Abstract
Three concepts for a 3000 t/a continuous autohydrolysis plant aiming at a full fractionation of agricultural residues (wheat straw as model substrate) into streams rich in xylose, glucose, and lignin, respectively, are developed. For this purpose, two kinetic models based on batch liquid-hot water experiments (30 mL, 170–230 °C, 10–60 min) were investigated. The solid hemicellulose conversion was described with a first-order rate model. The concentration and conversion profile investigation of the solid and liquid phases using the severity factor resulted in a linear equation describing the hemicellulose solubilization: XHC = (0.5839 ∗ log(R0) − 1.7027) ± 0.06. Here, specific process parameter boarders for the maximal hemicellulose concentration and furfural formation at log(R0) = 4.0 considering the liquid phase were identified. These findings were used to propose a multi-step hydrothermal processing approach, with intermediate sugar extraction, tailored to the compositional requirements of the product streams. Seven reactor types were evaluated for its feasibility; in this regard, the screw conveyor reactor (SCR) was evaluated as most promising. For the concepts, the SCR reactors were scaled, based on the generated data. It was found that the process temperature is a major parameter determining the reactor size. It is considered more important than the number of autohydrolysis treatment steps. In brief, the fractionation of agricultural residues using a multi-step continuous plant is evaluated to be very promising for the industrial application, regarding selectivity, handling, investment, and process costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
To tackle the depletion of crude oil regarding the feedstock for the chemical industry, the use of annual lignocellulose, e.g., wheat straw, sugarcane bagasse, and corn stover, possesses a high potential [1]. Despite the high potential, these materials take an insignificant role as a feedstock in the market today, mostly due to technical and economic challenges in processing. To promote a feedstock change in the chemical industry from crude oil to renewable and non-edible materials, more efficient refining processes and corresponding integrated plants are required.
Transportation of the feedstock to the processing plant is challenging due to the fluctuating availability, the low density, and poor flowability of the materials. Therefore, it is believed that future second-generation biorefineries will be realized in smaller scales compared with crude oil refineries and follow a rather decentralized approach.
The autohydrolysis process targeted in this work aims at the full fractionation of annual biomasses and utilizes water under high temperatures for this purpose. The autohydrolysis reactor development for decentral plants is the focus of this publication. For this purpose, knowledge about the chemical reactions, material flows, and energy consumption of the autohydrolysis unit is needed as well as the impact on downstream processing. In order to develop economically feasible processing concepts, plant-wide techno-economical investigations are required.
For the reactor design, the investigation of reaction phenomena and kinetic modeling regarding the utilized reactor type are the central tasks. In this work, reaction phenomena refer to the solubilization of the biopolymers, the physical and compositional changes in the solid matter, and the reaction pathways of the various dissolved molecules. A protocol for the evaluation of the reaction phenomena using the severity factor is presented, using data from laboratory batch experiments. In addition, the hemicellulose solubilization is modeled with the aim to evaluate different reactor types. In a second step, the models are used for the apparatus scaling on 3000 annual tons of wheat straw using preselected reactor types.
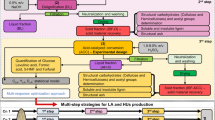
The selection of suitable apparatuses is undertaken in three steps: (1) Identification of technical process requirements, (2) Application thereof to seven different reactor types, as displayed in Fig. 1, and (3) development of an autohydrolysis process flow sheet. The reactor-type evaluation and plant concept study aim at the identification and quantification of causal relations for the design of an industrial autohydrolysis process for the full fractionation of annual lignocellulose. This work is supposed to serve as a signpost for the further process development and survey which reactor types and plant concepts bear potential for an economical process.
In Chapters 1.1 and 1.2, the principles of the targeted autohydrolysis process are given and the influence of the stream qualities on the downstream processes is described.
1.1 Autohydrolysis pretreatment
The pretreatment is also called aquasolv, hot water extraction, and liquid hot water. If saturated steam is used to heat the wet biomass, the process is sometimes named steam pretreatment. It aims at the selective removal of hemicellulose to recover it as a product and to produce a solid with beneficial properties for a consecutive enzymatic hydrolysis. The process uses the increasing ionic product of water at temperatures between 170 and 230 °C, which increases the concentrations of H3O+ and OH− ions. Thus, water acts as solvent and catalyst [2]. The acetyl side chains of hemicellulose are cleaved to release acetic acid during the process, which leads to a drop in the pH [3]. Usually, the pH does not drop lower than pH = 3. It is assumed that the biomass inherits a pH-buffering effect, which explains the deviation of the experimental pH data and the predictions using the concentration of organic acids.
The solubilization of hemicellulose is a combination of a chemical cleavage reaction of the glycoside bonds and dissolution and extraction of the produced fragments of shorter chain length [4]. The chain cleavage by hydrolysis is catalyzed by acids [5]. The release of organic acids during the process and acid-catalyzed cleavage reactions explains the name autohydrolysis. Cellulose is practically inert in the targeted temperature range (170–230 °C) [6]. The lignin fraction also remains to large extents in the solid phase. However, it is reported that lignin can change its position in the plant cell wall, lumps together, and forms droplets at the surface [7, 8]. In fixed-bed reactors, it was observed that small and spherical lignin-rich particles can be found in the liquid fraction [9]. The amount of lignin removed this way was reported to be up to 20 wt% [10]. Applying steam pretreatment, the lignin is reported to remain in the solid fraction, unless it is being exposed to a flow-through washing step [11, 12].
The dissolved oligomers undergo further acid-catalyzed hydrolysis to form monomers. These in turn can undergo dehydration reactions to form the degradations products furfural [5] from pentoses and hydroxymethylfurfural (HMF) from hexoses.
The acid-catalyzed formation of acid-“insoluble” heterogeneous polymers in autohydrolysis is reported to occur with furfural and intermediates of the xylose to furfural conversion [13], HMF [14], and dissolved lignin [15]. Those solid products can adhere to the biomass and be hardly distinguished from the mineral acid residue also called acid- “insoluble” lignin.
1.2 Integrated process design
For the design of an efficient pretreatment process, the upstream and downstream process units and the product requirements must be taken into account. The hydrolysate obtained from the autohydrolysis process can be used for the isolation of its components, production of furfural or sugar syrup, or fermented to biogas and organic acids respectively [16,17,18]. The pretreated solids, called cellulignin, shall be processed enzymatically to produce a high-quality lignin as well as a glucose-rich hydrolysate. In this work, processes are considered with a focus on the production of high-quality lignin. To produce highly digestible cellulignin, the aim is a full hemicellulose solubilization and the avoidance of condensation reactions. The formation of furfural indicates the loss of the product (xylose), the formation of a fermentation inhibitor (furfural) and possible condensation reactions [13]. Therefore, the formation of furfural is to be avoided.
Nevertheless, the particular application of the hydrolysate determines the requirements of hydrolysate composition. Taking into account the targeted high-quality lignin, optimal pretreatment conditions must be found. To name a few examples, for a consecutive fed-batch fermentation, it is assumed that high sugar concentrations and the absence of fermentation inhibitors, such as furfural, are more important parameters than the degree of the hemicellulose conversion itself. If the hydrolysate components should be further used and therefore purified, it is more important to minimize the fine particle content in the hydrolysate, than to decrease the furfural content. The utilization of the C5 sugar-rich hydrolysate is dependent on the market situation [16] and is to be investigated further in the future. Therefore, the pretreatment process should be flexible and adaptable to different product requirements coming from targeted applications of each fraction.
2 Materials and methods
2.1 Batch experiments
For the determination of the reaction kinetics experimental data from earlier published experiments were used [19]. Wheat straw pellets with a moisture content of approximately 10 wt% were used as substrate. Autohydrolysis experiments were conducted in 30 mL batch reactors at 50 bar at temperatures of 170 °C, 185 °C, 200 °C, 215 °C, and 230 °C for each 10, 20, 30, 45, 60, and 90 min. Each experiment was conducted in duplicate with 600 mg of dry biomass and deionized water (added to result in 30 mL reaction volume). Nitrogen gas was used to pressurize the reactors. An electrical heating jacket was applied to control the temperature. When the reactor temperature reached 10 °C below its set point, the reaction time was started. The reaction was stopped by cooling each reactor with an ice bath. The composition of the solid phase and the hydrolysate was measured as well as the remaining solid dry biomass.
2.2 Kinetics modeling
For the scaling of an autohydrolysis reactor, a mathematical description of the changing hemicellulose content in the solid phase during the process is required. For this propose, the experimental data from the batch screening (Section 2.1) were used. It is assumed that the temperature was homogeneously distributed in the reaction mixture and the hydrolysate composition did not show local gradients. The hemicellulose solubilization kinetics are commonly modeled as a pseudo-homogeneous reaction of first or second order [19], with various complexities. It is assumed that the mass transport can be neglected for the batch experiments and the modeled plant concepts. A comprehensive overview of hemicellulose hydrolysis models is given by Ruiz [20]. In this work, a model is required to calculate the hemicellulose solubilization independent of the liquid to solid mass ratio L/S of the experimental setup. With such a model, several reactors with different L/S can be scaled. The hemicellulose hydrolysis was modeled using first and second reaction orders using the hemicellulose mass fraction in the solid wHC and an Arrhenius temperature approach, see Eqs. (1)–(5). Here, k1 is the rate constant, n the reaction order, MHC the mass of hemicellulose in the solid, MdryBM the dry biomass, k1,0 the pre-exponential factor, EA the activation energy, R the universal gas constant, and T the temperature. The Arrhenius constants (pre-exponential factor and EA/R) were determined by the method of least squares using experimental values and those calculated with Eqs. (4) and (5).
The hemicellulose conversion XHC was calculated based on the determined hemicellulose mass fraction in the solid wHC and the degree of solubilization DS. The latter needs to be considered, since solid mass was partly liquefied altering the hemicellulose mass fraction. The degree of solubilization DS (eq. (6)) describes how much solid is liquefied during the autohydrolysis process. For the first- and second-order reaction models described above, all components but hemicellulose are treated as inert. Thus, the DS can be expressed based on the hemicellulose solid mass fraction wHC (compare Eqs. (7) and (8)). The conversion of hemicellulose describes the solubilized mass fraction of hemicellulose, see Eq. (9). For the experimental determination of XHC the experimentally determined DS and wHC are used. For the modeled solubilization, the hemicellulose conversion \( {X}_{HC}^{model} \) depends only on the calculated hemicellulose content (see Eq. (10)).
Evaluation of the model performance is determined using the root mean square error (RMSE).
Here, \( {Y}_{H,i,k}^{exp} \) are the experimental values, \( {Y}_{\mathrm{H},\mathrm{i},\mathrm{k}}^{\mathrm{mod}} \) are the values predicted by the model, and nexp is the number of data points.
For the comparison of different pretreatment severities, the severity factor R0 and its decadic logarithm log(R0) are used, see Eqs. (12) and (13). It combines the temperature T and the residence time t in a single variable. For consecutive pretreatments, the overall severity factor is the summation of the individual severity factors with m being the number of pretreatments (see Eq. (14)).
Based on the batch kinetics, a treatment strategy is developed and presented, which aims to achieve high hemicellulose yields, little hemicellulose loss reactions to furfural, and a high conversion of hemicellulose.
2.3 Bulk density measurement
For the calculation of reactor volume, the bulk density of the wheat straw pellets and of uncompressed wheat straw was determined. The pellets were swollen with cold water for 4 h and dried in a convective oven at 45 °C. To adjust the liquid to solid ratio approximately (precisely measured), 150 g of dried wheat straw was mixed with the according amount of cold tap water and stirred thoroughly. The mass of the probe that fits a beaker of known volume was determined in an adapted method according to DIN EN ISO 17828 [21]. The bulk density was modeled with a polynomial of second degree and as a function of the liquid to solid mass ratio L/S (eq. (15)). Here a, b, and c are constants, which are fitted with the method of least squares.
2.4 Reactor type and plant design evaluation
Different reactor types depicted in Fig. 1 were evaluated for the use in an industrial autohydrolysis pretreatment unit according to the design criteria developed in Section 3.3. Based on the reactor type evaluation and the reaction kinetics, several plant design concepts were created and are discussed in this work, according to the design criteria and their potential suitability for industrial processes.
2.5 Reactor scaling
The scale of 3000 t of annual lignocellulose per year was chosen and assumed to be adequate for the supply of lignin for a single customer. It represents a small industrial scale in a decentralized approach. The reaction parameters (temperature T and residence time t) were chosen according to the pretreatment strategy developed in this work (Sections 2.2 and 3.1). Taking the mass flow and the bulk density, the reaction volume Vr is calculated using a plug flow assumption eq. (16)). Here, Vr is the volume of the reaction mixture, \( {\dot{m}}_{slurry} \)is the mass flow of the straw and water mixture, and ρslurry its density.
The reactor volume VR is calculated using the reaction volume Vr and reactor volumetric fill level. The reactor diameter D is calculated from the reactor volume and the assumed length to diameter ratio L/D (Eq. (17)).
The minimum reactor wall thickness smin is determined using Barlow’s formula (Eq. (18)). This formula is universal and independent from specific construction methods. Here, p is the design pressure, which is 30 bar, and Rp0.2 is the 0.2% offset yield strength. This formula results in similar values than the description in the technical guideline AD2000.
To calculate the reduced solid dry mass at the outlet of a reactor, the mass flow of dry biomass MdryBM is corrected by using the DS and the severity factor compare Eqs. (19) and (20).
The mass of the reactor jacket mjacket is determined to enhance the understanding of the calculated reactor dimension, see Eq. (21). Here, ρsteel is the density of the construction steel.
Model parameter of the steel 1.4571 with 0.2% offset yield strength Rp0.2 equals 157 MPa at 250 °C according to DIN EN 10028-7 and a density of 8000 kg/m3. For the scaling of the screw conveyor reactor, a volumetric fill level of 40 vol% was assumed. The increase of the bulk density due to the pretreatment progress is neglected. The linear velocity vlin is calculated as the volumetric flow divided by the residence time.
3 Results and discussion
3.1 Kinetic modeling
The hemicellulose content is an important factor regarding the enzymatic digestibility of lignocellulose. Additionally, the hemicellulose fraction in the solid is regarded as a potentially valuable product. In order to predict the solubilization or conversion of the solid hemicellulose, a kinetic model describing the change of the hemicellulose solid composition wHC was set up, as described in Section 2.2. A fit of the Arrhenius constants to the experimental data for the first and second reaction orders was conducted. In Table 1 and Table 2 the Arrhenius constants, the RMSE, and the resulting rate constants are displayed. The initial hemicellulose solid fraction is wHC, 0 = 0.28. First and second order reaction model give a relatively good fit to the experimental data. At lower temperatures, first reaction order model gives slightly better results, and for higher temperatures, it is vice versa (not shown). The overall RMSE based on the fit of \( {k}_{1,0}^n \) and EA/R shows that, for the tested temperatures, the first-order reaction kinetics outperforms the second-order kinetics slightly. Therefore, the model for n = 1 will be used in Section 3.6.4 to determine the hemicellulose conversion XHC for the presented plant concepts. It is assumed that the calculation of the hemicellulose conversion with the batch model serves the purpose of this work to identify and evaluate promising reactor types and plant concepts suitable for an industrial process.
3.1.1 Severity factor analysis
The severity factor R0 (Eq. (12)) is used in the analysis of the reaction kinetics in the solid and liquid phases as well as in the description of chemical phenomena. The aim of this approach is to find a pretreatment strategy to fulfill the expected product and process requirements. The degree of solubilization of every experimental data point according to Eq. (6) is displayed in Fig. 2 as a function of log(R0). It can be seen that the different temperatures result in data that resemble a nearly linear dependency with a small plateau between 4.5 < log(R0) < 5.0. Also, the solid mass fraction of the main lignocellulose constituents results in continuous curves when plotted against log(R0) (Fig. 3). The courses of the curves are in good agreement with the theory of solubilization during autohydrolysis discussed in Chapter 1.1. The hemicellulose conversion XHC was calculated according to Eq. (9). In the linear part of the curve (3, 3 < log (R0) < 4, 46) a polynomial of first degree was fitted to calculate the hemicellulose conversion as a function of the severity factor log(R0):
Equation (22) was used to calculate the conversion in Section 3.5.
To identify the conditions at which hemicellulose solubilization occurs without the degradation to furfural, the hemicellulose and furfural concentrations of the hydrolysate are plotted against log(R0) (Fig. 4 (left)). It can be seen that with increasing severity, hemicellulose (monomers and oligomers) accumulates in the liquid phase. At log(R0) = 4.0, the presence of furfural is detected (detection limit of 100 mg/L); its concentration increases with the severity, whereas the hemicellulose concentration declines, since furfural is formed from hemicellulose, as discussed in Section 1.1. Thus, using the severity factor, the transition between solubilization-dominant (log(R0) < 4.0) and degradation-dominant (log(R0) > 4.0) conditions regarding hemicellulose can clearly be identified. Here, it has to be pointed out that more hemicellulose is lost than furfural is formed, supporting the assumption that both components react with each other to form condensation products. Once no hemicellulose is left, the furfural concentration starts to drop with the severity, most likely due to furfural resinification [13]. Similar considerations apply for glucose and its reaction product HMF, as can be seen in Fig. 4 (right). The glucose content (oligomers and monomers) in the hydrolysate stays constant until log(R0) = 4.5; at higher severity, HMF accumulates and a strong decline of the glucose content is detected.
After removing the hemicellulose from the biomass, the cellulose fraction is removed by enzymatic hydrolysis. It was shown that hydrothermal pretreatment improves the enzymatic digestibility due to increased surface area and low remaining content of the hemicellulose in the solids [12]. Thus, full hemicellulose solubilization and the avoidance of condensation reactions should be aimed by the process, since the condensation products may cover the particle surface and decrease the cellulose accessibility. At the same time, the formation of furfural indicates the loss of the product (xylose), the formation of a fermentation inhibitor (furfural), and possible condensation reactions [13]. Therefore, also the strategy of avoiding the formation of furfural will be considered during reactor evaluation.
3.2 Bulk density
The measured bulk density of untreated and pretreated wheat straw is displayed as a function of the liquid to solid ratio L/S in Fig. 5. The bulk density shows a strong increase with increasing L/S. The model constants for the bulk density of the untreated biomass are determined to be a = 13.044, b = 74.071, and c = 112.17. It can be noted that the pretreatment has only a small influence on the bulk density under the experimental conditions. The addition of water leads to almost no change in the bulk volume. This can be explained by the air in the bulk being replaced by water. To visualize this effect, the density is calculated using the dry biomass instead of the sample mass and displayed in Fig. 5 (diamonds). It can be seen that this dry matter-based density remains almost constant at 110 g/L. This behavior simplifies the reactor scaling, in regard to possible fluctuations in the dry matter content.
3.3 Design criteria
Based on the authors’ experience, a techno-economical assessment of a fixed-bed autohydrolysis scale-up study [16] and further publications, reactor and plant design criteria are developed and discussed below. Those will be used to evaluate different reactor types for the use in an autohydrolysis pretreatment unit.
3.3.1 Water consumption
The water consumption is of great importance, since heating of water requires a considerable amount of energy considering its high heat capacity. It is expressed as liquid to solid ratio L/S, which is often in the range of 1 to 10. Secondly, a large L/S leads to the dilution of the dissolved products. The required high concentration of the sugar stream will lead to the evaporation of the water, which in turn is regarded as energy intensive. Thirdly, the throughput of large amounts of water can lead to the need of treating large amounts of wastewater, which is to be avoided. The water consumption should be low.
3.3.2 Heat duty and integration
The usually large energy consumption of autohydrolysis processes is a major limitation towards its industrialization. In addition, the heat transfer should be fast and efficient for good process control and a reduction of the residence time. Additionally, the possibility to recover the process heat is beneficial. The heat transfer should be fast and there should be methods to integrate the heat.
3.3.3 Handling
This criterion targets the loading and cleaning processes of the reactor types. Time-consuming loading processes can lead to long downtimes of the process. To maintain a steady material flow, long downtimes can lead to a numbering up for a parallel production manner. Also, labor costs for the operation support this criterion. The handling of the reactor should be easy and fast.
3.3.4 Apparatus scalability and complexity
It is assumed to be beneficial to find an apparatus type that can be easily scaled to different sizes especially to industrial production scales. This criterion shall also be helpful to avoid the optimization of lab-scale apparatuses that will lead to a severe numbering up or are not economically scalable to an industrial size. With increasing diameter and pressure, the reactor wall thickness increases. Large reactor diameters and thick reactor walls can lead to large investment costs. The cost for high-pressure closing systems as a rule of thumb increases with its diameter and complexity. To reduce the investment cost the apparatus size should be small and complexity simple. Industrial scales should be possible with the selected reactor types and scalability should be high.
3.3.5 Batch versus continuous
Due to the high temperatures (170–230 °C) and the short residence times (5–30 min), the use of continuous apparatuses possesses outstanding advantages in terms of reactor size, downtime, energy consumption, and temperature stress to the reactor compared with batch processes. On the other hand, the design of a continuous high-pressure apparatus, with solid feeding and release equipment, is not trivial, especially when the residence time is high. In a batch process, the reaction mixture and the thick-walled pressure reactors have to be heated and cooled to the desired temperatures, which takes time and consumes thermal energy, which additionally is difficult to recycle. Also, the reactor must be loaded and unloaded, which costs time and effort. Nevertheless, in a batch reactor, the process severity can be easily adapted to account for changes in the substrate composition and to process other materials. If the process is realized in batch mode, the handling should be easy and fast and the heat consumption should be low and it should possess heat recycling possibilities. If the process is realized in continuous mode, the residence times should be short and the process parameters (L/S, T, t, substrate, particle size, etc.) adjustable.
3.4 Reactor-type evaluation
In this section, different reactor types will be assessed for the use in industrial autohydrolysis plant according to the design criteria in Section 3.3. Specific opportunities and challenges will be pointed out and discussed.
3.4.1 Batch reactor
A batch reactor shows a fast heat transfer, if direct steaming is used for heating and can operate at several L/S ratios. It is cheap to design and to scale and relatively simple to operate. The heat consumption is very large, the heat recovery is challenging, and the hemicellulose yield is limited to the degradation reactions.
3.4.2 Fixed-bed reactor
In the fixed-bed reactor, water is pumped through the biomass bed to solubilize products and remove them from the reaction zone. The inherent solid-liquid separation is the strength of the reactor, which results in a solid treatment time and a shorter liquid residence time. Thus, degradation reactions in the hydrolysate may be limited. The heat transfer based on saturated steam is fast and heats the bed homogeneously [22]. Cooling the outflowing hydrolysate to preheat the inflowing water offers a possibility to reduce the heat consumption significantly [22]. In addition, the formed steam due to the (slow) depressurization may be used to preheat the next load of biomass. Further issues are the convective heat transfer based on liquid water, the residence time distribution of the hydrolysate, the inhomogeneous solid pretreatment (temperature profile), and the usually high L/S. Additionally, the loading and unloading process might be challenging in a commercial process due to the poor flowability of the compressed biomass. The design and operation are well known and results in a good scalability but is prone to a numbering up for large industrial scales.
3.4.3 Solids mixer
A solids mixer is any type of vessel with agitation instruments to lift solid matter. It exists in a variety of shapes. Conical, vertical, and horizontal vessels are generally discussed. The use of agitation equipment improves the moisture and temperature homogeneity of the reaction mixture and simplifies the handling due to forced material flow. At the same time, the volumetric reactor loading is small to allow uncompressed material to be mixed. Thus, the resulting vessel is presumably large and scaling is limited. Low to medium L/S ratios can be realized as well as direct steam heating. Solids mixer can be used as continuous units in horizontal vessels using mixing paddles. Here, the back mixing will be presumably large, which leads to wide residence time distributions of both solid and liquid phases.
3.4.4 Horizontal batch reactor with rails
A horizontal direct steamed reactor with rails to load and unload the biomass on a wagon-like construction often is used for the impregnation or thermal modification of timber. The loading can be realized in a very time-efficient way and steam can be used for a fast heat transfer. A large length to diameter ratio allows thin walls. The mass transport and the volumetric reactor loading are poor. The latter is due to the uncompressed nature of the biomass and the poor volume usage of the reactor. Its design is complex regarding the wagon construction, the high-pressure reactor opening across the diameter, and the rail system outside the reactor. The material handling outside the reactor also is complex, since the biomass and water must be premixed and loaded onto the wagons. The necessity of unloading the material from the wagons adds to the plant complexity.
3.4.5 Extruder
The combination of thermal and mechanical treatment using an extruder is intensively studied for biorefinery processes in the past years [23]. The strength of this continuous process is the operation at very low L/S and it possesses superior heat and mass transfer properties. Due to the small diameters, high pressures and temperatures can be applied, which shortens the residence time. Therefore, a potentially high degree of controllability and an expected process intensification is expected. Additionally, the size reduction is beneficial for the subsequent enzymatic hydrolysis. Downsides are the large consumption of electrical energy and the small reaction volumes. Thus, the throughput and the residence times are coupled. Furthermore, residence times of 10 min and more are hardly realizable. Relevant throughputs can only be achieved with very fast reactions. The high equipment costs make an extruder cascade or numbering up economically challenging. For the production of bioethanol, the pretreatment in an extruder is promising, since a full fractionation of the sugars and the lignin is not required. Regarding multi-step processes, biomass washing and solid-liquid separation become important units, which is technically challenging with finely ground particles produced by an extruder. In unpublished experiments, it was shown that dry and wet extrusion of wheat straw can lead to a yield of 80% of particles smaller than 50 μm. On the one hand, this is challenging for washing and solid-liquid separation; on the other hand, it is promising for the enzymatic digestibility. Concluding a pretreatment plant aiming at a full fractionation may not be feasible by only using extruders, but it might be a suitable apparatus to combine with other units.
3.4.6 Screw conveyor reactor
A horizontal pressure reactor with an internal screw conveying mechanism is called a screw conveyor reactor (SCR). It possesses a top opening at one side and a bottom opening at the other side. This reactor type can be operated with several tubes stacked on top of each other and is equipped with a solids pressure feeder and a solids release system. For both, different machine types or solutions are available (see Section 3.6.5). The screw conveyor reactor is designed for industrial pulping of annual lignocellulose but is rather difficult to scale down. Operation at low but flexible L/S ratios is possible, heat transfer usually is realized with saturated steam. The steam pressure can also be used to control the reactor temperature. The moving action of the screw and the use of saturated steam allow for fast mass and heat transport. The screw elements move the material through the reactor and presumably reduce the residence time distribution significantly compared with a horizontal solids mixer. The material is processed in an uncompressed manner and takes approximately 40 vol% of the reactor volume. Thus, the reactor loading is limited. The setup inheres a certain complexity due to the connections of the different units. The length to diameter ratio is typically around 9, which allows for thin reactor walls. The number of stacked tubes for a reactor (compare Section 3.6) allows for a further reduction of the diameter. Also, the residence time and throughput are not directly coupled due to the possible stacking of the reactors. A disadvantage is the coupling of solids and liquid residence time. A countercurrent flow pattern can be realized by tilting the reactor and adding water at the top end. This was conducted in the autohydrolysis pretreatment of wheat straw [24]. In following publications of the development of this technology to a demonstration scale, the authors point out the high energy consumption due to the heating of large water streams and problems with the degradation of the dissolved hemicellulose. The authors of this work assume a wide residence time distribution of the hemicellulose in the liquid stream, which leads to the difficulties in controlling the degradation reactions. Nevertheless, the number of steps was reduced from three to two [25] and finally to one [26] due to economic and handling challenges. In the resulting single-step autohydrolysis treatment, a trade-off between heat duty, hemicellulose recovery, inhibitor formation, and improvement of enzymatic digestibility had to be found. A fiber-washing step is applied to separate the inhibitors prior to enzymatic hydrolysis.
3.4.7 Plug flow reactor
Finely ground lignocellulose can be conveyed into pressure reactors in the form of a slurry. Solid concentrations of 13.5 wt% can be realized [27], which equals a L/S = 7. This allows the use of a plug flow reactor (PFR), which is promising due to its very low complexity, low investment costs, and good scalability. The heat transfer can be realized with external heating/cooling fluids that allow an easy heat recovery and integration. Cooling the reaction medium provides heat to preheat the medium prior to the high-temperature zone. The residence time of solid and liquid phases are coupled. The particle size reduction is potentially energy intensive but also beneficial for the subsequent enzymatic hydrolysis. Low water consumption in this reactor type cannot be realized. In a full fractionation approach, the required solid/liquid separation with very fine particles is regarded as the main process limitation.
3.5 Reactor scaling
Based on the reactor type evaluation, we assume that the screw conveyor reactor (SCR) is the most promising reactor type regarding its flexibility and range of relevant processing parameters, as discussed in Section 3.4.6. Therefore, the SCR is dominantly used in the following conceptual design and scaling considerations are made for this reactor type in this section. Also, the extruder has promising features and therefore will be taken into account for the plant concept design.
For the dimensioning of a screw conveyor reactor (SCR) Eqs. (16)–(21) in Section 2.5 are used; the causal connection between the factors are displayed in Fig. 6.
Causal diagram of factors relevant for the dimensioning of screw conveyor reactors (SCR). Black letters: kinetics related factors; black background: reaction mixture related factors; white letters, bright background: reactor dimension and design-related factors. Solid line arrow: positive effect; dashed line arrow: negative effect; arrow thickness: effect strength
The effect strength and influence of one parameter on each other is evaluated for the case that all other factors remain constant. As discussed in Section 3.2, the effect of the water to solid ratio L/S on the bulk density is not accounted for. The colors are used to group the factor into kinetics (black letters), reaction mixture (black background), and reactor dimensions (white letters, bright background). It can be seen that the reactor volume VR is a very central factor, which is strongly influenced by temperature. Temperature has an indirect but very strong effect on many important factors, for instance through the water vapor pressure and reactor design pressure and through the residence time. For a desired conversion and recovery, the same severity factor can be realized with a higher temperature but a lower residence time, resulting in a smaller overall reactor volume and reactor mass. According to this argument, higher temperatures are generally preferred. On the other hand, higher temperatures result in larger vapor pressures, which affects the wall thickness as well as the feeding and release system and the sealing of the screw axis. Therefore, the temperature in a SCR reactor is expected to have an optimum regarding the investment costs. The effect of the process temperature both on the water vapor pressure and on the overall reactor jacket mass is shown in Fig. 7 (left). Here, a scale of 3000 t/a and the parameters described in Section 2.5 are used. Using a temperature of 215 °C compared with 185 °C reduces the jacket mass to 25%. Interestingly, the number of reactor tubes in a step has no influence on the overall reactor jacket mass (compare Fig. 6). The absence of a relation is beneficial in two ways: (1) Instead of using one reactor tube, several tubes can be stacked on top of each other. Thus, the size and wall thicknesses of the individual reactor decreases, which may make it easier to manufacture and install. (2) The linear velocity of the reaction mixture in the reactor contributes to the residence time distribution [28], thus is an important parameter. For a given mass feed rate and residence time, the velocity is determined by the reactor length and the number of tubes per stack. Thus, the linear velocity and therefore the residence time distribution can be influenced by the number of reactors without affecting the overall reactor size. Stacking several screw conveyor reactor tubes is done frequently in the industry [29].
(Left): Mass of reactor jackets per step versus the treatment temperature; The severity of the step is log(R0) = 4.00; Design pressure is vapor pressure times 1.5; Calculations are done for 1, 2, and 3 reactors per step; The curves perfectly overlap each other. (Right): Hemicellulose conversion versus the number of reactors in a cascade; each reactor step possesses a severity of log(R0) = 4.00; Error bars indicate XHC ± 0.06
Different from the number of tubes per step is the number of steps as in a cascade. Using more than one step is expected to increase the hemicellulose recovery. To calculate the hemicellulose conversion of a multi-step pretreatment, Eq. (22) and severity of log(R0) = 4.00 each step was used. A pretreatment severity of log(R0) = 4.00 was selected to solubilize the largest possible amount of hemicellulose without degrading it to furfural (compare Section 3.1.1). The result is displayed in Fig. 7 (right). With a number of steps of 1, 2, and 3, conversions of 64.0 wt%, 83.2 wt%, and 94.4 wt% are predicted, respectively. This model shows that not more than three steps are needed to solubilize and recover most of the hemicellulose, with no significant formation of furfural, if the sugars are completely separated between the steps (compare Section 3.6.3).
3.6 Autohydrolysis plant concepts
In this section, four autohydrolysis plant concepts for a 3000 t/a wheat straw scale are presented; in the last part, the reaction conditions and the autohydrolysis reactor dimensions are presented and discussed.
All concepts make use of continuously operated reactor types, namely the screw conveyor reactor (SCR) and an extruder. Fiber wash units are commonly used in the concepts to remove the dissolved sugar fraction from the solids, to recover it as a product, and to suppress the furfural formation in the next reactor step. In the concepts, washing is carried out at atmospheric pressure. Adequate extraction systems are to be chosen in dependence of the throughput, the extraction yield, the particle size, and the required hydrolysate concentration. Thus, it is left as a black box (see discussion in Section 3.6.5). Note, that a plug screw feeder resembles a mechanical dewatering device, which typically dries annual lignocellulose up to a dry matter content of 50 wt%. Assuming a dry matter content of 25% after the pretreatment and prior to pressing, 67% of the hydrolysate will be recovered already without washing. Additionally, all concepts feature a heat recovery scheme. The design concepts are to be interpreted as an early stage concept design based on extrapolating known principles and knowledge and therefore require further investigations. The first concept will be described rigorously; for the following concepts, only changes will be explained.
3.6.1 Concept 1—Cascade of SCR and extruder
This concept aims at a furfural free hydrolysate and a high yield and purity lignin. To achieve this, a mild pretreatment in an SCR is combined with a pretreatment/size reduction in an extruder (Fig. 8). Cut and water-soaked wheat straw is preheated making use of recycled steam in an atmospheric screw mixer. The biomass is forced into the pressure reactor (SCR) using a plug screw feeder. Saturated steam is used for a fast heating of the material and temperature control. Hot liquid water is used to control the water content, if required. For illustrative purposes, the reactor is shown with two reactor tubes. The pressure release is achieved with a discontinuous lock system. Here, the liquid water will evaporate to accelerate the material into a cyclone, where the forming steam is separated and recycled. The warm biomass is fed to the fiber wash unit, where the dissolved hemicellulose is extracted with water. The washed cellulignin is fed into an extruder to increase the accessible surface area for the following enzymatic hydrolysis. It has to be pointed out, that after the first SCR the overall solubilization reduces the dry solids mass flow for the second reactor significantly, which in turn reduces the required equipment size of the second step. The extruder can operate under atmospheric conditions (T < 100 °C) or a higher pressure and temperature to increase the hemicellulose conversion, by installing pressure feeder and release devices and electrical heating. If the fibers are not washed after extrusion, the solubilized hemicellulose would be transferred to the enzymatic hydrolysis.
3.6.2 Concept 2—SCR cascade
Next to a high lignin yield and purity, this concept focusses on a very high recovery of the hemicellulose fraction in the hydrolysate. Therefore, two steps, each in a SCR reactor, are applied with intermediate and final liquid separation by fiber washing, see Fig. 9, realizing a solid treatment time larger than the individual liquid treatments times. The conditions for the first step are chosen with regard to high hemicellulose recovery and to minimal furfural formation at the same time. The treatment conditions for the two steps can be chosen differently, for example, a mild temperature treatment followed by a high-temperature treatment (concept 2.1) or two high-temperature treatments after each other (concept 2.2) (compare the plant evaluation in Section 3.6.4).
Autohydrolysis plant concept 2: Two-step screw conveyor reactor with intermediate solid-liquid separation: Preheating and dosing screw, high-pressure feeder, autohydrolysis reactor one, discontinuous lock, aerocyclone, fiber washing unit, high-pressure feeder, autohydrolysis reactor two, discontinuous lock, aerocyclone, fiber washing unit, product tank
3.6.3 Concept 3—Cascade of three SCR and one extruder
This concept possesses three autohydrolysis units and a fine grinding unit, see Fig. 10. It is designed to show the maximum required complexity to achieve the highest hemicellulose conversion (three steps) and recoveries (washing units), and the highest lignin purity (extruder to improve the enzymatic digestibility). A discussion about the investment and process costs follows in the next sections.
3.6.4 Concepts evaluation
For the three concepts introduced above, scaling was performed according to Section 2.5; results are presented in Table 3. Here, log(R0,i) is the severity factor of each step, log(R0) is the overall severity factor of the plant, Vr is the overall reaction volume, and VR,i is the individual tube volume but not the step volume. Mjacket, step is the step reactor mass and Mjacket, total is the total reactor jacket mass adding up all tubes and steps for that concept.
The plant concept 1 (CSR and extruder) is designed to result in a very low complexity with the one-step SCR treatment and is similar to the Inbicon plant [26]. Here, an economical trade-off between the hemicellulose conversion, recovery, and degradation product formation must be found. To form a cascade introducing an extruder in the last step is a new approach. It aims at a high enzymatic digestibility but also could be used for the mechano-thermal pretreatment. The latter would require modifications to the extruder compared with the one used as atmospheric grinder.
For the reactor scaling, a temperature and residence time of 185 °C and 20 min, respectively, are chosen, which corresponds to a severity of log(R0) = 3.8. Using the reaction kinetics (compare Section 3.1), a hemicellulose conversion XHC of 40 wt%, no significant furfural formation, and a degree of solubilization DS of 26.2 wt% are expected. Applying the DS, the remaining dry solid flow after fiber washing is reduced from \( 400\ to\ 295\frac{kg}{h} \), which is fed to the extruder. Here, the extruder operates at atmospheric pressure. The SCR reactor volume was determined to be 1650 L that can be realized by stacking two reactors with a diameter, length, and wall thickness of 0.616 m, 5.54 m and 7.8 mm, respectively. This concept is designed to be robust and flexible in its processing parameters. With an increase of the reaction temperature to 200 °C, a higher severity factor can be reached and therefore increasing the hemicellulose conversion but also the furfural formation. The increase of the residence time also increases the severity but in turn reduces the throughput. The option to use the extruder as a second hydrothermal unit increases the robustness.
Concept 2 (CSR cascade) is calculated for two different sets of temperature and residence time but with the same severity factor. Concept 2.1 is a mild temperature treatment followed by a high temperature (185 °C and 200 °C); concept 2.2 are two consecutive harsh conditions (200 °C and 210 °C). Both concepts show the same severity for the first (log(R0,1) = 3.9) and the second (log(R0,2) = 4.25) step. In this way, both follow the developed pretreatment strategy (compare Section 3.1.1) and allow to investigate the influence of the process parameters on the reactor dimensions. The difference is very pronounced regarding the total jacket mass. The 15 °C more in step one of concept 2.2 results in a step jacket mass drop to 33% (compared with concept 2.1). In step two, an increase in temperature of 10 °C results in a step jacket mass drop to 50%, even though the volume flow is similar.
The robustness of concept 2.1 is expected to be higher than concept 2.2, since the temperatures could be increased to adapt to the severity factor. On the other hand, the reactor sizes of concept 2.2 are more favorable than those in concept 2.1. Anyway, in both concepts, a hemicellulose conversion of XHC = 90 wt% is reached with no expected furfural formation in step one and very low furfural formation in the second step. The enzymatic digestibility is expected to be high.
In concept three (three CSR and extruder), the three steps possess a severity of log(R0,i) = 3.99 to achieve maximum hemicellulose recovery without furfural formation. The final hemicellulose conversion is predicted to be XHC = 94 wt%. Additionally, using the extruder, a maximal enzymatic digestibility is expected. The temperature for each step is chosen to be 215 °C, which results in a residence time of 4 min for each step and therefore very small reactor sizes. The resulting total jacket mass is by far the smallest of all presented concepts, with 481 kg, followed by concept 2.2 (990 kg), concept 1 (1018 kg), and concept 2.1 (2248 kg).
For the investigation of the effect of the residence time on the reactor dimensions, the first step of concepts 1, 2.1, and 2.2 may be regarded, where the residence times are 10, 20, and 30 min, respectively. A larger residence time leads to a larger reaction volume that is treated per unit of time. Instead of increasing the reactor size, and therefore its minimum wall thickness, a staging approach was followed. The number of tubes per stack NR was increased with the residence time resulting in the same tube dimensions (D, L smin, vL) for all tubes. This displays that increasing NR is a powerful measure to limit the reactor dimensions. This measure is relevant for large throughputs and/or long residence times.
The discussed concepts show that the reaction temperature has a much stronger influence on the reactor size than the number of steps. Also, the number of tubes per stack has no influence on the step jacket mass in this framework (compare Section 3.5). The reactor or tube dimensions for all concepts are smaller than examples realized in the industry [29].
Regarding the autohydrolysis unit, the step number should be as small as possible with respect to the investment and process costs. With an increasing step number, the yields and purities of the product stream are expected to increase. Concluding, the selection of the most suitable plant concept depends on the particular stream applications, evaluating whether the increased qualities justify the increase of the step numbers and an overall economical evaluation.
For the design of an efficient autohydrolysis plant, further experimental and theoretical investigations must be made. The latter includes (periphery) equipment costs and overall energy demand.
For the investment costs, not only the size of the reactors is important but also the number of units. With an increasing step number, more high-pressure feeders, locks, cyclones, and fiber washing units must be installed. Also, piping becomes more complex and the space demand increases.
The processing costs highly depend on the energy consumption. The fiber wash units require warm water and therefore thermal energy. Also, the step number has a significant effect on the energy costs, since the overall liquid to solid ratio L/S increases with the step number.
3.6.5 Assumption for the reactor concepts
The plant concepts are based on kinetic data of laboratory batch reactors, thus the reactor and process type (batch vs. continuous), reactor size, heat transfer system, water to solid ratio are different. Therefore, it is obvious that experimental validation is necessary. Nevertheless, the assumptions and plausibility considerations are discussed in the following.
Heitz et al. [30] reported the furfural production of steam pretreated lignocellulose as a function of log(R0), which shows an increase after a severity of log(R0) = 4.0 is reached. This observation is in good agreement with the results reported in this work. The kinetics are investigated using particles with an average diameter of less than 1 mm, whereas in the concepts larger pieces are used. The particle size will affect the extraction behavior inside and outside the reactor. A clear trend on the particle size on the reaction velocity during hydrothermal processing was not reported, but rather on the enzymatic hydrolysis of the pretreated material [31]. The operation at higher solid concentrations will lead to a decrease in the pH value, hence the reactions will be accelerated [32]. The used model does not take this effect into account and therefore underestimates the reaction velocities. The biomass density depends on the particle size, the pretreatment conditions, and the actions of the high-pressure feeder [28]. Therefore, using a relatively low density of approximately 100 g/L, which does not change throughout the process, is assumed as a conservative choice. To control the reaction progress the residence time distribution is an important parameter. Sievers and Stickel [28] modeled the residence time distribution of an SCR, which can be used for prediction and reactor design optimization. Also, the control of the residence time itself is important. With increasing temperature, the residence times becomes shorter. In turn, the severity becomes more sensitive to the residence time. Reaction temperatures of SCRs are usually in the range 170 °C- 205 °C [25, 28]. With increasing vapor pressure, the sealing of the rotating screw shaft is becoming an increasing challenge. To tackle this challenge, a SCR can be equipped with an magnetic coupled drive for the screw [33]. Material feeding and release systems resemble an important part of the reactor setup considering the pressure sealing, its effect on the moisture content, the material structure, and size. Hot blown material (as in the presented concepts) may lead to problems in refeeding. Anyway, several feeding [34, 35] and release [36, 37] methods are investigated or developed regarding the processed material, material disruption, and heat recycling efficiency, which allows to adapt the presented concepts accordingly, for example by choosing cold blow pressure release system. Regarding the fiber washing, Söderström et al. [11] showed that a washing step in between two steam pretreatments of lignocellulose improved the recovery of hemicellulose components significantly, while leaving the glucose yield in a downstream enzymatic hydrolysis unaffected. Hongzhang et al. [38] investigated the fiber washing with water for steam pretreated wheat straw and simulated experimentally a countercurrent washing process. Here the hemicellulose extraction was investigated with varying solid concentrations, water temperature, and countercurrent washing steps, with promising results. Also, fiber and pulp washing is frequently applied in paper industry and therefore being well developed with a number of equipment producers.
4 Conclusions
Various autohydrolysis reactors and plant concepts were designed and evaluated regarding their potential to overcome current scale-up limitations. A two-step autohydrolysis pretreatment process is evaluated to be very promising for the full fractionation of the agricultural residues on industrial scale. This is mainly due to the chemical kinetics showing maximum hemicellulose concentration in the liquid phase at a severity of log(R0) = 4.0, and accumulating degradation product furfural at this severity and higher, while the necessary solubilization of hemicellulose is only reached at higher severities (XHC = 90 wt % , at log(R0) > 4.4). In three continuous plant concept designs the screw conveyor reactor was used, with intermediate sugar extraction. The reactor scaling for a 3000 t/a scale based on the quantified kinetics showed feasible reactor sizes. It was identified that the process temperature is the most important parameter affecting the reactor size, therefore should be selected relatively high (approximately 180–205 °C).
Abbreviations
- DS:
-
degree of solubilization
- HMF:
-
hydroxymethylfurfural
- PFR:
-
plug flow reactor
- RMSE:
-
root mean square error
- SCR:
-
screw conveyor reactor
- TUHH::
-
Hamburg University of Technology
- a,b,c :
-
constants (−)
- D :
-
reactor diameter (m)
- DS :
-
degree of solubilization (g/g)
- EA :
-
activation energy (J/kg)
- k1 :
-
rate constant (1/s; g/gs)
- k1,0 :
-
pre-exponential factor (1/s; g/gs)
- L :
-
reactor length (m)
- L/D :
-
length to diameter ratio (m/m)
- L/S :
-
liquid to solid ratio (kg/kg)
- Mdry,BM :
-
dry biomass (g)
- M dry,in :
-
mass flow of dry biomass into the reactor (kg/h)
- M dry,out :
-
mass flow of dry biomass out of the reactor (kg/h)
- M HC :
-
hemicellulose mass (g)
- M HC,0 :
-
initial hemicellulose mass (g)
- M S :
-
solid mass (g)
- \( {\dot{M}}_{slurry} \) :
-
slurry mass flow (kg/h)
- M S,0 :
-
initial solid mass (g)
- M jacket,step :
-
step jacket mass (kg)
- M jacket,total :
-
total jacket mass (kg)
- n :
-
reaction order (−)
- N R :
-
number of reactor tubes per stack (−)
- n exp :
-
number of experimental data points (−)
- p :
-
pressure (bar)
- R p0.2 0.2%:
-
offset yield strength
- R :
-
universal gas constant (J/kg K)
- R 0 :
-
severity factor overall
- R 0,i :
-
severity factor of step i
- s min :
-
minimal reactor wall thickness (mm)
- t :
-
residence time (min)
- T :
-
temperature (°C)
- v L :
-
linear velocity (mm/s)
- V r :
-
reaction volume (m3)
- V R,i :
-
reactor volume (m3)
- V R,i :
-
tube volume (m3)
- W HC :
-
hemicellulose solid mass fraction (g/g)
- X HC :
-
hemicellulose conversion (g/g)
- Y exp :
-
value from experiment
- Y mod :
-
value from model
- ρ slurry :
-
slurry density (kg/L)
- ρ bulk :
-
bulk density (kg/L)
- ρ steel :
-
steel density (kg/L)
References
Kamm B, Gruber PR, Kamm M (2010) Biorefineries - industrial processes and products: status quo and future directions. Wiley-VCH, Weinheim
Kruse A, Dinjus E (2007) Hot compressed water as reaction medium and reactant: properties and synthesis reactions. J Supercrit Fluids 39:362–380. https://doi.org/10.1016/j.supflu.2006.03.016
Garrote G, Domı́nguez H, Parajó JC (2002) Interpretation of deacetylation and hemicellulose hydrolysis during hydrothermal treatments on the basis of the severity factor. Process Biochem 37:1067–1073. https://doi.org/10.1016/S0032-9592(01)00315-6
Cocero MJ, Cabeza Á, Abad N, Adamovic T, Vaquerizo L, Martínez CM, Pazo-Cepeda MV (2018) Understanding biomass fractionation in subcritical & supercritical water. J Supercrit Fluids 133:550–565. https://doi.org/10.1016/j.supflu.2017.08.012
Zeitsch KJ (2000) 2. The reactions leading to furfural. In: Sugar Series. Elsevier, pp 3–7
Ingram T, Wörmeyer K, Lima JCI, Bockemühl V, Antranikian G, Brunner G, Smirnova I (2011) Comparison of different pretreatment methods for lignocellulosic materials. Part I: Conversion of rye straw to valuable products. Bioresour Technol 102:5221–5228. https://doi.org/10.1016/j.biortech.2011.02.005
Hansen MAT, Kristensen JB, Felby C, Jørgensen H (2011) Pretreatment and enzymatic hydrolysis of wheat straw (Triticum aestivum L.) – the impact of lignin relocation and plant tissues on enzymatic accessibility. Bioresour Technol 102:2804–2811. https://doi.org/10.1016/j.biortech.2010.10.030
Kristensen JB, Thygesen LG, Felby C, Jorgensen H, Elder T (2008) Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol Biofuels 1:5. https://doi.org/10.1186/1754-6834-1-5
Reynolds W, Singer H, Schug S, Smirnova I (2015) Hydrothermal flow-through treatment of wheat-straw: detailed characterization of fixed-bed properties and axial dispersion. Chem Eng J 281:696–703. https://doi.org/10.1016/j.cej.2015.06.117
Reynolds W, Kirsch C, Smirnova I (2015) Thermal-enzymatic hydrolysis of wheat straw in a single high pressure fixed bed. Chem-Ing-Tech 87:1305–1312. https://doi.org/10.1002/cite.201400192
Söderström J, Galbe M, Zacchi G (2004) Effect of washing on yield in one- and two-step steam pretreatment of softwood for production of ethanol. Biotechnol Prog 20:744–749. https://doi.org/10.1021/bp034353o
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1:119–134. https://doi.org/10.1002/bbb.4
Zeitsch KJ (2000) Furfural loss reactions. In: Sugar Series, vol 6. Elsevier, pp 19–22
Sumerskii IV, Krutov SM, Zarubin MY (2010) Humin-like substances formed under the conditions of industrial hydrolysis of wood. Russ J Appl Chem 83:320–327. https://doi.org/10.1134/S1070427210020266
Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quim Nova 26:863–871. https://doi.org/10.1590/S0100-40422003000600015
Schmidt LM, Pérez Martínez V, Kaltschmitt M (2018) Solvent-free lignin recovered by thermal-enzymatic treatment using fixed-bed reactor technology – economic assessment. Bioresource Techno 268:382–392. https://doi.org/10.1016/j.biortech.2018.07.107
Forstner J, Unkelbach G, Pindel E, Schweppe R (2012) Heterogen katalysierte Herstellung von Furfural aus Xylose. Chem-Ing-Tech 84:503–508. https://doi.org/10.1002/cite.201100178
Gairola K, Smirnova I (2012) Hydrothermal pentose to furfural conversion and simultaneous extraction with SC-CO2 – kinetics and application to biomass hydrolysates. Bioresour Technol 123:592–598. https://doi.org/10.1016/j.biortech.2012.07.031
Reynolds W, Smirnova I (2017) Hydrothermal flow-through treatment of wheat straw: coupled heat and mass transfer modeling with changing bed properties. J Supercrit Fluids 133:625–639. https://doi.org/10.1016/j.supflu.2017.08.001
Ruiz HA, Hedegaard Thomsen M, Trajano HL (2017) Hydrothermal processing in biorefineries. Springer International Publishing, Cham
DIN EN ISO 17828 (2015) Biogene Festbrennstoffe – Bestimmung der Schüttdichte (ISO 17828:2015). Deutsche Fassung EN ISO 17828
Kilpeläinen PO, Hautala SS, Byman OO, Tanner LJ, Korpinen RI, Lillandt MKJ, Pranovich AV, Kitunen VH, Willför SM, Ilvesniemi HS (2014) Pressurized hot water flow-through extraction system scale up from the laboratory to the pilot scale. Green Chem 16:3186–3194. https://doi.org/10.1039/C4GC00274A
Zheng J, Rehmann L (2014) Extrusion pretreatment of lignocellulosic biomass: a review. Int J Mol Sci 15:18967–18984. https://doi.org/10.3390/ijms151018967
Thomsen MH, Thygesen A, Jørgensen H et al (2006) Preliminary results on optimization of pilot scale pretreatment of wheat straw used in coproduction of bioethanol and electricity. Appl Biochem Biotechnol 129:13
Petersen MØ, Larsen J, Thomsen MH (2009) Optimization of hydrothermal pretreatment of wheat straw for production of bioethanol at low water consumption without addition of chemicals. Biomass Bioenergy 33:834–840. https://doi.org/10.1016/j.biombioe.2009.01.004
Larsen J, Haven MØ, Thirup L (2012) Inbicon makes lignocellulosic ethanol a commercial reality. Biomass Bioenergy 46:36–45. https://doi.org/10.1016/j.biombioe.2012.03.033
Makishima S, Mizuno M, Sato N, Shinji K, Suzuki M, Nozaki K, Takahashi F, Kanda T, Amano Y (2009) Development of continuous flow type hydrothermal reactor for hemicellulose fraction recovery from corncob. Bioresour Technol 100:2842–2848. https://doi.org/10.1016/j.biortech.2008.12.023
Sievers DA, Stickel JJ (2018) Modeling residence-time distribution in horizontal screw hydrolysis reactors. Chem Eng Sci 175:396–404. https://doi.org/10.1016/j.ces.2017.10.012
Zeitsch KJ (2000) Furfural processes. In: The chemistry and technology of furfural and its many by-products. Elsevier, pp 10, 36–74
Heitz M, Capek-Ménard E, Koeberle PG, Gagné J, Chornet E, Overend RP, Taylor JD, Yu E (1991) Fractionation of Populus tremuloides at the pilot plant scale: optimization of steam pretreatment conditions using the STAKE II technology. Bioresour Technol 35:23–32. https://doi.org/10.1016/0960-8524(91)90078-X
Zetzl C, Gairola K, Kirsch C, Perez-Cantu L, Smirnova I (2011) High pressure processes in biorefineries. Chem-Ing-Tech 83:1016–1025. https://doi.org/10.1002/cite.201100025
Laser M, Schulman D, Allen SG, Lichwa J, Antal MJ Jr, Lynd LR (2002) A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour Technol 81:33–44. https://doi.org/10.1016/S0960-8524(01)00103-1
조현수 (2010) Method of preparing aerogel using continuous countercurrent supercritical process and continuous countercurrent supercritical equipment. KR Patent 20100086297
Craven JM (2014) Energy efficient solids feed system for high pressure processes. Phd, University of Sheffield
Schell D (1988) High pressure solids feeding using a lockhopper system: design and operating experience. Appl Biochem Biotechnol 17:73–87. https://doi.org/10.1007/BF02779147
Carr WF (1967) Two-stage continuous digestion with removal of liquor in first stage and recirculation of liquor in second stage. US Patent 3,313,677
Ryham R (1990) Cold blow system for batch production of pulp. US Patent 4,975,148
Hongzhang C, Liying L (2007) Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresour Technol 98:666–676. https://doi.org/10.1016/j.biortech.2006.02.029
Acknowledgments
The authors are grateful to the German “Bundesministerium für Bildung und Forschung” (BMBF) for the financial support in the context of the German project cluster BIOREFINERY2021 (031A233).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Conrad, M., Häring, H. & Smirnova, I. Design of an industrial autohydrolysis pretreatment plant for annual lignocellulose. Biomass Conv. Bioref. 11, 2293–2310 (2021). https://doi.org/10.1007/s13399-019-00479-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00479-1