose is converted to hydrocarbon. The selectivity of hydrocarbon over γ-Al2O3 is 75.0%. The selectivity of hydrocarbon is 5.0% over ZSM-5 due to the strong acid property (> 300 °C). From the above discussion, it can be concluded that the presence of strong acid sites enhances their oxygen removal ability. The phenomenon shows the addition of TS-1 and γ-Al2O3 is contributed to the production of furans and hydrocarbon, respectively. In spite of minimum specific surface area and weakest acidity, the selectivity of furan over Nb2O5 is 20.7%. This is due to gas-phase dehydration at high temperature.
2.3 Characterization on used catalysts
The carbon deposited on used catalysts was characterized by means of TG technique. As shown in Fig. 8, the amount of carbon deposition was in the order of γ-Al2O3 > ZSM-5 > Nb2O5 > TS-1. For the γ-Al2O3, the sharp weight loss at 450 °C reflects a kind of carbon deposition on the active site. The DTG curves (Fig. 9) also show a strong peak due to the burnt from the carbon deposits by oxidation, and there was a gain in weight below 400 °C (Fig. 8) due to oxygen adsorption [32, 33]. Nb2O5 has few coke depositions due to the lowest specific surface area and weakest acid. Carbon deposits of ZSM-5 are higher than TS-1, which is due to the stronger Brønsted acid of ZSM-5 [34].
3 Conclusion
Furan compounds are produced from catalytic pyrolysis of cellulose over different catalysts. Due to its strong acidic surface property, hydrocarbon instead of furans is favorably produced over γ-Al2O3 probably via dehydration reaction. ZSM-5 with moderate acidic sites showed limited activity in epoxidation and subsequently a low selectivity to furans. TS-1, with weak acidic surface property performed opportune dehydration property and epoxidation property, results in a high selectivity towards furans. Although Nb2O5 also shows a weak surface property, the selectivity towards furans is low due to its low specific surface area.
The obtained results suggest that TS-1 is a good candidate for the production of furans by catalytic pyrolysis of cellulose. Further studies on the improvement of selectivity to furans will be done by loading different metals.
References
Chen L, Li X, Wen W, Jia J, Li G, Deng F (2012) The status, predicament and countermeasures of biomass secondary energy production in China. Renew Sust Energ Rev 16(8):6212–6219. https://doi.org/10.1016/j.rser.2012.07.006
Chheda JN, Dumesic JA (2007) An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal Today 123(1–4):59–70. https://doi.org/10.1016/j.cattod.2006.12.006
Ravishankara AR, Rudich Y, Pyle JA (2015) Role of chemistry in Earth’s climate. Chem Rev 115(10):3679–3681. https://doi.org/10.1021/acs.chemrev.5b00226
Joselin Herbert GM, Unni Krishnan A (2016) Quantifying environmental performance of biomass energy. Renew Sust Energ Rev 59:292–308. https://doi.org/10.1016/j.rser.2015.12.254
Shuit SH, Tan KT, Lee KT, Kamaruddin AH (2009) Oil palm biomass as a sustainable energy source: a Malaysian case study. Energy 34(9):1225–1235. https://doi.org/10.1016/j.energy.2009.05.008
Hall DO, Rosillocalle F, Degroot P (1992) Biomass energy - lessons from case-studies in developing-countries. Energy Policy 20(1):62–73. https://doi.org/10.1016/0301-4215(92)90148-u
Knapek J, Kralik T, Valentova M, Vorisek T (2015) Effectiveness of biomass for energy purposes: a fuel cycle approach. Wiley Interdiscip Rev Energy Environ 4(6):575–586. https://doi.org/10.1002/wene.164
Demirbas A (2004) Combustion characteristics of different biomass fuels. Prog Energy Combust Sci 30(2):219–230. https://doi.org/10.1016/j.pecs.2003.10.004
Herbst A, Janiak C (2016) Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J Chem 40(9):7958–7967. https://doi.org/10.1039/c6nj01399f
Chen S, Wojcieszak R, Dumeignil F, Marceau E, Royer S (2018) How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem Rev 118(22):11023–11117. https://doi.org/10.1021/acs.chemrev.8b00134
Yang J, Li S, Zhang L, Liu X, Wang J, Pan X, Li N, Wang A, Cong Y, Wang X, Zhang T (2017) Hydrodeoxygenation of furans over Pd-FeOx/SiO2 catalyst under atmospheric pressure. Appl Catal B Environ 201:266–277. https://doi.org/10.1016/j.apcatb.2016.08.045
Lu Q, Dong C-q, X-m Z, H-y T, Yang Y-p, Zhu X-f (2011) Selective fast pyrolysis of biomass impregnated with ZnCl2 to produce furfural: analytical Py-GC/MS study. J Anal Appl Pyrolysis 90(2):204–212. https://doi.org/10.1016/j.jaap.2010.12.007
Galadima A, Muraza O (2019) Zeolite catalyst design for the conversion of glucose to furans and other renewable fuels. Fuel 258:115851. https://doi.org/10.1016/j.fuel.2019.115851
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115(21):11559–11624. https://doi.org/10.1021/acs.chemrev.5b00155
Chen X, Chen Y, Chen Z, Zhu D, Yang H, Liu P, Li T, Chen H (2018) Catalytic fast pyrolysis of cellulose to produce furan compounds with SAPO type catalysts. J Anal Appl Pyrolysis 129:53–60. https://doi.org/10.1016/j.jaap.2017.12.004
Wang F, Wu HZ, Liu CL, Yang RZ, Dong WS (2013) Catalytic dehydration of fructose to 5-hydroxymethylfurfural over Nb2O5 catalyst in organic solvent. Carbohydr Res 368:78–83. https://doi.org/10.1016/j.carres.2012.12.021
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12–13):1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Tang B, Lu XH, Zhou D, Lei J, Niu ZH, Fan J, Xia QH (2012) Highly efficient epoxidation of styrene and α-pinene with air over Co2+-exchanged ZSM-5 and Beta zeolites. Catal Commun 21:68–71. https://doi.org/10.1016/j.catcom.2012.01.029
Song Z, Feng X, Sheng N, Lin D, Li Y, Liu Y, Chen X, Chen D, Zhou X, Yang C (2019) Cost-efficient core-shell TS-1/silicalite-1 supported Au catalysts: towards enhanced stability for propene epoxidation with H2 and O2. Chem Eng J 377. https://doi.org/10.1016/j.cej.2018.09.088
Serrano DP, Sanz R, Pizarro P, Moreno I, Medina S (2014) Hierarchical TS-1 zeolite as an efficient catalyst for oxidative desulphurization of hydrocarbon fractions. Appl Catal B Environ 146:35–42. https://doi.org/10.1016/j.apcatb.2013.05.025
Zhou Y, Chen Z, Gong H, Chen L, Yu H, Wu W (2019) Characteristics of dehydration during rice husk pyrolysis and catalytic mechanism of dehydration reaction with NiO/γ-Al2O3 as catalyst. Fuel 245:131–138. https://doi.org/10.1016/j.fuel.2019.02.059
Dai J, Zhong W, Yi W, Liu M, Mao L, Xu Q, Yin D (2016) Bifunctional H2WO4/TS-1 catalysts for direct conversion of cyclohexane to adipic acid: active sites and reaction steps. Appl Catal B Environ 192:325–341. https://doi.org/10.1016/j.apcatb.2016.04.005
Ewald S, Kolbeck M, Kratky T, Wolf M, Hinrichsen O (2019) On the deactivation of Ni-Al catalysts in CO2 methanation. Appl Catal A Gen 570:376–386. https://doi.org/10.1016/j.apcata.2018.10.033
Ren Y-g, Huang J, Lv Q, Xie Y, Lu A-H, Haruta M (2019) Dual-component gas pretreatment for Au/TS-1: enhanced propylene epoxidation with oxygen and hydrogen. Appl Catal A Gen 584. https://doi.org/10.1016/j.apcata.2019.117172
Ristic M, Popovic S, Music S (2004) Sol-gel synthesis and characterization of Nb2O5 powders. Mater Lett 58(21):2658–2663. https://doi.org/10.1016/j.matlet.2004.03.041
Khomane RB, Kulkarni BD, Paraskar A, Sainkar SR (2002) Synthesis, characterization and catalytic performance of titanium silicalite-1 prepared in micellar media. Mater Chem Phys 76(1):99–103. https://doi.org/10.1016/s0254-0584(01)00507-7
Zuo Y, Liu M, Zhang T, Hong L, Guo X, Song C, Chen Y, Zhu P, Jaye C, Fischer D (2015) Role of pentahedrally coordinated titanium in titanium silicalite-1 in propene epoxidation. RSC Adv 5(23):17897–17904. https://doi.org/10.1039/c5ra00194c
Song C-M, Yan Z-F (2008) Synthesis and characterization of M-ZSM-5 composites prepared from ZSM-5 zeolite. Asia Pac J Chem Eng 3(3):275–283. https://doi.org/10.1002/apj.146
Jensen H, Pedersen JH, Jorgensen JE, Pedersen JS, Joensen KD, Iversen SB, Sogaard EG (2006) Determination of size distributions in nanosized powders by TEM, XRD, and SAXS. J Exp Nanosci 1(3):355–373. https://doi.org/10.1080/17458080600752482
Gelves J-F, Dorkis L, Marquez M-A, Alvarez A-C, Gonzalez L-M, Villa A-L (2019) Activity of an iron Colombian natural zeolite as potential geo-catalyst for NH3-SCR of NOx. Catal Today 320:112–122. https://doi.org/10.1016/j.cattod.2018.01.025
Benaliouche F, Boucheffa Y, Ayrault P, Mignard S, Magnoux P (2008) NH3-TPD and FTIR spectroscopy of pyridine adsorption studies for characterization of Ag- and Cu-exchanged X zeolites. Microporous Mesoporous Mater 111(1–3):80–88. https://doi.org/10.1016/j.micromeso.2007.07.006
Liu BS, Au CT (2003) Sol-gel-generated La2NiO4 for CH4/CO2 reforming. Catal Lett 85(3–4):165–170. https://doi.org/10.1023/a:1022133511420
Liu BS, Jiang L, Sun H, Au CT (2007) XPS, XAES, and TG/DTA characterization of deposited carbon in methane dehydroaromatization over Ga–Mo/ZSM-5 catalyst. Appl Surf Sci 253(11):5092–5100. https://doi.org/10.1016/j.apsusc.2006.11.031
Chai S, Wang H, Liang Y, Xu B (2007) Sustainable production of acrolein: gas-phase dehydration of glycerol over Nb2O5 catalyst. J Catal 250(2):342–349. https://doi.org/10.1016/j.jcat.2007.06.016
Funding
The work was supported by the National Natural Science Foundation of China (No. 31870570, No. 31670599), the Fundamental Research Funds for the Central Universities (20720170043), and the Scientific Research Start-up Funding for Special Professor of Minjiang Scholars, Xiamen University, China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Catalytic cracking of volatiles from cellulose pyrolysis for furan production was performed.
• Four solid acidic catalysts including Nb2O5, ZSM-5, Al2O3, and TS-1 were used.
• TS-1 showed the highest selectivity of 43.9% for furan compounds.
Rights and permissions
About this article
Cite this article
Zhu, Y., Li, W., Huang, Y. et al. Catalytic pyrolysis of cellulose over solid acidic catalysts: an environment-friendly method for furan production. Biomass Conv. Bioref. 11, 2695–2702 (2021). https://doi.org/10.1007/s13399-020-00812-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00812-z