Abstract
The impact and interaction of light irradiance strength (light intensities), lighting cycle (photoperiod), and aeration rate on biomass concentration and lutein production efficacy of the microalga Chlorella salina in a closed laboratory-scale airlift photobioreactor were investigated via the response surface method. Among the factors assessed, light intensity and aeration rate had significant influence on cell concentration, though a concurrent increment in light intensity noticeably decreased the lutein content. All the parameters were observed to be statistically significant. Best operating conditions for the growth of alga was evaluated to be as follows: light intensity, 200 μmol m−2 s−1; photoperiod, 12:12 h L D; and the aeration rate, 3 lpm. These conditions could substantially enhance the microalgal growth rate (0.82 day−1) and biomass production (665.89 mg). Specific lutein productivity and a recovery of 9.73 mg/L/day were achieved at a day light cycle of 16 h. According to the results of the experimental design, the optimum conditions led to a twofold increase in biomass and lutein productivity compared with unoptimized condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lutein is a lipophilic carotenoid that has exhibited significant nutraceutical bioactivity, and antioxidant, anti-cancer effects on wide range of biological models. For this reason, lutein has been largely consumed with a market size of nearly $150 million in the USA, and the market size is yet expanding [1]. Traditionally, lutein is produced from marigold flowers (Tagetes erecta); however, due to the biological and operational benefits of microalgae, including higher lutein content, flexible cultivation conditions, fast growth, and the less labor required for operation, microalgae have become an interesting alternative for lutein production [2]. Since wild microalgae harvesting may have a negative ecological impact due to overexploitation of these natural sources, it is expected that carotenoid extraction from cultured organisms will have a dominant role to play in the coming years [3]. Despite the renewed interest in bioactive pigments, their concentrations, especially under optimal growth conditions, are often too low to make microalgae-based pigment production economically viable. Moreover, due to their simpler structure, energy is directed into photosynthesis, growth, and reproduction processes instead of the maintenance of differentiated structures [4], making microalgae organisms of interest for production of biomass and bioactive compounds.
The production of lutein in microalgae varies not only among species but also under different conditions, such as light intensity, nitrogen availability, physiological state of the culture, and other medium compositions. In some green microalgae (e.g., Dunaliella salina and Haematococcus pluvialis), specific stress conditions such as over-saturating light, nutrient deficiency, or high salt concentration induce the overproduction of secondary carotenoids, β-car, and astaxanthin [5,6,7]. Secondary pigments, including astaxanthin, β-car, and lutein, are known to play a scavenging role and prevent cell damage by reacting with reactive oxygen species (ROS) [8]. By contrast, the synthesis of primary pigments requires carbon dioxide and nutrients, such as inorganic nitrogen and phosphate in addition to light [9]. Clearly, when considering photoautotrophic microalgal cultivation, a key role is played by light availability and utilization [10]; hence, photobioreactors (PBRs) need to have a wide light-exposed surface, and a thin depth to avoid the presence of dark zones inside the culture. On the other hand, agitation during microalgae culture is needed to promote an adequate light-dark cycle regime, to increase oxygen mass transfer, and to help avoid detrimental conditions such as cell sedimentation or accumulation of oxygen in the medium [11].
For most bioreactors, the exterior surface is the only place exposed to light, called the photic zone. The rest that is unexposed to or with little light penetration is called the dark zone. Distinctive PBR geometries and designs result in varying light utilization and hydrodynamic circulation, which can affect light distribution and mixing inside the reactor. When microalgae are exposed to the light/dark zone within the PBR, the span under the light/dark zone alters the biomass production of the PBR. Air flow rate, mixing, biomass concentration, and light availability are the major parameters that influence growth superficially and productivity of microalgae in photobioreactors [12]. The mixing of the liquid in the reactor has an indirect influence on exposure of microalgae to light and dark phases [12]. High-density microalgae cultivation could be accomplished by establishing strategies to productively utilize the incident light energy. The extent of mixing is a decisive criterion that impinge light distribution and light availability in the system.
Among the physicochemical parameters that influence microalgal lutein synthesis, light intensity, CO2, and some medium components (nitrate, manganese, and copper) were identified as the primary factors critically influencing the growth rate and accumulation of lutein [13,14,15]. The biosynthesis of lutein can be improved by providing optimal mode of light intensity, as the carotenoid lutein is present in the photosynthetic antenna complexes [16]. This is evident from the study of Vaquero et al. [17], which reported that sudden change in light intensity either from low to moderate level or from moderate to low level enhanced the lutein content of microalga Coccomyxa onubensis. However, only limited studies so far have investigated the simultaneous effect of light intensity and mixing conditions on both cellular growth and lutein production in microalgae. The objective of the present study was to evaluate culture growth of Chlorella salina under different growth conditions (light intensity and mixing/agitation) and medium compositions to determine optimal conditions for the production of lutein in airlift PBRs.
2 Materials and methods
Chlorella salina was obtained from Central Institute of Brackish Aquaculture (CIBA), Chennai, Tamil Nadu. The algae were cultivated in a filter-sterilized (pore size 0.2 μm) Walne’s medium [18]. The cultures were pre-cultivated in a 200-mL Erlenmeyer flask containing 100 mL of medium at pH 8.0 and at 24 °C at a light intensity of 50–100 μmol photons m−2 s−1.
2.1 Operation of photobioreactor
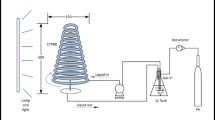
The microalgae were cultivated in a cylindrical airlift photobioreactor with a working volume of 8 L, which was continuously illuminated with external fluorescent light sources (TL5 light tubes, Philips) mounted on two opposite sides of the PBR, and the light intensity was measured using a quantum meter (LX 102 Lux Meter; HTC Instruments, India). The initial pH of the culture was 8.0. They were then divided into three groups grown under high light intensity (HL, 400 μmol m−2 s−1), medium light intensity (ML, 200 μmol m−2 s−1), and low light intensity (LL, 100 μmol m−2 s−1), respectively. The ranges of the parameters selected in the present study are depicted in Table 1. After inoculation, operating conditions of PBR were adjusted to specified values according to the experiment (Table 2). All experiments were performed in triplicates and expressed as mean with standard deviation. Samples were collected from the PBR at set time intervals to determine the cell concentration and lutein content. The microalgal cultures were harvested upon depletion of the nitrogen source in the medium.
2.2 Determination of cell growth
Cell growth estimated as cell number per mL was determined according to Chang et al. [19]. Cell culture of 30 μL was mixed with 10 μL of Lugol solution (Sigma-Aldrich, St. Louis, MO, USA). The mixture of 10 μL was loaded to the hemocytometer (Thoma, Bad Blankenburg, Germany) and the cell number was counted under microscopy (CX 41, Olympus, Japan). Each sample was counted twice and the average was calculated as the cell number of the sample.
Cell growth was also estimated as dry weight (d. wt.) per L. A total of 100 mL of cell culture was harvested in the sampling day and subjected to centrifugation at 12,000×g at 4 °C for 5 min. The pellet was fixed in liquid N2 and lyophilized at − 50 °C to obtain the dry weight.
The relative growth rate (106 cells day−1) was estimated as the difference in cell number between two culture times (day), μ = ln (N2/N1)/(X2 − X1), where N2 is the concentration of cells at time X2 and N1 is the concentration of cells at time X1.
2.3 Chromatographic analysis of lutein and other carotenoids
The HPLC determination of carotenoids was modified following the previous report [20]. The sample of approximately 0.5 × 106 cells was centrifuged at 12,000×g for 5 min at 4 °C, and the supernatant was discarded. Then, 1 mL of cold 80% acetone was added to the pellet for a 2-h extraction in the darkness and then extracted again with another 1 mL of 80% acetone. The two extracts were combined and passed through the 0.2-μm nylon filter. The filtrate was loaded into a screw tube filled with N2 gas and stored at − 20 °C freezer until analysis. The filtrate of 20 μL was injected into the HPLC system. The chromatographic analysis of acetone-extracted pigments was performed using a C18 column (5 mm, 150 mm × 46) on a Waters 2545 HPLC (Waters 2545, USA), at 30 °C. The mobile phase comprised of methanol/ acetonitrile (90:10 V/V). The injection volume was 20 μL, the solvent flow rate was 1.2 mL min−1, and the pigment detection was carried out at 445 nm [21].
2.4 Experimental design and statistical analysis
To investigate the interactive effects of the light intensity, light period, and aeration on the lutein productivity of the selected microalgal strain, central composite design (CCD) was used. In total, twenty batch experiments were designed and conducted following a response surface methodology (Table 2), which was performed in duplicate to optimize the levels of the selected variables. Data processing and calculations were carried out using a commercial statistical package, Design Expert at version 7.0.0, Stat-Ease, Minneapolis, to estimate the coefficients of the regression equation. The goodness of the fit of the model was validated using analysis of variance (ANOVA).
3 Results and discussion
3.1 Effect of the light intensity, irradiation regimes, and mixing/agitation on cell growth and biomass productivity
The selection of a specific strain with a high growth rate and high lutein productivity is crucial from a microalgal technology engineering perspective. In addition to lutein productivity, concentration of biomass, lutein content, and duration of cultivation must also be considered for fast and efficient production of lutein from algae. Chlorella species are well-known for their capability of lutein production. To know the significance and practical application values, the effects of different light intensities, illumination period, and aeration/mixing strategies on the cell growth and lutein production in Chlorella salina have further investigated in the present study.
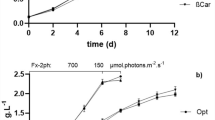
The growth profile of C. salina was determined in each experiment using direct counting and dry cell weight methods. Lutein production was determined spectrophotometrically and reported as milligrams per gram of dry cell weight. The maximum values for biomass production were measured in each experiment and were shown in Fig. 1.
The difference of growth rates among the HL, ML, and LL cultures indicated that 200 μmol m−2 s−1 was more suitable for the growth of C. salina than other light intensities. As shown in Fig. 1, the linear growth in all aerated cultures started at 3 days and lasted for 7 to 10 days. The control showed little growth initially, and then, the cell count decreased gradually. At 200 μmol m−2 s−1, the biomass concentration and growth rate reached maximum values of 0.82 g/L and 0.20/day, respectively. The biomass productivity was enhanced by about threefold with an increment in the intensity of light, and a maximum productivity of about 0.108 g/L/day was noticed at 200 μmol m−2 s−1. It indicates a better availability of light inside the reactor. An increased growth rate corresponding to the light intensity was also noted in Dunaliella tertiolecta when it was cultivated at 100, 200, and 350 μmol photons m−2 s−1 [22].
Other than varying the light intensity, the experimentation also takes into account the photoperiod (light/dark cycle) variation. It was found that the highest biomass of 0.103 g/L/day was recorded with light-dark cycle of 12:12 h while the highest percentage of lutein production was obtained at 16:8 h for C. salina at 200 μmol m−2 s−1 light intensity. However, there was no significant difference in dry weight among the growth conditions. This indicates that light could alter cellular metabolism but have no effect on cell growth. For all light conditions, the biomass concentration increased with the residence time, as already reported by Martinez et al. [23] for Scenedesmus obliquus, as well as for other species like Chlorella vulgaris, Chlorella protothecoides, etc. [24,25,26].
Further, the experiment is also carried out by applying at different aeration rates: 1, 3, and 5 lpm. The initial biomass concentration was 0.08 g/L for each experiment; the biomass concentration and the growth rate were sampled at 24-h interval. With the aeration rate of 3 lpm, the culture spent 6–8 days of log phase with a specific growth rate of 0.548/day. The growth rate of C. salina increased from 0.426 to 0.548/day with the increase in aeration. The productivity decreased significantly in lower flow rate because the turbulence caused by air circulation (bubbling) was insufficient to resist the auto-flocculation of the microalgae and provided poor nutrient mixing for cell cultivation [27, 28].
3.2 The effect of light intensity, irradiation regimes, and mixing/agitation on lutein productivity
The culture conditions that rendered the highest cell concentration are not the same where the highest lutein concentration was observed. With an increase in light intensity, the lutein content was increased at first, reaching the highest at 30% of DW under the light intensity of 200 μmol m−2 s−1 which was a twofold increase of that in the dark condition. After that, as the light intensity continued to increase, the lutein content began to decrease. These results indicated that a light intensity of 200 μmol m−2 s−1 is the most suitable for lutein production. The highest lutein content obtained was 6.2 + 0.32 mg/g, which was higher than the reported values, around 3–5 mg/g [25, 29]. At low irradiance, fewer oxygen radicals are generated, whereas under high irradiance cells are unable to utilize all the energy generated that implies a decrease in lutein for high irradiance, and this may be due to the functional group in the lutein molecule [30]. There was no coherence between growth rates, and lutein contents reveal that the most favorable conditions for growth were generally not concomitant with those with higher pigment content.
At the end of the investigation, the increase in pigment amount continued, and studies showed that light regime had an effect on lutein productivity of C. salina. Light had no obvious effect on lutein accumulation for the first 24 h, and the positive effect of light on lutein accumulation occurred only after 24 to 36 h. A light/dark cycle of 16:8 h shows a 1.5-fold increase in the productivity of lutein than the other experimented light/dark cycles. The specific lutein content of 9.86 mg/g yields the highest and suggests that 16:8 h is the best period for lutein accumulation. These were in agreement with the study of Mahale and Chaugule [31]; the optimal photoperiod is between 12:12 to 16:8 h for most of the cultures. These changes in pigments depending on the provided photoperiod could be related to the photo-adaptation mechanism [32].
Figure 2 shows the variation in the biomass and lutein productivity at different aeration rates. This plays crucial roles such as reducing photo-limitation or shelf-shading in high-density cultures, homogenously distributing the nutrients in the culture medium, and increasing CO2 dissolution and O2 evolution [33]. In general, at low aeration rate (1 lpm), the degree of photosynthesis was low due to scarce mixing of broth. With an increase in flow rate from 1 to 3 lpm, lutein productivity improved for all light intensities. This can be attributed to better mixing at high flow rate, thus improving the rate of photosynthesis. Thus, aeration rate of 3 lpm found to be adequate for specific lutein productivity. Any further increase beyond 3 lpm reduced or had no effect on specific lutein content, indicating 3 lpm to be the optimum aeration rate for the productivity of lutein. This is because higher flow rates tend to reduce the retention time of gas bubbles and thus the utilization of CO2 by the microalgal cells [34].
3.3 Data analysis and evaluation of the models
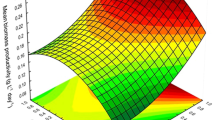
All experiments were designed and analyzed by RSM, a statistical technique used to design the experiments. CCD consisting of 20 sets of experiments is designed for optimizing the growth of C. salina with respect to biomass at incubation period of 18 days. All experiments were done in triplet form. The results of the predicted values were almost similar in comparison with the actual values of the experimental results. This model gave an optimized value of aeration rate, light intensity, and light period (LP) in respect of its growth or biomass. The results of all optimized condition of microalgae were shown in Fig. 3a, b, and c.
a The fitted RSM model for microalga biomass concentration in the designed irradiance strength, light hours per day, and aeration rate. b The fitted RSM model for lutein content in the designed irradiance strength, light hours per day, and aeration rate. c The fitted RSM model for lutein productivity in the designed irradiance strength, light hours per day, and aeration rate
Final biomass concentration, lutein content, and productivity of C. salina cultivated under different light intensities, photoperiods, and aeration rate are shown in Table 2. It can be noted that C. salina had the ability to grow under all the investigated levels. The data are fitted into the quantitative surface models using a second-order polynomial regression equation [35]. Tables 3, 4, and 5 show the analysis of variance of the fitted quadratic polynomial model for biomass production, lutein production, and lutein productivity. The application of regression analysis of the experimental data using the Design Expert software (version 7.0.0; Stat-Ease, Inc., Minneapolis, MN, USA) yielded the following quadratic regression equations for biomass production (Eq. (1), lutein content (Eq. (2), and lutein productivity (Eq. (3).
where X1, X2, and X3 are the coded terms for the three independent variables denoted as light intensity, photoperiod, and aeration rate, respectively. The p value from the ANOVA shows the significance of each variable. The smaller the p value, the more significant is the corresponding coefficient. All mutual interaction terms were found to be insignificant in both models (p > 0.05). Adequate precision measures the signal-to-noise ratio. A ratio greater than 4 is desirable. R2 (coefficient of regression) is the proportion of variation in the depended variable explained by the regression model. Adjusted R2 is the coefficient of determination adjusted for the number of independent variables in the regression model [43]. From the analysis of variances for the experimental data (Tables 3 and 4), it showed that the R2 value of the models were 0.98, 0.97, and 0.97 which indicating that 98% and 97% of the experimental data can be fitted with the model-predicted value. High values of R2 obtained in for all the models indicated that they would accurately predict the relationships between the parameters. Further, the “Predicted R2” values were in reasonable agreement with the “Adjusted R2” which confirmed that models could be used to navigate the design space. Results acquired from the graphs by CCD indicate that light intensity and photoperiod have noticeable effects on algal biomass concentration and lutein productivity. In fact, there is a correlation between the light energy captured and carbon fixation which eventually causes variations in biomass production.
3.4 Model validation and experimental confirmation
The second-order polynomial model obtained described in Eqs. (1, (2, and (3 were utilized for response optimization by using CCD. The maximum lutein and biomass production as 10.15 mg g−1 and 1.15 g L−1, respectively, were determined at the optimum conditions: light intensity of 200 μmol m−2 s−1, light period of 12:12, and aeration of 3 lpm. Suggested culture conditions favored improved the production of lutein and biomass. The obtained amount of lutein and biomass production under streamlined conditions exhibited 95.89% and 97.88% productivity, respectively, showing an excellent correlation with the predicted value, and thus indicating the competence of the fitted model.
4 Conclusion
A laboratory-scale externally illuminated airlift photobioreactor was used in the present work to evaluate lutein productivity of microalga C. salina. Twenty sets of experiments were conducted in accordance with CCD in order to study and optimize the effect of three environmental factors on growth kinetics and lutein productivity. The optimum values were the following: light intensity, 200 μmol m−2 s−1; photoperiod, 12:12 h, 16:8 h; and aeration rate, 3 lpm. Under these conditions, highest lutein productivity of 9.86 mg/(L day) and RC biomass concentration of 565.89 mg/(L day) were obtained. The experimental data obtained for p were in great concurrence with the projected data by the expressed numerical model. C. salina was cultivated under medium-low light intensities, and 16:8 h light supplementation yielded desirable lutein productivity.
References
Ceron MC, Campos I, Sanchez JF, Acien FG, Molina E, Fernandez-Sevilla JM (2008) Recovery of lutein from microalgae biomass: development of a process for Scenedesmus almeriensis biomass. J Agric Food Chem 56:11761–11766
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34(8):1396–1412
Murray PM, Moane S, Collins C, Beletskaya T, Thomas OP, Duarte AWF, Nobre FS, Owoyemi IO, Pagnocca FC, Settle LD, McHugh E, Causse E, Perez-Lopez P, Feijoo G, Moreira MT, Rubiolo J, Leiros M, Botana LM, Walsh DJ (2013) Sustainable production of biologically active molecules of marine based origin. New Biotechnol 30:839–850
Walker TL, Purton S, Becker DK, Collet C (2005) Microalgae as bioreactors. Plant Cell Rep 24:629–641
Lamers PP, Van de Laak CCW, Kaasenbrood PS, Lorier J, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648
Lamers PP, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2012) Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliellaalina, a unicellular green microalga. J Biotechnol 162:21–27
Wang B, Zarka A, Trebst A, Boussiba S (2003) Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J Phycol 39:1116–1124
Telfer A, Pascal A, Gall A (2008) Carotenoids in photosynthesis. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Carotenoids, vol 4. Birkhäuser Basel. pp. 265-308
Mulders KJM, Lamers PP, Martens DE, Wijffels RH (2014) Phototrophic pigment production with microalgae: biological constraints and opportunities. J Phycol 50:229–242
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89:1275–1288
Brindley Alias C, Garcia-MaleaLopez MC, AcienFernandez FG, Fernandez Sevilla JM, Garcia Sanchez JL, Molina Grima E (2004) Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol Bioeng 87:723–733
Bitog JP, Lee IB, Lee CG, Kim KS, Hwang HS, Hong SW, Seo IH, Kwon KS, Mostafa E (2011) Application of computational fluid dynamics for modeling and designing photobioreactors for microalgae production: a review. Comput Electron Agric 76:131–147
Dineshkumar R, Dhanarajan G, Dash SK, Sen R (2015a) An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res 7:24–32
Dineshkumar R, Dash SK, Sen R (2015b) Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv 5:73381–73394
Xie Y, Ho SH, Chen CN, Chen CY, Ng IS, Jing KJ, Chang JS, Lu Y (2013) Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: effects of nitrate concentration, light intensity and fed-batch operation. Bioresour Technol 144:435–444
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light and nitrogen starvation on the content and composition of carotenoids of the green microalga Parietochloris incisa. Russ J Plant Physiol 55:455–462
Vaquero I, Mogedas B, Ruiz-Dominguez MC, Vega JM, Vílchez C (2014) Light- mediated lutein enrichment of an acid environment microalga. Algal Res 6:70–77
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilus. Fish Invest London Ser 2 26(5):1–62
Chang HL, Tseng YL, Ho KL, Shie SC, Wu PS, Hsu YT, Lee TM (2014) Reactive oxygen species modulate the differential expression of methionine sulfoxide reductase genes in Chlamydomonas reinhardtii by high light illumination. Plant Physiol 144:225–237
Chang HL, Hsu YT, Kang CY, Lee TM (2013) Nitric oxide down-regulation of carotenoid synthesis and PSII activity in relation to very high light-induced singlet oxygen production and oxidative stress in Chlamydomonas reinhardtii. Plant Cell Physiol 53:445–456
Polle JE, Niyogi KK, Melis A (2001) Absence of lutein, violaxanthin and neoxanthin affects the functional chlorophyll antenna size of photosystem-II but not that of photosystem-I in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol 42:482–491
Tang H, Abunasser N, Garcia MED, Chen M, Simon Ng KY, Salley SO (2010) Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl Energy 88:3324–3330
Martinez ME, Jimnez JM, Yousfi FE (1999) Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour Technol 67:233–240
Barbosa MJ, Zijffers JW, Nisworo A, Vaes W, van Schoonhoven J, Wijffels RH (2005) Optimization of biomass, vitamins, and carotenoid yield on light energy in a flatpanel reactor using the A-stat technique. Biotechnol Bioeng 89(2):233–242
Ramos Tercero EA, Sforza E, Morandini M, Bertucco A (2014) Cultivation of Chlorella protothecoides with urban wastewater in continuous photobioreactor: biomass productivity and nutrient removal. Appl Biochem Biotechnol 172:1470–1485
Zijffers JWF, Schippers KJ, Zheng K, Janssen M, Tramper J, Wijffels RH (2010) Maximum photosynthetic yield of green microalgae in photobioreactors. Mar Biotechnol 12(6):708–718
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Wong YK, Yung KKL, Tsang YF, Xia Y, Wang L, Ho KC (2015b) Scenedesmus quadricauda for nutrient removal and lipid production in wastewater. Water Environ Res 87(12):2037–2044
Cordero BF, Obraztsova I, Couso I, Leon R, Vargas MA, Rodriguez H (2011) Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar Drugs 9(9):1607–1624
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Mahale VE, Chaugule BB (2013) Optimization of freshwater green alga Scenedesmus incrassatulus for biomass production and augmentation of fatty acids under abiotic stress conditions. Phykos 43(1):22–31
Sharma R, Singh GP, Sharma VK (2012) Effect of culture conditions on growth and biochemical profile of Chlorella vulgaris. J Plant Pathol Microbiol 3(5)
Anjos M, Fernandes BD, Vicente AA, Teixeira JA, Dragone G (2013) Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour Technol 139:149–154
Fan LH, Zhang YT, Cheng LH, Zhang L, Tang DS, Chen HL (2007) Optimization of carbon dioxide fixation by Chlorella vulgaris cultivated in a membrane-photobioreactor. Chem Eng Technol 30:1094–1099
Gai C, Zhang Y, Chen WT, Zhang P, Dong Y (2014) Energy and nutrient recovery efficiencies in biocrude oil produced via hydrothermal liquefaction of Chlorella pyrenoidosa. RSC Adv 4:16958
Del Campo JA, Moreno J, Rodriguez H, Vargas MA, Rivas J, Guerrero MG (2000) Carotenoid content of chlorophycean microalgae. Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76:51–59
Del Campo JA, Rodríguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 81:289–295
Wei D, Chen F, Chen G, Zhang X, Liu L, Zhang H (2008) Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci China C Life Sci 51:1088–1093
Sanchez JF, Fernandez-Sevilla JM, Acien FG, Ceron MC, Perez-Parra J, Molina-Grima E (2008) Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79:719–729
Sanchez JF, Fernandez-Sevilla JM, Acien FG, Rueda A, Perez-Parra J, Molina E (2008) Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem 43:398–405
Garbayo I, Cuaresma M, Vílchez C, Vega JM (2008) Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process Biochem 43:1158–1161
Shi XM, Zhang XW, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym Microb Technol 27(3–5):312–318
Ferreira SC, Bruns R, Ferreira H, Matos G, David J, Brandao G, da Silva E, Portugal L, Dos Reis P, Souza A (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597(2):179–186
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gayathri, S., Rajasree, S.R.R., Suman, T.Y. et al. Induction of β, ε-carotene-3, 3′-diol (lutein) production in green algae Chlorella salina with airlift photobioreactor: interaction of different aeration and light-related strategies. Biomass Conv. Bioref. 11, 2003–2012 (2021). https://doi.org/10.1007/s13399-019-00580-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00580-5