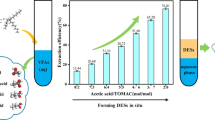
Abstract
Green chemistry era forces scientists to develop and apply new media in recovery of high-added value materials. It is interesting to utilize deep eutectic liquids (DELs), which have advantages of biodegradability and very low toxicity and ease of use, in separating these fine materials from a complex environment. DELs have been specially designed and used for the extraction of recovery of acetic acid (AA) from its aqueous solutions. Two DELs containing the same hydrogen bond donor-HBD (glycerol) and two different hydrogen bond acceptor-HBA (a quaternary ammonium salt and an amine-based) have been designed with a molar ratio of 1:2. The tailor-designed extractants were diluted with diethyl adipate (DEA), diethyl malonate (DEM), and diethyl succinate (DES), respectively. Extraction efficiency of the diluents has been increased more than 4 times. To compare the results with that of the ionic liquid (IL), 1-hexyl-3-methylimidazolium bromide has been used in the same organic solvents. Efficiencies of the DELs have surpassed 1.4 to 4 times over the IL.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With regard to green chemistry, although the development of solvent-free processes seems to be excellent, it is very difficult to successfully commercialize a solvent-free process. It is almost inevitable to use solvents because of their important role in the dissolution of solids, in mass and heat transfer, in changing viscosity, and in separation and purification steps. It is obvious that most of the practical processes require a solvent. This makes the green solvents attractive. Therefore, ionic liquids (ILs) attracted attention as green solvents [1]. However, considering other parameters such as toxicity and price, ILs were insufficient to classify as green solvents [2]. So, attempts have been evolved for relatively cheap solvents with lower detrimental to the environment. In this study, a new ionic liquid family called deep eutectic liquid (DEL), which is very new and rapidly emerged, has been investigated. DELs are sometimes referred to the fourth-generation ionic liquid although they are not considered to be ionic liquids, because they do not consist entirely of ionic species [3]. DELs are eutectic mixtures which are easily prepared by mixing two or three low-cost components (such as quaternary ammonium salts, amides, organic acids, and polyalcohol) [4]. A DEL is a fluid containing two or three inexpensive and reliable components to form an eutectic mixture with a lower melting point than the individual components through hydrogen bond interactions [5]. DELs are generally liquid at temperatures below 100 °C [6]. Furthermore, the relevant liquids exhibit physicochemical properties similar to those of conventional ionic liquids, but DELs are much cheaper and environmentally friendly. Due to the concerned advantages, there is a growing interest in many of the research areas such as catalysis, organic synthesis, extraction processes, electrochemistry, and material chemistry [7].
Carboxylic acids have been studied in a wide variety of scientific papers since they are utilized in many commercial products such as detergents, surfactants, pharmaceuticals, cosmetics, and food [8]. On the other hand, recovery of these valuable materials from renewable resources such as fermentation media is an attractive research area. In this study, recovery of acetic acid (AA) from its aqueous solutions has been chosen as the investigation media. Several extractant systems such as amines dissolved in diluents were applied to investigate the reactive extraction of AA from its aqueous media [9]. Hong and Hong (2005) used tri-n-octylamine in 1-octanol to extract AA [10]. Caşcaval et al. also used the same amine in dichloromethane, butyl acetate, and n-heptane with/without 1-octanol for similar reasons [9]. Aliphatic tertiary amine (trioctylamine) diluted with octane [11] and 2-ethyl-hexanol [12] were utilized to recover AA from pyrolysis oil, respectively. Differently, triisooctylamine in decanol was also exploited as an extractant to recover AA from pre-hydrolysis liquor in pulp production [13]. Alternatively, ionic liquids (phosphonium and imidazolium types) in several diluents have started to be used as extractant system to extract AA from various media [14]. The first purpose of the present study is to synthesize these original solvents (DELs), which are also considered as design products. In the second step, such novel solvents have been used to obtain AA from its aqueous solutions. Finally, the findings of DELs have been compared to those of an IL for control reasons.
2 Materials and methods
2.1 Chemical materials
Table 1 summarizes the chemical materials used in the present study with their cast registry number, suppliers, and purity properties. Deionized water was obtained by a Millipore Direct-Q3 ultrapure water system (≥ 18 M Ω cm).
2.2 Synthesis of deep eutectic liquids
Deep eutectic liquids are mixtures prepared by combining a quaternary ammonium salt (hydrogen bond acceptor-HBA) and a hydrogen bond donor (HBD). The relevant solvent systems were synthesized by heating and mixing. The components constituting the mixture (Table 2) are placed in a closed flask and stirred at 80 °C until a clear liquid is obtained with a magnetic stirrer [15]. The mixture of choline chloride and glycerol (1:2, molar ratio) has been called as DEL1, while N,N′-dimethyl urea and glycerol (1:2, molar ratio) has been DEL2.
2.3 Procedure for reactive extraction
First, 10% (v/v) of AA-water solution was prepared. In order to evaluate the initial concentration, different levels (10, 8, 6, 4, and 2%, v/v) of aqueous AA solution were prepared. About 5 mL of AA solution and 5 mL of extractant system containing DEL1, DEL2, and IL in diluents such as DEA, DEM, and DES were collected in an Erlenmeyer flask (50 mL). After the heterogeneous mixture was shaked (NUVE ST-402) at 25 °C for 3 h, a centrifugation process at 4000 rpm was performed for 15 min for the separation of the layers in liquid-liquid extraction. AA concentration in the extractant system was quantified according to the material balance through NaOH solution (0.1 mol L−1) depending on titration with uncertainty of 1%.
2.4 Fourier transformed infrared-attenuated total reflectance
Fourier transformed infrared-attenuated total reflectance (FTIR-ATR) spectra were used to characterize the extractants by means of a FTIR spectrometer (Bruker Optic GmbH, Ettlingen, Germany).
3 Results and discussions
3.1 Recovery of acetic acid with pure diluents
In order to state the success of an extraction process, distribution coefficient (D) and the efficiency (%) of the solvent system can be used.
D is calculated by the AA content in the extractant phase (CAA, extractant phase) divided by the AA content in the aqueous phase (CAA, aqueous phase):
The following equation is used regarding efficiency (%) by applying the initial concentration of the acid solution (CAA,0):
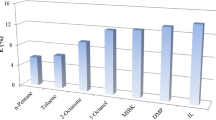
The carbon chain of the selected diluents increased in the order of DEA > DES > DEM. As seen in Fig. 1, extraction efficiency increased by the decrease in the carbon number of the diluent. This finding is consistent with that of Uslu (2009), where formic acid recovery decreased by the increase in the carbon chain length of the diluent used with trioctylamine [16]. Similarly, Uslu et al. also reported that levulinic acid extraction was enhanced by the decrease of the carbon number in the alcohol diluents [17]. The shorter the chain length, the more soluble the acid in the solvent system [18].
3.2 Extraction performance of DELs
Figure 2a and b show the effect of volume fractions (20, 40, 60, and 80%, v/v) of DEL1 and DEL2 on the distribution coefficients of the extraction systems in different diluents, respectively. Initial acid concentrations were 1.92 and 1.79 mol L−1 for each DEL. As seen in Table 3, acid recovery raised by increase in DEL concentration in the solvent system. Similar results were also reported for several carboxylic acids from aqueous media by different extractants (such as phosphorous-bonded oxygen donor extractants and high molecular weight aliphatic amines) in several diluents [8, 19, 20].
Another indicator for demonstration of extraction performance is known as loading factor (Z) [20]. It is calculated by the AA content in the extractant phase divided by the DEL content in the extractant phase (CDEL, extractant phase):
Figure 3a and b show the effect of volume fractions (20, 40, 60, and 80%, v/v) of DEL1 and DEL2 on the loading factors of the extraction system in different diluents, respectively. Expectedly, loading factors decreased by the volume fractions of each DEL as seen in Table 4 [20, 21].
3.3 Effect of initial AA concentration on the extraction yield
Figure 4a and b demonstrate the effect of initial acid concentrations (0.66, 0.96, 1.19, 1.47, and 2.03 mol L−1) on the distribution coefficients of the extraction systems in different diluents, respectively. The concentration of the DELs in the system was 60% (v/v). Both of the DELs showed the same trend toward the initial AA concentration (Table 5). Increasing the AA content in the aqueous solution resulted in less yield for both systems in each diluent. This finding is reasonable since carboxylic acid concentration in fermentation broths is known to be relatively low in practice [19].
3.4 Comparative findings of DELs and IL
DEM had the highest extraction yield (16.98%) comparing to DES (14.94%) and DEA (9.95% efficiency) as reported above. However, the most effective improvement was observed when DEA was used as the diluent for both DELs (Fig. 5). The extraction performance of the DEA was increased 3.1 times over the pure diluent by adding 60% DEL1. Regarding DEL2, its performance increased by 4.4 times. The performances of the DELs have surpassed that of the IL. This is the most promising finding of the study, where a novel tailor-designed solvent has been found to be successful. Even though ILs have known to be green solvents owing to their low vapor pressures and high boiling points, their membership of green chemistry has become controversial in the current literature [22]. Indeed, many reports have shown that ILs are toxic in dangerous dimensions through a very poor biodegradability [23,24,25]. On the other hand, synthesis of IL is very difficult to achieve with an environmentally friendly method since salt and solvent are used in large quantities. When high prices are added to these negative situations, it is almost impossible to carry ILs to the industrial dimensions. Nowadays, new concepts are strongly required in order to use these systems in a more reasonable way. As already seen in Fig. 5, DELs with higher yields are candidates to be new generation solvents to cope with the problems of ILs such as high cost and toxicity [3].
3.5 FTIR results of extractant
The characteristics peaks of the selected diluents (DEM, DES, and DEA) are given in Fig. 6a–c as reported in our previous study [26]. FTIR spectrums of IL and the designed solvents (DEL1 and DEL2) are given in Fig. 7. Since HBD used in the mixtures is the same in both DELs, they show similar spectrums with few exceptions (Fig. 7a and b). Peaks over 3200–3600 cm−1 correspondence to O–H groups of glycerol in both DELs (Fig. 7a and b) are also attributed to amine-based structure of DEL2. The stretching over 600–1400 also cm−1 while stretching bonds on 3000–3400 cm−1 are ascribed to N–H as a result of N,N′-dimethyl urea of DEL2 (Fig. 7b) [27]. Moreover, range between 1580 and 1650 cm−1 corresponds to C–H bending [28]. C–I stretching of halo compound in choline chloride (Fig. 7a) is observed at 500–600 cm−1. On the other hand, strong C-Br stretching of IL was seen over 515–690 cm−1as seen in Fig. 7c. Stretching over 1266–1342 cm−1 is a sign of C–N of aromatic amine in IL (Fig. 7c).
Figure 8a and b display the FTIR spectrums of the extractant systems in DES after extraction of AA from aqueous solutions. Similarly, both DELs show similar spectrums due to the same HBD used in the mixtures. The medium stretching over 1395–1440 cm−1 is ascribed to O–H bending of carboxylic acid extracted. The weak broad on 2700–3200 cm−1 corresponds to O–H.
4 Conclusions
Two DELs have been synthesized using glycerol as hydrogen bond donor and a quaternary ammonium salt and amine-based hydrogen bond acceptor. The designed solvents have proven to be an efficient candidate to extract acetic acid from its aqueous media by increasing the efficiency by 4.4 times over the pure organic solvent (without applying DEL). Considering the disadvantages of ionic liquids such as toxicity, poor biological degradability, and high cost (almost 5 to 20 times over the price of a conventional organic solvent), deep eutectic liquids will be a good alternative to cosmetic, pharmaceutical, or food applications with opportunities such as low price, water stability, and high atom economy (100%) to synthesize. Furthermore, properties such as nonvolatility and nonflammability make the storage of these DELs feasible for industrial applications.
References
Zhang Q, De Oliveira VK, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108. https://doi.org/10.1039/c2cs35178a
Tang B, Zhang H, Row KH (2015) Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J Sep Sci 38:1053–1064. https://doi.org/10.1002/jssc.201401347
Radošević K, Cvjetko Bubalo M, Gaurina Srček V et al (2015) Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol Environ Saf 112:46–53. https://doi.org/10.1016/J.ECOENV.2014.09.034
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Paiva A, Craveiro R, Aroso I et al (2014) Natural deep eutectic solvents – solvents for the 21st century. ACS Sustain Chem Eng 2:1063–1071. https://doi.org/10.1021/sc500096j
Andrew P. Abbott *, David Boothby, Glen Capper, et al (2004) Deep eutectic solvents formed between choline chloride and carboxylic Acids: Versatile Alternatives to Ionic Liquids. doi: https://doi.org/10.1021/JA048266J
Durand E, Lecomte J, Villeneuve P (2013) Deep eutectic solvents: synthesis, application, and focus on lipase-catalyzed reactions. Eur J Lipid Sci Technol 115:379–385. https://doi.org/10.1002/ejlt.201200416
Hong YK, Hong WH, Han DH (2001) Application of reactive extraction to recovery of carboxylic acids. Biotechnol Bioprocess Eng 6:386–394. https://doi.org/10.1007/BF02932319
Cas -Caval, D, Lenut -A Kloetzer † (2011) Influence of organic phase polarity on interfacial mechanism and efficiency of reactive extraction of acetic acid with tri-n-octylamine. J Chem Eng Data 56:2521–2526. doi: https://doi.org/10.1021/je200044y
Hong YK, Hong WH (2005) Removal of acetic acid from aqueous solutions containing succinic acid and acetic acid by tri-n-octylamine. Sep Purif Technol 42:151–157. https://doi.org/10.1016/J.SEPPUR.2004.03.015
Mahfud FH, van Geel FP, Venderbosch RH, Heeres HJ (2008) Acetic acid recovery from fast pyrolysis oil. An exploratory study on liquid-liquid reactive extraction using aliphatic tertiary amines. Sep Sci Technol 43:3056–3074. https://doi.org/10.1080/01496390802222509
Rasrendra CB, Girisuta B, van de Bovenkamp HH et al (2011) Recovery of acetic acid from an aqueous pyrolysis oil phase by reactive extraction using tri-n-octylamine. Chem Eng J 176–177:244–252. https://doi.org/10.1016/J.CEJ.2011.08.082
Yang G, Jahan MS, Ahsan L, Zheng L, Ni Y (2013) Recovery of acetic acid from pre-hydrolysis liquor of hardwood Kraft-based dissolving pulp production process by reactive extraction with triisooctylamine. Bioresour Technol 138:253–258. https://doi.org/10.1016/J.BIORTECH.2013.03.164
Li X, Kersten SRA, Schuur B (2017) Extraction of acetic acid, glycolaldehyde and acetol from aqueous solutions mimicking pyrolysis oil cuts using ionic liquids. Sep Purif Technol 175:498–505. https://doi.org/10.1016/J.SEPPUR.2016.10.023
Duan L, Dou L-L, Guo L, et al Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. doi: https://doi.org/10.1021/acssuschemeng.6b00091
Uslu H (2009) Reactive extraction of formic acid by using tri octyl amine (TOA). Sep Sci Technol 44:1784–1798. https://doi.org/10.1080/01496390902775893
Uslu H, I ˙ Smail Kırbas¸larkırbas¸lar S¸, Wasewar KL Reactive extraction of levulinic acid by amberlite LA-2 extractant. doi: https://doi.org/10.1021/je800261j
Kırbaşlar Şİ, Şahin S, Bilgin M (2006) (liquid + liquid) equilibria of (water + propionic acid + alcohol) ternary systems. J Chem Thermodyn 38:1503–1509. https://doi.org/10.1016/J.JCT.2006.05.001
Wasewar KL, Heesink ABM, Versteeg GF, Pangarkar VG (2002) Reactive extraction of lactic acid using alamine 336 in MIBK: equilibria and kinetics. J Biotechnol 97:59–68. https://doi.org/10.1016/S0168-1656(02)00057-3
Ahin S, Bayazit S, Bilgin M, et al Investigation of formic acid separation from aqueous solution by reactive extraction: effects of extractant and diluent. doi: https://doi.org/10.1021/je9006635
Uslu H, Datta D (2015) Experimental and theoretical investigations on the reactive extraction of itaconic (2-methylidenebutanedioic) acid using trioctylamine (N,N-dioctyloctan-1-amine). doi: https://doi.org/10.1021/je501131j
Deetlefs M, Seddon KR (2009) Assessing the greenness of some typical laboratory ionic liquid preparations. https://doi.org/10.1039/b915049h
Romero A, Santos A, Tojo J, Rodríguez A (2008) Toxicity and biodegradability of imidazolium ionic liquids. J Hazard Mater 151:268–273. https://doi.org/10.1016/J.JHAZMAT.2007.10.079
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150. https://doi.org/10.1039/B006677J
Cvjetko Bubalo M, Radošević K, Radojčić Redovniković I et al (2014) A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol Environ Saf 99:1–12. https://doi.org/10.1016/J.ECOENV.2013.10.019
Şahin S, Elhussein EAA, Bilgin M et al (2018) Investigation of extractive interaction between ionic liquids and carbamazepine. J Mol Liq 268:523–528. https://doi.org/10.1016/J.MOLLIQ.2018.07.088
AlOmar MK, Hayyan M, Alsaadi MA et al (2016) Glycerol-based deep eutectic solvents: physical properties. J Mol Liq 215:98–103. https://doi.org/10.1016/J.MOLLIQ.2015.11.032
Infrared Spectroscopy Absorption Table - Chemistry LibreTexts
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Şahin, S., Kurtulbaş, E. An advanced approach for the recovery of acetic acid from its aqueous media: deep eutectic liquids versus ionic liquids. Biomass Conv. Bioref. 12, 341–349 (2022). https://doi.org/10.1007/s13399-019-00599-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00599-8