Abstract
In this research, equilibrium, kinetics, and adsorption mechanism of methylene blue on a relatively large surface area (1437 m2 g−1) Azolla-derived micro- and mesoporous carbon has been investigated. The porous activated carbon was characterized using X-ray diffraction (XRD), Fourier transform infrared (FTIR), Raman and also X-ray photoelectron spectroscopy (XPS), N2 sorption, and high-resolution transmission electron microscopy. Remaining methylene blue solution revealed that the adsorption energy and adsorption capacity of the porous carbon are 21.76 kJ mol−1 and 1930 mg g−1, respectively. Based on the kinetics results, pseudo-second-order kinetic model is dominant during the adsorption process. Diffusion of methylene blue in the porosities and in the space between the graphitic planes of carbons is the rate-limiting parameters. Adsorption of methylene blue into the graphitic planes and the micro-porosities leads to some compressive stresses on the graphitic planes. The relative graphitization value of the prepared carbons will decrease during adsorption process. FTIR and XPS results demonstrate the effect of surface nitrogen groups on the adsorption of methylene blue. During the adsorption process, a relative percent of pyridinic nitrogen will decrease, pyrrolic and graphitic nitrogen of graphitic layers will eliminate and N-(C)3 and H-N-(C)2 nitrogen appear.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dye contaminants are of the main pollution sources of water and their presence can result in serious problems for many forms of life. These toxic substances have been utilized in different industries such as printing, paper, textile, food, cosmetics and electroplating [1]. Considerable amounts of wastewater are generated during the utilization of dyes in the industrial processes [2]. Therefore, improper disposal of these contaminants can bring about serious environmental problems [3].
Methylene blue (MB), one of these toxic dyes, is widely used in the large number of industries as a colorant agent. Although this compound is not classified as so hazardous material, it can cause permanent eye damages in human and animals. On inhalation, it can lead to short period of rapid or difficult breathing. Ingestion of methylene blue results in the burning sensation and may give rise to several problems such as nausea, vomiting, profuse sweating, mental confusion, and methemoglobinemia [1,2,3]. Therefore, removal of this harmful organic component from the wastewaters is of great necessity [4].
Several processes such as trickling filter [5], membrane filtration [6], activated sludge [7], chemical coagulation, and adsorption [8, 9] have been developed for removing of dye contaminants from wastewaters. Among them, adsorption has been paid more attentions due to the effectiveness, low cost, facile process, relatively low cost operating factors, and also the possibility of the recycling process [9]. Different adsorbents such as natural zeolites [10], mesoporous SiO2 [11, 12], metal organic frameworks (MOFs) [13], graphene-based materials [14], and artificially [15, 16] and naturally (biomass and waste material) derived porous carbons [17, 18] have been developed for this purpose. Natural precursor–derived porous carbons which are cost-effective can be obtained by physical (with CO2 or steam) or chemical activation of the carbon precursors. Chemical approaches were performed with activating agents such as NaOH, KOH, ZnCl2, and H3PO4 in order to induce micro- and mesopores in the carbon containing materials and remove some of the unfavorable contents of the char [19,20,21]. Porosities, especially the micro-porosities, increase the surface area of the prepared activated carbons. Carbon source, activating agent, activation temperature, and the ratio of activating agent to carbon source are the main parameters which affect the characteristics of the porosities, i.e., mean pore size, distribution, and total volume of the porosities. These, in turn, determine the surface area of the prepared activated carbons. The surface area of various activated carbons differs from several hundred to about 3000 m2 g−1 [22,23,24].
Applying activated carbons, the adsorption behavior of methylene blue has extensively been investigated both thermodynamically and kinetically [25, 26]. These studies revealed that the adsorption capacities of the activated carbons depend on several parameters such as porosity size (meso- and especially micropores), porosity distribution, total pore volume, and also on the heteroatoms (boron, nitrogen, oxygen, sulfur, and phosphor) contents of the adsorbents [27,28,29]. To the best of the authors’ knowledge, there is not any straight dependency between the specific surface area of the adsorbent and the adsorption capacity. Boron-doped biomass-derived porous carbon with moderate surface area amount of about 650 m2 g−1 shows high adsorption capacity of about 2440 mg g−1 [27] and on the other hand, activated carbons with very high surface area amounts of 1534, 1937, 2086 and 2573 m2 g−1 reveal moderate amount of adsorption capacity (704, 916, 385 and 570 mg g−1, respectively) [30,31,32,33]. The applicability of several adsorption theories has also been studied for the adsorption process and it has been found that Langmuir adsorption theory (monolayer chemical adsorption of the adsorbate on the activated carbon) is the most acceptable theory [34,35,36]. This finding has also been approved by the pseudo-second-order kinetic theory [37, 38]. Thermodynamic studies revealed that the adsorption process of methylene blue on porous carbons, like namely all other dyes, is endothermic. Also, the entropy of the adsorption reaction will be increased during the dye removal process [16, 35, 39].
Although there are numerous studies about the adsorption behavior of porous carbons, to the best of the author’s knowledge, there is no any characteristic view about the adsorption process regarding pre- and post-adsorption process. In other words, there is no any deep structural insight on the adsorption process. The present study focuses on the relation between the adsorption behaviors of Azolla-derived porous carbons and their structural characteristics before and after the absorption process. The experimental data have been collected from the adsorption of methylene blue on the relatively high surface area Azolla-derived porous carbons. Based on the fitness results, the best isotherm and also the adsorption energy of methylene blue on Azolla-derived activated carbon were determined. Following this, pseudo-first- and pseudo-second-order kinetics model were applied to better understand the adsorption process. For better insight about the structural effects of the adsorption process, XRD, FTIR, Raman, and XPS analyses have been conducted on the prepared activated carbons before and after the adsorption process.
2 Experimental
2.1 Materials
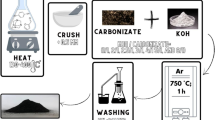
All chemicals and reagents were of analytical grade and used as received without any further purification. Azolla biomass was collected from Anzali Lagoon. In order to remove any contaminants from this biomass, it was washed several times before treatment. Sodium hydroxide was purchased from Merck and nitric acid (37%) (HNO3) and also methylene blue was purchased from Dr. Mojallali™ Company.
2.2 Synthesis of activated carbon
Porous activated carbons have been prepared using an alkaline approach which is categorized as a chemical activation process. At first, Azolla biomass was pre-carbonized for 3 h at 300 °C under argon atmosphere. Obtained biochar was mixed with sodium hydroxide (NaOH) with the weight ratio of 1 to 3 (biochar to NaOH). Mixing treatment was continued until homogenization. The obtained mixture was pyrolyzed for 3 h at 600 °C in a tubular electric furnace, under argon atmosphere. The black product was washed with nitric acid solution (1 M) to remove the activation agents and other impurities. This treatment was followed by washing using deionized water until neutral pH, (Az-C).
3 Characterization
PXRD patterns were collected on a Philips (Holland) PW1730 diffractometer with Cu Kα (λ = 1.54 Å). N2 adsorption–desorption isotherm was conducted on a BELSORP mini II equipment at 77 K, degassing time and temperature of which are 6 h and 150 °C, respectively. Raman spectroscopy was conducted on a Takram micro-Raman spectrometer (Teksan™, Iran). FTIR spectroscopy was accomplished on a Shimadzo 8400s Spectrometer. X-ray photoelectron spectroscopy (XPS) measurements were carried out on an Al Ka source (XPS Spectrometer Kratos AXIS Supra). High-resolution transmission electron micrographs were obtained on a 300-kV FEI (US) TITAN microscope (HR-TEM). The absorbance of MB solution was measured by an UV-1700 (Shimadzu, Japan) UV–visible spectrophotometer.
3.1 Adsorption of methylene blue
Kinetics and thermodynamics of MB adsorption on the Azolla-derived porous carbon have been investigated during the adsorption process. In a typical process, 5 mg of porous carbon powders was added to different volumes (from 10 to 40 ml) of MB aqueous solutions. The concentration of all the solutions was fixed at 400 mg l−1. The adsorption processes were continued until equilibrium establishment (up to 45 min). All the experiments were conducted at ambient temperature.
Using the following equation, the adsorption capacity of each experiment at every adsorption time was determined [40]:
In this expression, Qt (mg g−1) is the time-dependent adsorbed amount of dyes, C0 and Ct (mg l−1) are the solute concentrations at the beginning and after the selected time, respectively, V (ml) is the volume of dye solution which was fixed at 20 ml, and m (mg) is the mass of the porous activated carbon.
The fitness of four well-known adsorption isotherms models, i.e., Langmuir, Freundlich, Temkin and Dubinin–Radushkevich, has been investigated for the obtained equilibrium solution concentrations. Parameters including in these isotherms have been introduced as follows:
-
a)
Langmuir’s isotherm:
Langmuir isotherm which is obeyed in systems with homogeneous interaction between the adsorbent and the adsorbate can be introduced as:
In the above equation, Ce (mg l−1) is the equilibrium concentration of the solution, Qe (mg g−1) is the amount of adsorbed dye when equilibrium condition was established, Qm (mg g−1) is the maximum adsorption capacity, and KL (l mg−1) is the Langmuir constant [16].
-
b)
Freundlich isotherm:
Freundlich isotherm is followed in heterogeneous adsorption systems. This isotherm can be written as:
KF (mg g−1) and n are constant coefficients and relate to the absorption capacity and absorption intensity, respectively [16].
-
c)
Temkin isotherm:
Temkin isotherm equation which based on heterogeneous adsorption behavior is as follows:
where KT and bT are the equilibrium binding (l mg−1) and the Temkin isotherm constants, respectively.
Dubinin–Radushkevich (D–R) isotherm is as follows.
This isotherm, which is applied for heterogeneous adsorption systems, can be presented as:
In these expressions, β (mol2 kJ−2) is D–R isotherm constant, ε is the Polanyi potential, and Ea (kJ mol−1) is the adsorption energy. T (k) is the adsorption temperature and also R with the value of 8.314 is the gas universal constant (J mol−1 k−1) [26].
Adsorption kinetics of the prepared activated carbon for methylene blue has also been investigated. Two adsorption kinetics models, namely pseudo-first- and pseudo-second-order, were applied to investigate their applicability on the experimental adsorption data.
In order to investigate the adsorption mechanism of methylene blue on the Azolla-derived activated carbon, XRD, FTIR, Raman, and XPS analyses have been conducted before and after the methylene blue adsorption on the prepared carbons. For this purpose, 30 mg Azolla-derived porous carbons was added to 20 and 40 ml of 400 mg l−1 of methylene blue solutions, (Az-C-20 and Az-C-40, respectively). After completion of the adsorption process and establishing of equilibrium, the carbon and adsorbed methylene blue mixtures were filtered and dried for further characterization. This adsorption process results in the addition of 8 and 16 mg of methylene blue into the 30 mg activated carbon.
4 Results and discussion
4.1 Equilibrium and kinetics of adsorption behavior
Equilibrium adsorption capacity results of MB on Az-C have been demonstrated in Fig. 1a. Linear forms of Langmuir, Freundlich, Temkin and Dubinin–Radushkevich [37, 41] isotherms were also included in Fig. 1b–e. The obtained data from these plots have been shown in Table 1. Comparison between these results reveals that there is a better consistency between the methylene blue adsorption behavior of Az-C with the Langmuir (homogeneous monolayer adsorption) model. Therefore, it can be concluded that all the adsorption sites of the prepared activated carbon have similar adsorption conditions (both energetically and availability). This consistency also implies that the functional groups were homogeneously distributed on the surface of Az-C. Considering the adsorption process, both in aqueous and gaseous atmospheres, Langmuir is more common model which is followed with the adsorbent–adsorbate systems [15, 16]. Based on the Langmuir isotherm, the adsorption capacity of Azolla-derived porous carbon is about 1930 mg g−1 which is one of the highest adsorption capacity of porous activated carbon for methylene blue [15, 42]. This high value is attributed to both surface physical (high specific surface area, meso- and micro-porosities) and chemical (nitrogen-containing functional groups) characteristics of the activated carbon [42]. The equilibrium parameter (RL) can be calculated based on Eq. 8:
a Adsorption capacity of methylene blue on Azolla-derived porous carbon (Az-C) in concomitant with the Langmuir and Freundlich plots of the experimental results, b linearized forms of Langmuir, c Freundlich, d Temkin, and e Dubinin–Radushkevich (D–R) isotherms for the adsorption of methylene blue on Az-C
RL is about 0.00003 which is approximately equal to zero and therefore implies irreversible adsorption of methylene blue on Az-C [43]. Monolayer and limiting value of methylene blue adsorption and also very low equilibrium parameter predict the chemical adsorption mechanism of this adsorbate on Az-C. This prediction can further be approved by the mean adsorption energy. From expression 7, the mean adsorption energy of methylene blue on Az-C is 21.76 kJ mol−1 which is so greater than 8 kJ mol−1 which is the top limit for physical adsorption. This finding emphasizes on the strong chemical interaction between the adsorbent and the adsorbate [38, 44].
The kinetic behavior of Az-C aqueous solution of methylene blue system was also investigated for initial methylene blue concentration of 400 mg l−1. The kinetic plots of this system are presented in Fig. 2a–f. From Fig. 2a, b, it can be observed that after 15 min about 95% of methylene blue was adsorbed on Az-C and the equilibrium condition is established after about 45 min. The high adsorption rate of methylene blue on Az-C implies high driving force for interaction between dye molecules and the adsorbent. For better insight into the kinetics of the adsorption process, pseudo-first- and pseudo-second-order kinetic models were applied to the experimental results. These two models can be expressed as follows, respectively [9, 38, 44]:
The results of these analyses can be followed in Fig. 2c, d. Better agreement between the experimental results and the pseudo-second-order kinetic model can be observed. This finding emphasizes that the adsorption process is chemical. The same behavior has been proposed for the adsorption of methylene blue on the several other adsorbents [37, 38, 44, 45]. For better insight on the rate-limiting step [external transport (film diffusion) or intra-particle diffusion], two other models (intra-particle diffusion and Boyd diffusion) were fitted to the experimental results. Intra-particle diffusion model is described as follows:
where qt (mg g−1) is the adsorption capacity at time t, ki (mg g−1 min−0.5) is the rate constant of the intra-particle diffusion model and C is a constant [44]. Two parameters of this model, i.e., ki and C, can easily be determined by the slope and intercept of qt versus t0.5 [9, 45]. If the graph pass through the origin and also has a good fitting with the experimental data points (R2~1), the rate-limiting step in the adsorption process is intra-particle diffusion of the dye ions. From Fig. 2e, it is clear that despite fair fitting of the experimental data points with the model, the results did not pass through the origin; therefore, at this point, one cannot decide for the intra-particle diffusion as the rate controlling step and Boyd model should be examined. This model is described as:
The plot of Bt versus t is called the Boyd plot. If a straight line passing the origin with high R2 value is achieved during this data fitting, intra-particle diffusion is the rate-limiting step [9]. From the Boyd plot of methylene blue adsorption on Az-C, Fig. 2f, this is the case for this adsorption process. Little deviation of the plot from the origin (0.07) seems negligible. Considering the high adsorption capacity, chemical nature of the adsorption process, and intra-particle diffusion of the adsorbate as the rate-limiting step, it can be concluded that physical and chemical characteristics of the adsorbent and their variations during the adsorption process are so determinative.
4.2 Structural interpretation of the adsorption mechanism
The HR-TEM micrograph of Az-C in concomitant with N2 adsorption–desorption isotherm, BJH, and micropore (MP) plots is presented in Fig. 3a–d. Worm-like porous structure which comprises of large amounts of micropores can be observed in the prepared Azolla-derived porous carbons (Fig. 3a). Total surface area of this porous structure is about 1437.6 m2 g−1 which was obtained from BET analysis of the adsorption–desorption data. Also, the total pore volume of this structure is 0.74 cm3 g−1 (Fig. 3b). Comparing Fig. 3c and d, it will be determined that, in consistence with Fig. 3a, micropores have greater amount than meso- and macropores. Micropore surface area and pore volume are 1352.2 m2 g−1 and 0.599 cm3 g−1, respectively. In fact, approximately 94% of the surface area and 80% of the pore volume of this biomass-derived carbon come from micropores. High surface area of this porous carbon is a prerequisite for high adsorption capacity, especially for Langmuir model of adsorption. In addition, intra-particle diffusion as the limiting rate step for MB adsorption (Fig. 2f) [42] can be attributed to the meso-sized porosities which facilities the diffusion of cationic dye ions and to the high density of micropores with diameters less than 1 nm (Fig. 3c).
XRD, Raman, and FTIR spectra of Az-C before and after adsorption of MB has been demonstrated in Fig. 4a–c. Methylene blue adsorption on the prepared activated carbon resulted in a shift of (002) crystallographic plane of the turbostratic carbon from about 22 to about 25° (Fig. 4a) [46]. This shift to higher 2θ can be attributed to the induction of compressive stress on the graphite crystallites. Chemical interaction of methylene blue molecules with the surface functional groups and atoms of Az-C and also their diffusion into the micro-porosities of Az-C can be considered as the reason for this compressive stress. A blue shift of X-ray diffraction patterns of the adsorbed porous carbons to higher 2θ also implies that no intercalation of methylene blue molecules occurred into the (002) planes. This may be attributed to the low value of the adsorbed methylene blue ions on the porous carbon [47]. In fact, several mechanisms may be active during the adsorption process of MB from the contaminated water. These are adsorption of cationic ions on the surface functional groups by physical or chemical interactions, entrapping of them in to the micro-porosities and intercalation of MB ions between the crystal planes (absorption). If intercalation was the active mechanism, it would induce tensile stress on the crystallographic planes of the porous carbon. This tensile stress, in turn, would lead to red shift in the characteristic peaks of porous carbon [48]. Considering the blue shift of the characteristic peaks and also the same trend observed during water decolorization (Fig. 2), it can be concluded that intercalation (and absorption) is not the prevailing mechanism of water decolorization by Azolla-derived porous carbon. From Raman spectra of Az-C before and after MB adsorption (Fig. 4b and inset), it is observed that graphite band (G-band) is blue shifted (from 1593 to 1597.75 cm−1) which means a decrease in C = C graphite band length (compressive stress on graphite structure) which is in agreement with the XRD results. At the same time, the relative graphitization value of the carbon structure (\( \frac{I_D}{I_G}\Big) \) increases from 0.88 for Az-C to 0.93 for Az-C-20 and 0.97 for Az-C-40. This increase in relative graphitization value means that adsorption of methylene blue will result in the increase in the defects of the carbon structure. Comparing the FTIR spectra of Az-C and methylene-infiltrated Az-C (Az-C-20 and Az-C-40), it can be deduced that there is two distinct shift in the location of characteristic peaks of Az-C, which are shown with arrows in Fig. 4c. The first shift is occurred for the peaks attributed to C-O bands (1050 cm−1) [30]. Increasing the amount of the adsorbed methylene blue increases the wavenumber of the characteristic peak of C-O band to about 1160 cm−1. The intensity of this peak decreases during the adsorption process. Also, the characteristic peaks of C=C and N-H bands at around 1570–1650 cm−1 [49] shifts to higher wavenumbers and also, the intensity of this peak increases. Other characteristic peaks of Az-C seem without any notable changes during adsorption process. The change in the intensity and the wavenumber of these functional groups emphasizes the involvement of C-O, N-H, and C=C groups on adsorption process [9, 38]. Considering these observed shifts in the characteristic peaks of some functional groups (in FTIR analysis) and crystal plains (in XRD and Raman analyses) emphasizes on the strong electrostatic attraction or chemical bonding between a (some) group(s) of the adsorbed dye and a (some) functional group(s) of the adsorbent.
XPS results can give better insight about the surface chemistry of the prepared porous carbon and also chemical interaction between the methylene blue and the adsorbent. Deconvoluted quantitative XPS results of C1s, N1s, O1s, and S2p spectra of Azolla-derived porous carbon before and after the adsorption process have been shown in Table 2. Except with some difference between C, O, and S surface functionalities of Az-C before and after the adsorption treatment, it seems that nitrogen surface chemistry is substantially different in these three specimens. N1s spectra of Azolla-derived porous carbon before and after adsorption of methylene blue have been demonstrated in Fig. 5. It is clear that, as a result of the adsorption process, pyrrolic nitrogen group diminished and N-(C)3 and H-N-(C)2 nitrogen groups peak appeared. During the adsorption process, the relative percent of this new peak increased from 39.2 to 79.7 which is in agreement with the amounts of the adsorbed methylene blue. Therefore, this peak can be attributed to the nitrogen groups in the adsorbed dye. In addition and by continuation of the adsorption process, graphitic nitrogen also diminished. The relative percent of pyridinic nitrogen group also decreased. But this decrease is not as high as that of pyrrolic and graphitic nitrogen groups. Therefore, it can be inferred that ionized nitrogen atoms of methylene blue which have positive electrical charge and a vacant 2p orbital, chemically, interact with pyrrolic, graphitic, and to some extent with pyridinic nitrogen surface sites of Az-C. It seems that the adsorbed dye works like a coating and prevents the detection of these functional groups. Elaborating the detailed mechanism of this interaction is out of the scope of the present research.
5 Conclusion
In the present research, equilibrium, kinetics, and structural changes during the adsorption of methylene blue on a relatively large surface area (1437 m2 g−1) Azolla-derived porous carbon was investigated. Several adsorption theories, Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich were investigated for the adsorption process of methylene blue on the prepared porous carbon. These considerations were declared that Langmuir (monolayer, chemical, and homogeneous) adsorption theory is consistence with the experimental results with high adsorption capacity of about 1930 mg g−1. Adsorption energy of methylene on the prepared porous carbon was determined to be about 21.76 kJ mol−1 which further confirm the chemical adsorption. From kinetics point of view, pseudo-second-order kinetic model is dominant in this adsorption process. This finding further emphasizes on chemical nature of the adsorption process. Applying intra-particle and Boyd diffusion models, it was determined that the rate-limiting step in this process is the diffusion of methylene blue in the meso- and micro-porosities of the prepared carbons. Based on X-ray diffraction results before and after the adsorption process, there is no any sign (induction of tensile stress in the graphitic planes) for intercalation of methylene blue in the inter-crystalline plane spaces of the porous carbon. Adsorption process results in the blue shift of X-ray diffraction peak of (002) graphitic planes from about 22 to about 25° which means the induction of compressive stresses on the graphitic planes. This compressive stresses come from the diffusion of methylene blue in to the meso- and micro-porosities of the porous carbon structure. Adsorption of methylene blue leads to the reduction of relative graphitization of the prepared carbon. From FTIR and XPS results, it was declared that nitrogen functional groups are the main functional groups which involve in the adsorption process of methylene blue. During the adsorption process, the ratio of pyridinic nitrogen groups decreased and pyrrolic and graphitic nitrogen groups eliminated from the surface of Azolla-derived porous carbon. At the same time, two new functional nitrogen groups, namely and N-(C)3 and H-N-(C)2, appeared in the surface of the prepared adsorbent.
References
Liu T, Li Y, Du Q, Sun J, Jiao Y, Yang G, Wang Z, Xia Y, Zhang W, Wang K, Zhu H, Wu D (2012) Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf B: Biointerfaces 90:197–203
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Hameed BH, Ahmad AL, Latiff KNA (2007) Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dyes Pigments 75:143–149
Kornaros M, Lyberatos G (2006) Biological treatment of wastewaters from a dye manufacturing company using a trickling filter. J Hazard Mater 136:95–102
Rashidi HR, Sulaiman NMN, Hashim NA, Hassan CRC, Ramli MR (2015) Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalin Water Treat 55:86–95
Djafer A, Djafer L, Maimoun B, Iddou A, Mostefai SK, Ayral A (2017) Reuse of waste activated sludge for textile dyeing wastewater treatment by biosorption: performance optimization and comparison. Water Environ J 31:105–112
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manag 93:154–168
Sharma P, Hussain N, Borah DJ, Das MR (2013) Kinetics and adsorption behavior of the methyl blue at the graphene oxide/reduced graphene oxide nanosheet–water interface: a comparative study. J Chem Eng Data 58:3477–3488
Han R, Zhang J, Han P, Wang Y, Zhao Z, Tang M (2009) Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504
Lee YS, Jang W, Koo HY, Choi WS (2015) Facile synthesis of mesoporous SiO2 nanoparticles using the mobility differences of etchants. RSC Adv 5:26223–26230
Mathew A, Parambadath S, Barnabas MJ, Song HJ, Kim J-S, Park SS, Ha C-S (2016) Rhodamine 6G assisted adsorption of metanil yellow over succinamic acid functionalized MCM-41. Dyes Pigments 131:177–185
Lin S, Song Z, Che G, Ren A, Li P, Liu C, Zhang J (2014) Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater 193:27–34
Chen Y, Song X, Zhao T, Xiao Y, Wang Y, Chen X (2018) A phosphorylethanolamine-functionalized super-hydrophilic 3D graphene-based foam filter for water purification. J Hazard Mater 343:298–303
He X, Male KB, Nesterenko PN, Brabazon D, Paull B, Luong JHT (2013) Adsorption and desorption of methylene blue on porous carbon monoliths and Nanocrystalline cellulose. ACS Appl Mater Interfaces 5:8796–8804
Jin Q, Li Y, Yang D, Cui J (2018) Chitosan-derived three-dimensional porous carbon for fast removal of methylene blue from wastewater. RSC Adv 8:1255–1264
Jung K-W, Choi BH, Hwang M-J, Jeong T-U, Ahn K-H (2016) Fabrication of granular activated carbons derived from spent coffee grounds by entrapment in calcium alginate beads for adsorption of acid orange 7 and methylene blue. Bioresour Technol 219:185–195
Tsoncheva T, Mileva A, Tsyntsarski B, Paneva D, Spassova I, Kovacheva D, Velinov N, Karashanova D, Georgieva B, Petrov N (2018) Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenergy 109:135–146
Spessato L, Bedin KC, Cazetta AL, Souza IPAF, Duarte VA, Crespo LHS, Silva MC, Pontes RM, Almeida VC (2019) KOH-super activated carbon from biomass waste: insights into the paracetamol adsorption mechanism and thermal regeneration cycles. J Hazard Mater 371:499–505
Liu J, Liu B, Wang C, Huang Z, Hu L, Ke X, Liu L, Shi Z, Guo Z (2017) Walnut shell–derived activated carbon: synthesis and its application in the sulfur cathode for lithium–sulfur batteries. J Alloys Compd 718:373–378
Liu X, He C, Yu X, Bai Y, Ye L, Wang B, Zhang L (2018) Net-like porous activated carbon materials from shrimp shell by solution-processed carbonization and H3PO4 activation for methylene blue adsorption. Powder Technol 326:181–189
Acosta R, Fierro V, Martinez de Yuso A, Nabarlatz D, Celzard A (2016) Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 149:168–176
Önal Y, Akmil-Başar C, Sarıcı-Özdemir Ç, Erdoğan S (2007) Textural development of sugar beet bagasse activated with ZnCl2. J Hazard Mater 142:138–143
Xu M, Li D, Yan Y, Guo T, Pang H, Xue H (2017) Porous high specific surface area-activated carbon with co-doping N S and P for high-performance supercapacitors. RSC Adv 7:43780–43788
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004
Fan S, Tang J, Wang Y, Li H, Zhang H, Tang J, Wang Z, Li X (2016) Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: kinetics, isotherm, thermodynamic and mechanism. J Mol Liq 220:432–441
Xin W, Song Y, Peng J, Liu R, Han L (2017) Synthesis of biomass-derived Mesoporous carbon with super adsorption performance by an aqueous cooperative assemble route. ACS Sustain Chem Eng 5:2312–2319
Yu R, Shi Y, Yang D, Liu Y, Qu J, Yu Z-Z (2017) Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-Spectrum and rapid adsorption of water contaminants. ACS Appl Mater Interfaces 9:21809–21819
Lee JH, Lee HJ, Choi JW (2017) Unveiling anomalous CO2-to-N2 selectivity of graphene oxide. Phys Chem Chem Phys 19:22743–22748
Bedin KC, Martins AC, Cazetta AL, Pezoti O, Almeida VC (2016) KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem Eng J 286:476–484
Cazetta AL, Vargas AMM, Nogami EM, Kunita MH, Guilherme MR, Martins AC, Silva TL, Moraes JCG, Almeida VC (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174:117–125
Liu R-L, Liu Y, Zhou X-Y, Zhang Z-Q, Zhang J, Dang F-Q (2014) Biomass-derived highly porous functional carbon fabricated by using a free-standing template for efficient removal of methylene blue. Bioresour Technol 154:138–147
Pezoti O, Cazetta AL, Bedin KC, Souza LS, Martins AC, Silva TL, Santos Júnior OO, Visentainer JV, Almeida VC (2016) NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies. Chem Eng J 288:778–788
Islam MA, Sabar S, Benhouria A, Khanday WA, Asif M, Hameed BH (2017) Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption. J Taiwan Inst Chem Eng 74:96–104
Li Z, Wang G, Zhai K, He C, Li Q, Guo P (2018) Methylene blue adsorption from aqueous solution by loofah sponge-based porous carbons. Colloids Surf A Physicochem Eng Asp 538:28–35
Marrakchi F, Auta M, Khanday WA, Hameed BH (2017) High-surface-area and nitrogen-rich mesoporous carbon material from fishery waste for effective adsorption of methylene blue. Powder Technol 321:428–434
Elmoubarki R, Mahjoubi FZ, Tounsadi H, Moustadraf J, Abdennouri M, Zouhri A, El Albani A, Barka N (2015) Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Resour Ind 9:16–29
Gürses A, Hassani A, Kıranşan M, Açışlı Ö, Karaca S (2014) Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: kinetic, thermodynamic and equilibrium approach. J Water Process Eng 2:10–21
Islam MA, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285
Foo KY, Hameed BH (2012) Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour Technol 104:679–686
Wang S, Zhai Y-Y, Gao Q, Luo W-J, Xia H, Zhou C-G (2014) Highly efficient removal of acid red 18 from aqueous solution by magnetically retrievable chitosan/carbon nanotube: batch study, isotherms, kinetics, and thermodynamics. J Chem Eng Data 59:39–51
Xu S, Lv Y, Zeng X, Cao D (2017) ZIF-derived nitrogen-doped porous carbons as highly efficient adsorbents for removal of organic compounds from wastewater. Chem Eng J 323:502–511
Maneerung T, Liew J, Dai Y, Kawi S, Chong C, Wang C-H (2016) Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: kinetics, isotherms and thermodynamic studies. Bioresour Technol 200:350–359
Duran C, Ozdes D, Gundogdu A, Senturk HB (2011) Kinetics and isotherm analysis of basic dyes adsorption onto almond Shell (Prunus dulcis) as a low cost adsorbent. J Chem Eng Data 56:2136–2147
Chen L, Bai B (2013) Equilibrium, kinetic, thermodynamic, and in situ regeneration studies about methylene blue adsorption by the raspberry-like TiO2@yeast microspheres. Ind Eng Chem Res 52:15568–15577
Tang H, Yan D, Lu T, Pan L (2017) Sulfur-doped carbon spheres with hierarchical micro/mesopores as anode materials for sodium-ion batteries. Electrochim Acta 241:63–72
Chang P-H, Jiang W-T, Li Z (2018) Mechanism of tyramine adsorption on Ca-montmorillonite. Sci Total Environ 642:198–207
Lv G, Chang P-H, Xing X, Jiang W-T, Jean J-S, Li Z (2017) Investigation of intercalation of diphenhydramine into the interlayer of smectite by XRD FTIR, TG-DTG analyses and molecular simulation. Arab J Chem 10:855–861
Miranda AF, Biswas B, Ramkumar N, Singh R, Kumar J, James A, Roddick F, Lal B, Subudhi S, Bhaskar T, Mouradov A (2016) Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol Biofuels 9:221
Perazzolo V, Durante C, Pilot R, Paduano A, Zheng J, Rizzi GA, Martucci A, Granozzi G, Gennaro A (2015) Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 95:949–963
Tan A-D, Wan K, Wang Y-F, Fu Z-Y, Liang Z-X (2018) N, S-containing MOF-derived dual-doped mesoporous carbon as a highly effective oxygen reduction reaction electrocatalyst. Catal Sci Technol 8:335–343
Li X, Fang Y, Zhao S, Wu J, Li F, Tian M, Long X, Jin J, Ma J (2016) Nitrogen-doped mesoporous carbon nanosheet/carbon nanotube hybrids as metal-free bi-functional electrocatalysts for water oxidation and oxygen reduction. J Mater Chem A 4:13133–13141
Li Y, Wang G, Wei T, Fan Z, Yan P (2016) Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 19:165–175
Kim CK, Choi IT, Kang SH, Kim HK (2017) Anchovy-derived nitrogen and sulfur co-doped porous carbon materials for high-performance supercapacitors and dye-sensitized solar cells. RSC Adv 7:35565–35574
Acknowledgments
We greatly appreciate the help of Rodrigo Fernández-Pacheco from the Universidad de Zaragoza (Spain).
Funding
This research work has been conducted under the kind support of Iran Nanotechnology Initiative Council of Iran University of Science and Technology (IUST).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motejadded Emrooz, H.B., Maleki, M., Rashidi, A. et al. Adsorption mechanism of a cationic dye on a biomass-derived micro- and mesoporous carbon: structural, kinetic, and equilibrium insight. Biomass Conv. Bioref. 11, 943–954 (2021). https://doi.org/10.1007/s13399-019-00584-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00584-1