Abstract
In order to enhance the adsorption capacity of activated carbon (AC) from cocoa pod husk (CPH) and reuse the solution after the acid-leaching of CPH as a liquid fertilizer, CPH was first leached by acid and then used as a precursor (CPH-A) for preparing ACs by physical activation at a temperature range of 650–850 °C in this work. Based on the proximate analysis, mineral compositions, thermogravimetric analysis, and thermochemical properties, the differences between CPH and CPH-A were investigated. The chemical and pore properties of the resulting ACs were further studied. The results show that the pretreatment of CPH with hydrochloric acid led to removal of over 90% of the ash content in the CPH, mainly composed of potassium minerals. The Brunauer-Emmet-Teller (BET) surface area of the AC derived from CPH-A at 650 °C is 355.8 m2/g, significantly larger than that (i.e., 1.1 m2/g) of the AC derived from CPH. The higher activation temperature (e.g., 900 °C) is beneficial to the pore development of the resulting AC (e.g., BET surface area ˃ 1300 m2/g). In addition, the carbon (C) and sulfur (S) contents of the resulting ACs indicate an increasing trend as the temperature increased from 650 to 850 °C, but a decreasing trend in the hydrogen (H), nitrogen (N), and oxygen (O) contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carbon materials have been widely used in a variety of industrial and environmental applications such as energy materials in supercapacitors and adsorption materials for purification/remediation [1]. In this regard, activated carbon (AC), used as adsorbents, antidotes, and catalysts (or catalyst supports), may be one of the most important carbon materials for removal of contaminants and for purification of valuable chemicals [2]. Practically, the carbonaceous precursors for producing ACs are mainly based on coals, coconut shell, and wood because they have high carbon contents, large amounts (i.e., low-cost raw materials), or low ash compositions (inorganics) [3]. However, commercial AC products are relatively expensive, suggesting that it is necessary to find new low-cost starting materials for producing them. Therefore, many researchers have recently adopted the reuse of agricultural by-products (biowaste materials, or agricultural residues) as precursors for AC production [4,5,6]. Due to their amounts in abundance, many efforts on upgrading their available reuses have attracted much attention. On the other hand, some of them discarded directly into soils as organic fertilizer could cause environmental problems, including greenhouse gas (i.e., CO2 and CH4) emissions, unpleasant odor, and inoculum sources for plant diseases (e.g., black pod rod) because of their lignocellulosic components and biodecomposition features [7].
Cocoa is an important fruit in the tropical countries because its seeds (beans) are commonly processed to make chocolate products and other foodstuffs. However, the cocoa industry will generate large amounts of agricultural by-products during the cocoa bean-producing process. Cocoa pod husk (CPH) is the most significant one. It represents over 50% of the whole weight of the cocoa fruit [8, 9]. More significantly, the mass ratio of CPH to cocoa beans may be up to 10 [10], accounting for over 10 million tons of CPH being generated every year [11]. Although CPH was often discarded as an organic fertilizer on the farm, it has some alternative applications [12], which include animal feed, food antioxidants, biomass fuel for boilers, potash (from CPH) for soft soap manufacturing, filling material for bioplastics and biocomposite, biosorbent for removal of pollutants in water, precursor for AC production, dietary fibers. Based on its lignocellulosic content and plentiful amount, CPH seems to be one of excellent precursors for AC production, suggesting that there is the potential for its value-added utilization.
The methods for manufacturing AC include physical activation and chemical activation. Although the latter process can be carried out in a single step, thus providing a lower activation temperature (energy consumption), a higher carbon yield, and larger pore properties [13], the former is a cleaner process commonly used in the industrial production because it does not involve the release of chemical activating agents such as zinc chloride (ZnCl2) [2]. Regarding the researches on the reuse of CPH as a low-cost precursor for producing AC by physical activation, only a few investigators have conducted in recent years. The CPH-based AC was produced at carbonization of 800 °C followed by CO2 activation at 850 °C, showing that the largest surface area of the resulting AC was about 560 m2/g [14,15,16]. The authors further treated the CPH-based AC with hydrochloric acid (HCl), resulting in higher surface area because of ash leaching and new pore development. The biomass acid pretreatment studies also reported similar findings [17, 18]. Furthermore, other authors investigated the CPH-based AC production using carbonization at 500 °C and subsequent CO2 activation at 700 °C for 2 h [19]. Their results indicate that the resulting AC only offers the surface area of 503 m2/g.
The ash content in the precursor also plays a vital role in the pore properties. In some circumstances, these minerals in the precursor or its resulting AC will hinder pore development during activation, or act as a catalyst during steam regeneration [20]. Due to the significant potassium content involved in CPH, the acid-leaching solution may be reused as a liquid fertilizer. In this work, CPH was initially treated with inorganic acid (HCl) to permit the reduction of ash content because of leaching by acid dissolution. In order to compare the pore properties of ACs from original CPH and leached CPH, a series of physical (CO2) activation experiments were performed at different temperatures (i.e., 650, 750, and 850 °C) held for 30 min. Regarding their variations on the chemical and pore properties, the resulting ACs were further characterized by the N2 adsorption-desorption isotherms and elemental analyses.
2 Materials and methods
2.1 Materials
The CPH samples were collected from a local cocoa farm in Neipu Township (Pingtung County, Taiwan). To avoid the material deterioration and fermentation odor generated, the wet samples were put into an oven set at about 105 °C for several days (denoted as-received CPH). Subsequently, this dried CPH was crushed and then screened to get the fraction of − 20/+ 40 mesh (particle size 0.63 mm on average) as the feedstock samples, which were used in the thermochemical characterization measurements and physical activation experiments.
2.2 Acid pretreatment
Acid pretreatment (i.e., acid-leaching) was performed to remove minerals (i.e., ash) from the CPH sample by mixing 100 ml 3 M HCl and 10 g feedstock in a 250-ml Erlenmeyer flask. The mixture solution was stirred on a hot plate (Corning Co., USA; Model: PC-420D) at about 80 °C (353 K) for 30 min, and then cooled to room temperature for decanting (solid-liquid separation). Finally, the solid fraction was washed with hot (about 90 °C) deionized water (150 ml each batch) for three times using a vacuum filter. The de-ashed CPH sample (labeled as “CPH-A”) was then dried in a heated air circulation oven at about 105 °C (378 K) overnight. The washing also plays a role on the increase of the pore properties of the AC from CPH-A because of the removal of water-soluble components in the AC precursor [17].
2.3 Thermochemical characterization analyses of CPH and CPH-A
2.3.1 Proximate analysis
According to the Standard Test Method set by the American Society for Testing and Materials (ASTM), the proximate analysis was used to determine the contents (wt%, dry basis) of volatile matter, ash, and fixed carbon. The fixed carbon is determined by subtracting the weight percentages of volatile matter and ash from a sample. Volatile matter was determined by the difference between the CPH mass and its residual mass after heating at 950 °C for 7 min under inert atmosphere. The ash contents of the precursors (i.e., CPH and CPH-A) play a vital role in the production and characterization of AC. The samples were burnt in the muffle furnace at about 750 °C for a minimum of 4 h. Ash composition was further analyzed with an inductively coupled plasma-optical emission spectrometer (ICP-OES).
2.3.2 Ultimate (elemental) analysis
In general, the ultimate analysis refers to the contents of organic elements except for its moisture and inorganic constituents (ash) [21]. An elemental analyzer (vario EL III; Elementar Co., Germany) was used to determine its elemental concentrations, including carbon (C), hydrogen (H), nitrogen (N), sulfur (S), and oxygen (O). In this analysis, each sample (1–3 mg) was analyzed in duplicate, showing that the repeated measurement error is within 1% of the mean value.
2.3.3 Thermogravimetric analysis
In order to evaluate the pyrolysis conditions for producing char under the inert atmosphere, the thermogravimetric analysis (TGA) curves for the dried CPH and CPH-A samples were obtained using a thermal analyzer (TGA-51; Shimadzu Co., Japan). The sample of known weight (about 0.2 g) was placed in a quartz crucible and then heated from room temperature to 1000 °C at a constant rate of 10 °C/min under a N2 flow environment (50 cm3/min). Furthermore, the derivative thermogravimetric (DTG) analysis curves can be obtained by converting mass loss data into mass loss rate as a function of temperature.
2.3.4 Higher heating value analysis
It is well known that the ash content of biomass lowers its calorific value or higher heating value (HHV). Herein, the HHV is a measured value of the specific energy of combustion for unit mass of a biomass (or fuel) burned in oxygen in an adiabatic bomb. In the work, the HHVs of dried CPH and CPH-A samples (about 0.5 g) were obtained by a calorimeter (Calorimeter ASSY 6200; Parr Instrument Co., USA).
2.3.5 Trace element analysis
The inorganic elements in the ash are alkali metals, alkaline earth metals, transition metals, and other elements, depending on its starting material. An inductively coupled plasma-optical emission spectrometer (ICP-OES) (Agilent 725; Agilent Co., USA) was used for the determination of 20 relevant elements. They include arsenic (As), aluminum (Al), barium (Ba), calcium (Ca), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), nickel (Ni), phosphorus (P), lead (Pb), silicon (Si), strontium (Sr), titanium (Ti), and zinc (Zn). Prior to the ICP-OES analysis, the concentrated HNO3/HCl/HF solution was employed for the dissolution of the dried CPH and CPH-A samples (about 0.05 g) in a microwave digester at two stages (first 170 °C for 20 min, then 190 °C for 20 min).
2.4 Physical activation experiments
In order to produce AC with a cleaner process, the method of physical activation was employed using gasifying gas carbon dioxide (CO2) in this work. However, this process involves the carbonization of the starting precursor at temperature below 600 °C, followed by the gasification at higher temperature (above 700 °C). In this study, a series of carbonization-activation experiments was carried out to produce the CPH-based ACs from the CPH and CPH-A samples in a vertical fixed-bed furnace [22, 23]. For each experiment, about 5 g of the dried CPH sample was poured into a mesh-made holder, and then placed at the center of the reaction system. The precursor was subjected to the carbonization at a ramp of 10 °C/min under N2 flow (500 cm3/min) by heating to 500 °C. Subsequently, the activation of the resulting carbon was carried out by switching to a CO2 flow (50 cm3/min), heating to specified temperature (i.e., 650, 750, or 850 °C) and then maintaining for 30 min. The yields of the resulting ACs are within the range 18–38 wt%, indicating a decreasing trend with activation temperature increased. According to the CPH precursor and activation temperature, the CPH-based AC products in this work were coded as AC-CPH-650, AC-CPH-650, AC-CPH-A-650, AC-CPH-A-750, and AC-CPH-A-850, respectively.
2.5 Characterization of CPH-based activated carbons
Pore properties of CPH-based ACs were measured by the surface area and porosity analyzer (ASAP 2020; Micromeritics Co., USA). The nitrogen molecules adsorbed by monolayer coverage must be determined in order to calculate the specific surface area (SSA). In this regard, the method for SSA (denoted as SBET) is based on the Brunauer-Emmett-Teller (BET) theory. The BET equation was applied to the relative pressure (P/P0) range of 0.05–0.25 in this work [24]. The total pore volume (Vt) was determined by the N2 adsorption isotherm at P/P0 close to unity (i.e., 0.95). It was suggested that all the pores are of straight and cylindrical geometry. The average pore diameter can be roughly calculated from Vt and SBET [25, 26]. Regarding the microporous properties, the t-plot method was employed to permit the determination of micropore volume and surface area [26]. The pore size distribution was obtained from the desorption branch of the N2 adsorption-desorption isotherm by the modified Kelvin equation, called as the Barrett-Joyner-Halenda (BJH) method [26].
As described above, the ultimate analysis of the CPH-based AC is expressed in terms of its weight percentages (on a dry basis) of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sulfur (S) by an elemental analyzer (vario EL III; Elementar Co., Germany).
3 Results and discussion
3.1 Thermochemical characterization of CPH and CPH-A
Table 1 indicates the data in terms of the proximate analysis, ultimate analysis, and calorific value for the precursors (i.e., CPH and CPH-A) used in the AC production. The value (i.e., 9.8 wt%) of ash content in the CPH agrees very well with those found in the literature [28]. Clearly, the acid-leaching of CPH with 3 M HCl results in removal of over 90% of the ash content. It is well known that the ash constituents in the biomass consist mainly of silica, alumina, iron, alkaline, and alkaline earth metals [29]. Consequently, ash was easily leached out by acid solution. On the other hand, it indicates the increased fixed carbon in the CPH-A, representing more carbon fractions left after the drive-out of volatiles during the acid treatment. This result is consistent with the data on ultimate analysis and calorific value (Table 1). For example, the carbon content of CPH-A (50.23 wt%) is obviously larger than that of CPH (43.25 wt%).
In order to verify the difference of inorganic compositions between CPH and CPH-A, Table 2 lists the total contents of main inorganic elements and trace metals. It shows that inorganic element in the CPH is mainly K, which could be the presence of oxides, carbonates, bicarbonates, or hydroxides [30]. Minor inorganic elements are Mg, P, Ca, and Mn. It is noteworthy that trace amounts of heavy metals and other elements (Table 2), including Al, As, Ba, Cd, Co, Cr, Cu, Fe, Na, Ni, Pb, Si, Sr, Ti, and Zn, were found in CPH and CPH-A. Similar data have been found in the previous study [27] and other researches [31, 32]. These findings suggest that the emissions of toxic metals from the combustion of cocoa processing by-product will be negligible.
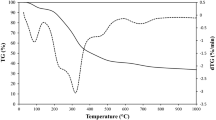
The thermogravimetric and its derivative thermogravimetric (TG/DTG) curves of the dried CPH (blue lines) and CPH-A (red lines) precursors at a heating rate of 10 °C/min can be seen in Fig. 1. Obviously, it revealed the slight weight loss at temperatures less than 200 °C, which is probably due to the releases of adsorbed water and poorly bonded materials. As shown by sharp DTG peaks, a significant weight loss subsequently occurs at 200–400 °C, which is corresponding to the release of the volatile matter from the thermal decomposition of the hemicellulose and cellulose contained in the biomass precursor. These results suggest that the carbonization condition should be set at the temperature higher than 400 °C to prepare a carbon-rich product used in the next activation process. On the other hand, the TGA/DTA curves in the CPH-A sample are shifted to higher temperatures by about 30 °C, indicating that it has a higher thermal stability than the CPH sample between 200 and 400 °C. This result could be attributed to the acid-leaching treatment in the CPH-A sample, leading to the crystallinity of cellulosic biomass increased [17].
3.2 Pore properties of CPH-based activated carbons
Table 3 summarizes the pore properties of the CPH-based ACs obtained from the CPH and CPH-A at different activation temperatures. Obviously, their pore properties indicate an increasing trend with activation temperature. For example, the BET surface area significantly increased from 1.1 m2/g (AC-CPH-650) to 289.5 m2/g (AC-CPH-750). The activation at higher temperature would enhance the diffusion and reaction of gasification gas (CO2) in the physical activation and thus increase the pore properties of the resulting AC. On the other hand, these data in Table 3 further show that the acid pretreatment has resulted in more developed pores, thus increasing the specific surface area and pore volume of the resulting AC. It is seen by the fact that the BET surface area significantly increased from 289.5 m2/g (AC-CPH-750) to 427.8 m2/g (AC-CPH-A-750). Comparing the data in Table 3 also demonstrates that the average pore width gradually decreases with increasing activating temperature, indicating the development of more fine pores at higher temperature from 650 to 850 °C. To assure the higher pore properties of the resulting ACs at higher temperature and longer time in the CO2 activation experiments, AC-CPH-A-900 was produced at 900 °C, holding 90 min. Due to the serious burn-off at higher activation temperature and longer holding time, the BET surface area of AC-CPH-A-900 increases significantly to about 1330 m2/g, but its yield reduces rapidly to 8.0%, as compared to the yield of 26.4% for AC-CPH-A-850.
Figure 2a, b shows the corresponding N2 adsorption-desorption isotherms (at − 196 °C) and the Barrett-Joyner-Halenda (BJH) desorption pore size distribution curves of the resulting ACs, respectively. These AC products prepared from the CPH and CPH-A at different activation temperatures exhibit the shape of the type IV isotherm according to the classification by the International Union of Pure and Applied Chemistry (IUPAC) [26]. In the case of physical adsorption, this type is often encountered in microporous materials where the high adsorption uptake is observed at low relative pressures because of the narrow pore width and the high adsorption potential. These features are also demonstrated in the pore size distribution curves, where the pores of the resulting ACs have narrow pore size distributions at less than 2.0 nm (20 Å) depicted in Fig. 2b. It is interesting that the mesoporous structure is also present in the resulting ACs; there are slight hysteresis loops for AC-CPH-750 and AC-CPH-A-850 when relative pressure increases to 0.4 or more. This result provides the fact that the rigorous reaction at higher activation temperatures is beneficial to the development of mesoporous structure, thus converting the narrowly distributed micropores into the wider pore size distribution with little mesopores created [2].
3.3 Chemical properties of CPH-based activated carbons
Table 4 provides the data on the elemental analysis of the resulting ACs produced from CPH and CPH-A. Obviously, the CPH-A-based AC products are rich in carbon (O), ranging from 85 to 90%. Compared to the data in Tables 1 and 4, this can be further confirmed by the increase in the content of C from 50 to 85% or more, clarifying that the contents of O and H in the lignocellulosic precursor (i.e., CPH-A) significantly decrease during the activation process. In addition, the precursor (i.e., CPH) for producing AC contains a significant ash content, which should be attributed to the fact that most of the potassium-containing minerals are likely remained in the resulting ACs, thus resulting in relatively high in oxygen contents (> 30 wt%) and low in carbon contents (< 60 wt%). Furthermore, increasing the activation temperature from 650 to 850 °C will induce more gasification reaction, thus indicating an increase in the C and S contents of the resulting ACs (Table 3), and a decreasing trend of H, N, and O contents.
4 Conclusions
Based on the proximate analysis, mineral compositions, thermogravimetric analysis, and thermochemical properties, the chemical and thermochemical differences between cocoa pod husk (CPH) and acid-leaching CPH (CPH-A) were investigated in the present study. It shows that the pretreatment of CPH with hydrochloric acid led to removal of over 90% of the ash content in the CPH, mainly composed of potassium minerals. This work further studied the effect of CPH-A on the pore properties and chemical compositions of the resulting ACs, which were prepared from the physical activation at different activation temperatures (650–850 °C). The Brunauer-Emmet-Teller (BET) surface area of the AC derived from CPH-A at 650 °C is 355.8 m2/g, significantly larger than that (i.e., 1.1 m2/g) of the AC derived from CPH. In addition, the activation at higher temperature is beneficial to the pore development of the resulting ACs. These results suggest that the de-ashed CPH provides an excellent precursor for preparing microporous ACs. More significantly, the solution after the acid-leaching of CPH may be reused as a liquid fertilizer because it is abundant in potassium ions.
References
Rufford TE, Fiset E, Hulicova-Jurcakova D, Zhu ZH (2014) Biomass-derived carbons for supercapacitor electrodes. In: Rufford TE, Zhu J, Hulicova-Jurcakova D (eds) Green carbon materials: advances and applications. Pan Stanford, Singapore, pp 93–113. https://doi.org/10.1201/b15651-5
Marsh H, Rodriguez-Reinoso F (2006) Activated carbon. Elsevier, Amsterdam
Bansal RC, Donnet JB, Stoeckli F (1988) Active carbon. Marcel Dekker, New York
Ioannsidou O, Zabaniotou A (2007) Agricultural residues as precursor for activated carbon production—a review. Renew Sust Energ Rev 11(9):1966–2005. https://doi.org/10.1016/j.rser.2006.03.013
Abioye AM, Ani FN (2015) Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: a review. Renew Sust Energ Rev 52:1282–1293. https://doi.org/10.1016/j.rser.2015.07.129
Yahya MA, Al-Qodah Z, Ngah CMZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sust Energ Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Vriesmann LC, Amboni RDM, Petkowicz CLO (2011) Cacao pod husks (Theobroma cacao L.): composition and hot-water-soluble pectins. Ind Crop Prod 3:1173–1181
Cruz G, Pirila M, Huuhtanen M, Carrion L, Alvarenga E, Keiski R (2012) Production of activated carbon from cocoa (Theobroma cacao) pod husk. J Civ Environ Eng 2:1–6
Vriesmann LC, Teofilo RF, Petkowicz CLO (2012) Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT–Food Sci Technol 49(1):108–116. https://doi.org/10.1016/j.lwt.2012.04.018
Figueira A, Janick J, BeMiller JN (1993) New products from Theobroma cacao: seed pulp and pod gum. In: Janick J, Simon JE (eds) New crops. Wiley, New York, pp 475–478
Okiyama DCG, Navarro SLB, Rodrigues CEC (2017) Cocoa shell and its compounds: applications in the food industry. Trends Food Sci Technol 63:103–112. https://doi.org/10.1016/j.tifs.2017.03.007
Oddoye EOK, Agyente-Badu CK, Gyedu-Akoto E (2013) Cocoa and its by-products: identification and utilization. In: Watson RR, Preedy VR, Zibadi S (eds) Chocolate in health and nutrition. Springer, New York, pp 23–37. https://doi.org/10.1007/978-1-61779-803-0_3
Ma Y (2017) Comparison of activated carbons prepared from wheat straw via ZnCl2 and KOH activation. Waste Biomass Valor 8(3):549–559. https://doi.org/10.1007/s12649-016-9640-z
Ahmad F, Daud WMAW, Ahmad MA, Radzi R (2011) Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: kinetics and equilibrium studies. Chem Eng J 178:461–467
Ahmad F, Daud WMAW, Ahmad MA, Radzi R (2012) Cocoa (Theobroma cacco) shell-based activated carbons by CO2 activation in removing of cationic dye from aqueous solution: kinetics and equilibrium studies. Chem Eng Res Des 90(10):1480–1490. https://doi.org/10.1016/j.cherd.2012.01.017
Ahmad F, Daud WMAW, Ahmad MA, Radzi R, Azmi AA (2013) The effect of CO2 activation, on porosity and surface functional groups of cocoa (Theobroma cacco)—shell based activated carbon. J Environ Chem Eng 1(3):378–388. https://doi.org/10.1016/j.jece.2013.06.004
Zhou S, Yang Q, Runge TM (2015) Ambient-temperature sulfuric acid pretreatment to alter structure and improve enzymatic digestibility of alfalfa stems. Ind Crop Prod 70:410–416. https://doi.org/10.1016/j.indcrop.2015.03.068
Zhou S, Runge TM (2015) Mechanism of improved cellulosic bio-ethanol production from alfalfa stems via ambient-temperature acid pretreatment. Bioresour Technol 193:288–296. https://doi.org/10.1016/j.biortech.2015.06.096
Bello OS, Siang TT, Ahmad MA (2012) Adsorption of Remazol Brilliant Violet-5R reactive dye from aqueous solution by cocoa pod husk-based activated carbon: kinetics, equilibrium and thermodynamic studies. Asia Pac J Chem Eng 7(3):378–388. https://doi.org/10.1002/apj.557
Suzuki M (1990) Adsorption engineering. Elsevier, Amsterdam
Basu P (2013) Biomass gasification, pyrolysis and torrefaction, 2nd edn. Academic Press, San Diego
Tsai WT, Liu SC (2013) Effect of temperature on thermochemical property and true density of torrefied coffee residue. J Anal Appl Pyrol 102:47–52. https://doi.org/10.1016/j.jaap.2013.04.003
Touray N, Tsai WT, Chen HL, Liu SC (2014) Thermochemical and pore properties of goat-manure-derived biochars prepared from different pyrolysis temperatures. J Anal Appl Pyrol 109:116–122. https://doi.org/10.1016/j.jaap.2014.07.004
Gregg SJ, Sing KSW (1982) Adsorption, surface area, and porosity. Academic Press, London
Smith JM (1981) Chemical engineering kinetics, 3rd edn. McGraw-Hill, New York
Lowell S, Shields JE, Thomas MA, Thommes M (2006) Characterization of porous solids and powders: surface area, pore size and density. Springer, Dordrecht
Tsai CH, Tsai WT, Liu SC, Lin YQ (2017) Thermochemical characterization of biochar from cocoa pod husk prepared at low pyrolysis temperature. Biomass Convers Biorefin https://doi.org/10.1007/s13399-017-0259-5
Simpson BK, Oldham JH, Martin AM (1985) Extraction of potash from cocoa pod husks. Agric Wastes 13(1):69–73. https://doi.org/10.1016/0141-4607(85)90013-7
Jenkins BM, Baxter LL, Miles Jr TR, Miles TR (1985) Combustion properties of biomass. Fuel Process Technol 54:17–46
Afrane G (1992) Leaching of caustic potash from cocoa husk ash. Bioresour Technol 41(2):101–104. https://doi.org/10.1016/0960-8524(92)90177-Y
Vriesmann LC, Amboni RDMC, Petkowicz CLO (2011) Cacao pod husks (Theobroma cacao L.): composition and hot-water-soluble pectins. Ind Crop Prod 34(1):1173–1181. https://doi.org/10.1016/j.indcrop.2011.04.004
Martinez-Angel JD, Villamizar-Gallardo RA, Ortiz-Rodriguez OO (2015) Characterization and evaluation of cocoa (Theobroma cacao L.) pod husk as a renewable energy source. Agrociencia 49:329–345
Acknowledgements
The authors acknowledge Li-Jing Viscarb Co. (Pingtung, Taiwan) for providing grants under the support by the Small Business Innovation Research (SBIR) project from the Pingtung County Government. We also thank the Instrument Centers at National Chung Hsing University for the elemental analysis (EA) and National Ching-Hwa University for the inductively coupled plasma-optical emission spectrometry (ICP-OES). In addition, we also thank Mr. Yu-Quan Lin (Department of Biomechatronics Engineering, National Pingtung University of Science and Technology) for analytical and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, WT., Huang, PC. Characterization of acid-leaching cocoa pod husk (CPH) and its resulting activated carbon. Biomass Conv. Bioref. 8, 521–528 (2018). https://doi.org/10.1007/s13399-018-0302-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0302-1