Abstract
India generated around 1.45 lakh tonne of municipal solid waste (MSW) per day, out of which 40% is organic biodegradable waste, which has food waste as the major component. Scientific conversion of this food waste to energy is always challenging. In the present study, anaerobic digestibility of food waste as a mono digestion substrate and co-digestion of food waste with water hyacinth were tested and analyzed in a batch type anaerobic digester of capacity 60 l. Four different samples, i.e., only food waste, only water hyacinth, and with food waste to water hyacinth in the ratio of 15:2 and 8:3 to maintain total solids contain equal in all samples were analyzed for the anaerobic digestion (AD). Biogas yield for the above four samples were found to be 370.85 (ml/g VS), 320.54 (ml/g VS), 286.50 (ml/g VS), and 298.83 (ml/g VS), respectively. The average methane content was found to be 68.3%, 58.2%, 52.1%, and 65.4%, respectively whereas CO2 content was found to be 30.2%, 40.9%, 46.6%, and 33.3%, respectively, using gas chromatography. The temperature variation for anaerobic digester was measured in the range of 32 to 43 °C during the experiment without supplying any external heat. pH value of all samples was ranged between 6.5 and 7.5 at the end of the experiment. The results of this study conclude that co-digestion of food waste with water hyacinth has higher operational stability compared to mono digestion of food waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Food waste can be a useful resource for energy and manure rather than a burden on the municipality. Worldwide, one-third of food produced is wasted daily according to the Food and Agriculture Organization (FAO) [1]. In India alone, 40% of the total food produced is wasted which is worth of INR 50000 crore [2]. Food waste is generated in every stage of the food chain, starting from pre- and post-harvesting waste to waste from restaurant and household which can be separated at the source and utilized. Waste generated from earlier stages can be reused as animal feed and for fertilizer production [3]. But food waste generated from the later stages of the food chain is disposed of by landfilling, incineration, composting, and anaerobic digestion (AD) [4]. The landfilling of food waste generates greenhouse gas (GHG) emission, and percolated contaminants result in soil and groundwater pollution. Incineration and composting of food waste are not an effective option because of high moisture content (MC) while AD is a more adequate solution for the disposal of food waste because it generates biogas and digestate slurry which can be used as fertilizer [5].
Anaerobic digestion is a progression of serial process in which microorganism or bacteria degrade organic material or biomass in the absence of gaseous oxygen and lead to the formation of biogas and digestate slurry [6]. AD is a process containing various stages which include hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Biomass containing vast organic polymers is first broken down into their smaller constituent parts of monomers like sugars, glucose, fatty acids, and amino acids [7]. The way toward breaking these chains and dissolving the smaller molecules into solution is called hydrolysis. The chemical reaction of hydrolysis is given by Eq. 1.
Hydrolysis is followed by the acid-forming step acidogenesis in which there is a further breakdown of the rest of the parts by acidogenic (fermentative) microorganisms. Products of acidogenesis are higher organic acids like propionic acid, butyric acid, and acetic acid (CH3COOH), with hydrogen, carbon dioxide (CO2), short-chain volatile fatty acids (VFA), alcohol, and ammonia (NH3). In this second phase, the following reaction takes place (Eqs. 2–6):
The third phase of AD is acetogenesis in which basic atoms made through the acidogenesis stage are further digestate by acetogens to produce largely acetic acid and also carbon dioxide and hydrogen. Acetogenesis takes place in the following way (Eqs. 7–11):
The last step of AD, methanogens utilize the intermediate products of the preceding stages (acidogenesis and acetogenesis) and convert them into methane (CH4), CO2, some traces of hydrogen sulfide (H2S) and water (H2O). Methane formation takes place by the following reaction (Eqs. 12–13):
Methanogenesis is a critical step in the entire AD process, and its biochemical reactions are the slowest and time-consuming compared with other phases of AD. Methane-producing bacteria are strict anaerobes and are vulnerable to even small amounts of oxygen. Methanogenesis is sensitive to both high as well as low pH and occurs between pH 6 and 8.5 [8].
The organic material characteristics and digestion process parameters are important information for the process of AD. These parameters affect the biogas production, process stability, and digestate slurry quality during AD. It includes total solids (TS), volatile solids (VS), moisture content (MC), C/N ratio, particle size, initial pH value, temperature, and biodegradability. Biomass biodegradability is defined as the total reduction in TS and VS during the digestion and total biogas production per kg of biomass [7]. Biogas or methane yield of biomass is defined as biogas or methane production per kg of VS. Food waste has high lipid content and lipid-rich waste is considered to be highly attractive for AD since the methane potential of lipids (1014 l/kg VS) is much higher than that of carbohydrates (370 l/kg VS) and proteins (740 l/kg TS) [9]. Hence, food waste containing high lipid content has higher methane yield compared with low lipid biomass such as fruits and vegetables. For the mixed food waste as studied by Cho et al. [10], the methane yield was found to be 472 ml/g VS and for the mixed traditional Korean food (10–15% boiled rice, 65–70% vegetables, and 15–20% meat and eggs), the same was observed to be 489 ml/g VS [11]. Furthermore, Zhang et al. [8] measured methane yield for restaurant food waste as 440 ml/g VS whereas methane yield from the AD of food waste with lime mud from papermaking process was found to be 272.8 ml/g VS [12]. However, the AD of food waste can be difficult to digest as a mono substrate at a higher organic loading rate because of rapid acid formation during the digestion which creates instability in the AD process. Instability of food waste is mainly due to pH drop during the rapid acid formation in the acidogenesis stage which makes an unsuitable environment for methanogenic bacteria. Methanogenesis is sensitive to both high as well as low pH and occurs between 6 and 8.5 [8]. Co-digestion of food waste with a substrate having a high C/N ratio or lignocellulosic characteristics like rice husk has more stable AD compared with mono digestion of food waste [13]. Addition of substrate having a high C/N ratio removes limitations due to ammonia inhibition [14]. It was reported that when lime mud from papermaking process was added in proportion 10 g/l with food waste at 19.8 g VS/l in AD system, lime mud acts as buffer agent and inorganic nutrient, and methane yield was observed 272.8 ml/g VS [12].
The use of water hyacinth as a substrate for co-digestion of food waste will reduce the environmental problems caused by this aquatic weed along with social and economic benefit at the mass level. Water hyacinth which is low in lignin content (10%) (cellulose (25%) and hemicellulose (33%)) is also a potential substrate for the co-digestion with food waste [15]. However, the applicability of water hyacinth as a buffering agent for AD is hardly reported to date. In this context, the objective of this study was to determine the biodegradability of food waste as mono digestion and co-digestion with water hyacinth under mesophilic condition.
2 Experimental setup
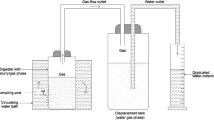
Batch mode floating dome type biogas plant having simple installation, low maintenance, constant gas pressure, and less scum problem were established at the Renewable and Sustainable Energy Lab, Mechanical Engineering Department, S V National Institute of Technology, Surat, India. Four batch type biogas plants with digester capacity of 60 l and gas holder capacity of 35 l were designed and developed. The schematic of the experimental setup is shown in Fig. 1. Continuous measurement of parameters was ensured by equipping experimental setup with the thermocouple, pH sensor, pressure gauge, and displacement scale. A J-type thermocouple was used to measure the temperature of digestate during the experimentation period. Thermocouples were calibrated by using standard thermometer before the experiment started. To measure pH value, samples were taken out from the outlet provided with a digester and were measured regularly by using a digital pH meter. Pressure gauges were installed on the gas holder to monitor gas pressure. To measure daily generated biogas, dome displacement scale was used. The scale was attached to the gas holder and the difference between the heights was monitored periodically. The gas outlet was provided to take a sample and use extra biogas generated from AD in various thermal applications. Gas samples were collected once in a week to measure the biogas compositions using gas sampling balloons. Samples were analyzed into gas chromatography to measure the percentage of CH4 and CO2. Furthermore, a characterization study was also performed on food waste sample and TS and VS were measured by a standard procedure. Ultimate analysis of food waste and water hyacinth was performed to measure the value of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) in LeCo TruSpec CHNS analyzer. The stability of the AD process was evaluated in terms of variation in pH value. Performance of the AD process was evaluated in terms of biodegradability of food waste as mono digestion and co-digestion with water hyacinth. Biodegradability was evaluated in terms of reduction in TS and VS percentage and total biogas yield (ml/g VS).
2.1 Feeding substrate
Food waste was used as the main substrate for AD during this experiment. The required amount of food waste was collected and segregated food waste was crushed to reduce the particle size and to achieve uniform mixing of food waste. Samples were collected for characterization study after crushing and food waste was mixed with water in the proportion of food waste to water 1:1.2 for the smooth operation of AD. Water hyacinth was collected from Tapi River, Surat, India. Characteristic analysis of green water hyacinth was performed to measure TS, VS, and MC. Green water hyacinth was cleaned and dried in the solar drier to remove MC. Water hyacinth was dried for 2 days until the MC reduced to 6.5%. Dried water hyacinth was pulverized for uniform mixing and was mixed with water in a ratio of dry water hyacinth to water 1:10. The inoculum was collected from already existed biogas plant based on fruits and vegetable waste. Every sample of the anaerobic digester was fed with two parts as feeding substrate and one part as inoculum. Four different samples of feeding substrate were prepared for performance analysis as mentioned in Table 1. Sample 1 was fed with only food waste while sample 2 and sample 3 were fed with the mixture of food waste and water hyacinth in a ratio of food waste (wet basis) to water hyacinth (dry basis) 15:2 and 8:3, respectively, and sample 4 was fed with only water hyacinth.
3 Result and discussion
3.1 Characterization of food waste
The average value of MC, TS, VS, VS/TS, C/N ratio, initial pH, and temperature of food waste samples are shown Table 2 along with different characteristics mentioned in previous studies on food waste. It was observed that VS/TS remains almost constant in food waste. Initial pH value of food waste was measured 4.9 which conclude that the food waste required the addition of inorganic nutrient or buffer agent to stabilize the AD. Lime was added to food waste in quantity of 10 g/l as a buffering agent which showed stable digestion of food waste during the present study. C/N ratio of food waste was observed 14.82 on a dry basis which is adequate for AD as mentioned by Zhang et al. [17].
The characteristic of water hyacinth was measured before and after drying, and the value of MC, TS, VS, and VS/TS for green water hyacinth were measure 89.38%, 10.62%, 8.86%, and 83.46%, respectively. The MC of water hyacinth was reduced to 6.5% by using a solar dryer. Green water hyacinth was kept into the solar dryer for 2 days and characteristics of dry water hyacinth were measured. The value of MC, TS, VS, and VS/TS for dry water hyacinth was observed to be 6.5%, 93.5%, 83.76%, and 89.58%, respectively. The C/N ratio for dry water hyacinth was observed 25.32 in the present study which makes water hyacinth a suitable option for co-digestion with food waste.
3.2 Variation of temperature and pH
The variations in the temperature of the anaerobic digester with digestion time for all four samples were measured two times a day during morning and afternoon. Daily change in the average value of temperature with digestion time is shown in Fig. 2. It was observed that temperature inside the anaerobic digester was higher than atmospheric temperature because of biological reaction which generates higher temperature. From the variation of temperature value, the average temperature ranged from 32 to 43 °C which shows that the AD was in the mesophilic range of temperature [7, 18]. There is no external heat that is supplied to the anaerobic digester to control the temperature, but because of the atmospheric condition temperature of digester remained in mesophilic condition during the study.
Variation of pH value during the retention period is shown in Fig. 3. Because of the high biodegradability of food waste sample 1, it was observed that pH value was decreased rapidly during the initial stage of AD. For sample 1 in which digestion of food waste alone took place, a rapid drop of pH value was observed. During the digestion period of 12 days, the pH of sample 1 digester dropped from 5.5 to 4.3. But with over a period of time, it was improved to 6.9 at the end of the experiment. For sample 2 which contained food waste and water hyacinth, pH value was dropped from 6.61 to 5.00 during the digestion period of 15 days, but because of the presence of water hyacinth, pH value increase to 7.20 at the end of the experiment. Same has observed in sample 3 in which pH value ranged from 6.7 to 5.44 in 15 days of the retention period. It can be deduced that water hyacinth acts as organic nutrient and buffer agent to stabilize AD by maintaining pH value in the optimum range. Also, it was observed that with an increase in the proportion of water hyacinth with food waste, variation in pH value decreases. For sample 4 in which digestion of water hyacinth alone took place, pH value was observed in the optimum range of AD over a period of the experiment.
3.3 TS and VS reduction
Biodegradability of a feedstock or biomass is measured in terms of reduction in TS and VS of biomass during the retention period of AD. The initial and final value of TS and VS with percentage reduction is shown in Table 3. It can be deduced that with an increase in water hyacinth proportion in feedstock, biodegradability decreases but the stability of anaerobic process was increased. That attributes to the lignocellulosic characteristics and low lignin content of water hyacinth.
3.4 Biogas production
The biogas production (liter/day) of different samples during the retention period of 40 days is shown in Fig. 4. As shown in Fig. 4, the highest biogas production for sample 1, 2, 3, and 4 were 85.26 l, 53.26 l, 54.01 l, and 49.67 l during 12 to 15 days of the retention period. It can be concluded that biogas production from the kitchen waste was highest among all samples. Biogas production was higher after 10 days of the retention period and it was almost negligible after 33 days of the retention period. Biogas yield (ml/g VS) of different samples is shown in Fig. 5 and it can be deduced that maximum biogas yield was observed in the case of sample 1. Sample 4 showed higher biogas yield while it had lower biogas production than sample 2 and sample 3 during the retention period of 12 to 15 days. From Fig. 4 and Fig. 5, it can be concluded that the co-digestion of water hyacinth with food waste slightly reduces the biogas production and biogas yield but a variation of pH value showed that stability of performance was better compared with mono digestion of food waste. An average methane percentage of biogas for sample 1, 2, 3, and 4 were found to be 68.3%, 58.2%, 52.1% and 65.4%, respectively, during the present study.
Variation of cumulative biogas production with retention period is shown in Fig. 6 and it can be concluded that total biogas production was higher for AD of food waste compared with co-digestion of food waste and water hyacinth and also water hyacinth alone. Cumulative production of biogas during retention period was measured 1281.28 l, 977.55 l, 957.16 l, 928.01 l for sample 1, 2, 3, and 4, respectively. Cumulative biogas yield for food waste alone is 370.85 (ml/g VS) while in previous studies, the mixed food waste studied by Cho et al. [10], the methane yield was found to be 472 ml/g VS. For the mixed traditional Korean food (10–15% boiled rice, 65–70% vegetables, and 15–20% meat and eggs), the same was observed to be 489 ml/g VS [11]. Furthermore, Zhang et al. [8] measured methane yield for restaurant food waste as 440 ml/g VS whereas methane yield from the AD of food waste with lime mud from papermaking process was found to be 272.8 ml/g VS [12]. Compared with other studies, biogas yield was less in the present study because of different food waste compositions according to the region. It was found that the cumulative biogas yield for sample 2 and sample 3 with co-digestion of food waste were 286.50 (ml/g VS) and 298.83 (ml/g VS). Cumulative biogas yield for water hyacinth alone was measure 320.54 (ml/g VS) during the retention period. Cumulative biogas yield for co-digestion of food waste with water hyacinth for sample 2 and sample 3 was 22.74% and 19.41% less than cumulative biogas yield of food waste alone during the retention period. From Fig. 6, it can be concluded that 95% of biogas produced by 33 days of the retention period, and it was completely stopped after 40 days of the retention period. As observed in Figs. 3, 5, and 6, initially with a rapid drop in pH value, because of the high biodegradability of food waste, biogas yield was less and biogas production was increasing gradually. Same was observed in all four samples during experimentation. But after 12 days as pH value was improving, the rate of biogas yield also increased and increase in biogas production was steep. It can be deduced that with supplement of proper buffer agent like water hyacinth to improve pH value of digestate, biogas yield and biogas production from food waste increase.
4 Conclusion
Characteristic analysis of food waste concludes that food waste is a potential substrate for AD. Water hyacinth is a suitable substrate for co-digestion of food waste and it has more stability. The following conclusions were drawn from the present study:
-
During the retention period, TS for sample 1, 2, 3, and 4 reduced by 71.45%, 67.44%, 61.09%, and 67.10%, respectively, and VS reduced by 70.22%, 69.43%, 60.54%, and 66.02%, respectively.
-
Biogas yield accounted for samples 1, 2, 3, and 4 to be 370.85 (ml/g VS), 320.54 (ml/g VS), 286.50 (ml/g VS), and 298.83 (ml/g VS), respectively.
pH variation during the retention period shows that mono digestion of food waste was unstable because of rapid pH drop, but co-digestion of food waste with water hyacinth has great stability during AD. It concludes that during co-digestion, water hyacinth acts as a buffering agent which maintained pH in the optimum range and is a good option for a smooth operation of the biogas plant.
References
Gustavsson J, Cederberg C, Sonesson U, et al (2011) Global food losses and food waste
Searchinger T, Waite R, Hanson C, et al (2018) Creating a sustainable food future: synthesis report
Joshi R, Ahmed S (2016) Status and challenges of municipal solid waste management in India: a review. Cogent Environ Sci 2:1–18. https://doi.org/10.1080/23311843.2016.1139434
Kumar V, Pandit RK (2013) Problems of solid waste management in Indian cities. Int J Sci Res Publ 3:2250–3153
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sust Energ Rev 38:383–392. https://doi.org/10.1016/j.rser.2014.05.038
Banks CJ, Chesshire M, Heaven S, Arnold R (2011) Anaerobic digestion of source-segregated domestic food waste: performance assessment by mass and energy balance. Bioresour Technol 102:612–620. https://doi.org/10.1016/j.biortech.2010.08.005
Zhang C, Xiao G, Peng L, Su H, Tan T (2013) The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol 129:170–176. https://doi.org/10.1016/j.biortech.2012.10.138
Zhang R, El-Mashad HM, Hartman K et al (2007) Characterization of food waste as feedstock for anaerobic digestion. Bioresour Technol 98:929–935. https://doi.org/10.1016/j.biortech.2006.02.039
Kim HW, Han SK, Shin HS (2003) The optimisation of food waste addition as a co-substrate in anaerobic digestion of sewage sludge. Waste Manag Res 21:515–526. https://doi.org/10.1177/0734242X0302100604
Cho JK, Park SC, Chang HN (1995) Biochemical methane potential and solid state anaerobic digestion of Korean food wastes. Bioresour Technol 52:245–253. https://doi.org/10.1016/0960-8524(95)00031-9
Heo NH, Park SC, Kang PH (2004) Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J Environ Sci Health A 39:1739–1756. https://doi.org/10.1081/ESE-120037874
Zhang J, Wang Q, Jiang J (2013) Lime mud from paper-making process addition to food waste synergistically enhances hydrogen fermentation performance. Int J Hydrog Energy 38:2738–2745. https://doi.org/10.1016/j.ijhydene.2012.12.048
Haider MR, Zeshan YS et al (2015) Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour Technol 190:451–457. https://doi.org/10.1016/j.biortech.2015.02.105
Zhang L, Lee YW, Jahng D (2011) Anaerobic co-digestion of food waste and piggery wastewater: focusing on the role of trace elements. Bioresour Technol 102:5048–5059. https://doi.org/10.1016/j.biortech.2011.01.082
Prasad R, Yadav KD (2016) Water hyacinth as a potential natural resource
Zhang J, Wang Q, Zheng P, Wang Y (2014) Anaerobic digestion of food waste stabilized by lime mud from papermaking process. Bioresour Technol 170:270–277. https://doi.org/10.1016/j.biortech.2014.08.003
Zhang Y, Banks CJ, Heaven S (2012) Co-digestion of source segregated domestic food waste to improve process stability. Bioresour Technol 114:168–178. https://doi.org/10.1016/j.biortech.2012.03.040
Chen X, Romano RT, Zhang R (2010) Anaerobic digestion of food wastes for biogas production. Int J Agric Biol Eng 3:61–72. https://doi.org/10.3965/j.issn.1934-6344.2010.04.061-072
El-Mashad HM, Zeeman G, Van Loon WKP, et al (2004) Effect of temperature and temperature fluctuation on thermophilic anaerobic digestion of cattle manure. Bioresour Technol 95:191–201. https://doi.org/10.1016/j.biortech.2003.07.013
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zala, M., Solanki, R., Bhale, P.V. et al. Experimental investigation on anaerobic co-digestion of food waste and water hyacinth in batch type reactor under mesophilic condition. Biomass Conv. Bioref. 10, 707–714 (2020). https://doi.org/10.1007/s13399-019-00522-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00522-1