Abstract
The incorporation of semi-crystalline polymers as additives with small-molecule organic semiconductors has emerged as a pioneering method for the alteration of crystallization processes, thin film morphologies, and charge carrier mobility within organic semiconductor matrices. In this paper, we utilize the intrinsic attributes of polyethylene oxide (PEO), acting as a semi-crystalline polymer additive, to modulate the crystallization, phase segregation and charge transport of 6,13-bis (triisopropylsilyl) pentacene (TIPS pentacene). To understand the synergistic effects between varying molecular weights (8, 100, 300 and 900 K) of PEO and the crystallization behavior of TIPS pentacene, we conducted a quantitative analysis of the films' relative crystallinity and crystallographic morphology employing X-ray diffraction (XRD) and optical microscopy. Our findings indicate that higher molecular weight PEOs (300K and 900K) exhibit reduced molecular chain activity, resulting in lower crystallinity at increased doping ratios. Furthermore, attributes such as a high dielectric constant and a substantial melting point, combined with favorable thermoplastic properties, predispose these films to a more susceptible phase separation within the crystalline matrix. Conversely, films with lower molecular weight PEOs (8 and 100 K) showed lesser impact from molecular chain dynamics, leading to enhanced crystal morphology, higher crystallinity, and improved charge carrier mobility by up to 11 times. This substantial enhancement underscores the potential of employing low molecular weight semi-crystalline polymers as additive agents in the development of advanced organic semiconductor devices.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Recent advancements in the research of organic thin-film transistors (OTFTs) have been substantial, particularly in the development of various solution-processing techniques [1,2,3,4,5,6,7,8,9,10,11,12,13]. However, the random orientation of crystals and variations in carrier mobility have created a huge challenge for the application in organic electronics [14,15,16,17,18,19,20,21,22]. To address these issues, researchers have been focusing on optimizing the molecular design and deposition techniques to enhance the performance and consistency of these materials. Therefore, TIPS pentacene, as a p-type organic semiconductor, has become a benchmark semiconductor [23,24,25,26,27,28,29] for a large number of related studies. For example, Kneppe et al. [24] selected 6,13-bis(triisopropylsilyl)pentacene (TIPS pentacene), which is adapted to a low-temperature, low-cost solution-processing mode, as the preferred choice for the preparation of ultrashort-channel transistors. Not only that, Jousselin-Oba et al. [30] utilized TIPS pentacene's excellent compatibility between stability and solubility to prepare two surrounding synthetic materials, tetracenotetracene (TT) and pentacenopentacene (PP), for modifying the π-topology of molecular organic semiconductors.
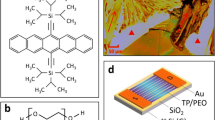
The molecular structure of TIPS pentacene is shown in Fig. S1a. Due to its acene unit and substrate side-pair arrangement, the bulky side groups on both sides disrupt the edge-to-face interactions of the aromatic ring [31,32,33,34,35,36,37], and further destroy the herringbone packing motifs, which makes TIPS pentacene more resistant to oxidation and degradation [38,39,40,41]. Meanwhile, the carbon–carbon triple bond keeps bulky side group substitution at a distance from the aromatic ring [42,43,44]. This makes π-π stacking between TIPS pentacene molecules possible, and results in improved π-orbital coupling and charge carrier mobility. Due to these great potentials, TIPS pentacene has been showcased in various high performance organic electronic applications [45,46,47,48,49,50,51]. For instance, Ajayan et al. [52] utilized TIPS pentacene for flexible/wearable electronics applications in view of the fact that it provides good air stability and high hole mobility in OTFTs. Wang et al. [53] employed the strong charge transport capability inherent in TIPS pentacene as a high-quality crystalline thin film for fabricating an ultrasensitive NO2 sensor. Additionally, Singh et al. [54] prepared a low-cost heterojunction photodiode by utilizing the high carrier mobility and air stability of TIPS pentacene for application in UV photodetectors.
However, better crystal morphology as well as higher charge transport is usually obtained when TIPS pentacene is mixed and crystallized with polymers, primarily due to the following reasons. Firstly, the polymer additive improves the morphological homogeneity of the organic semiconductor crystals, resulting in an increase in the consistency of the transistor's performance [55, 56]. Secondly, organic semiconductors with polymer additives, such as poly (α-methylstyrene) (PαMS) and polystyrene (PS), have the potential to initiate and promote vertical phase separation [57,58,59]. For example, Ohe et al. [60] investigated the phase separation dependent electrical performance of OTFTs in relation to the different molecular weights of PαMS as polymer additives. It was concluded that the driving force of phase separation could be explained by the Flori-Huggins theory. Chae et al. [61] also reported a significant vertical phase separation with the addition of PαMS to TIPS pentacene, which enhanced the field-effect mobility of the OTFTs while further reducing its electrical hysteresis. As for the polymer additive PS, Bharti et al. [62] reported phase separation induced high carrier mobility and demonstrated electrically stable OTFTs by blending TIPS pentacene with PS.Coincidentally, the OTFTs prepared by Rahi et al. [63] using PS as a polymer additive blended with TIPS pentacene also produced phase separation, and the devices also possessed high electrical stability at different temperatures. These phase separation phenomena occur because the stability of OTFTs in air is improved when the polymer separation leads to the formation of an encapsulation layer at the air interface, separating the semiconductor crystal layer from oxygen and water [64, 65]. In addition, the phase separation also enriches a large number of organic semiconductors at the charge-transfer interface, which further improves the performance of semiconductor devices [66].
Compared to amorphous polymers, relatively few studies have been conducted on blending semicrystalline polymers as additives for the preparation of OTFTs. Semicrystalline polymers compete with organic semiconductors for crystallization when they are blended together, thus providing in-situ crystallization regulation that amorphous polymers cannot provide. In addition, the use of non-conjugated semi-crystalline polymers avoids polymer-induced π-π interactions during the crystallization process [67].
Herein, semi-crystalline polymers, poly(ethylene oxide) (PEO), a non-conjugated semi-crystalline polymer (molecular structure shown in Figure S1b), is chosen to manufacture and is available in a wide range of molecular weights. Moreover, PEO has extensive applications in construction materials, pharmaceutical production, lightweight textiles, and oil and mining industries [68]. PEO as a semicrystalline polymer is mixed with organic semiconductor TIPS pentacene as an additive, which allowed effective control of the semiconductor crystallization and charge transport. In particular, four molecular weights of PEO, each with five different mixing ratios, were studied for their effect on the crystallinity, crystal morphology and charge carrier mobility of TIPS pentacene mixing.
The polymer material PEO was purchased from Sigma Aldrich. The small molecule organic semiconductor TIPS pentacene was purchased from Tokyo Chemical Industry (TCI). A total of four different molecular weights of PEO, including 8K, 100K, 300K and 900K, were used in this work. Firstly, two separate solutions with a concentration of 3 mg/mL were prepared using toluene as the solvent and TIPS pentacene and PEO as solutes, respectively. The solution of TIPS pentacene was stirred for 30 min at ambient temperature, while the solution of PEO was heated and stirred for 30 min at 70 ℃ to ensure that all PEO was dissolved. Next, to find the optimal weight ratio of PEO, PEO was added to TIPS pentacene at different ratios, including 1, 3, 5, 10 and 20%. The mixed solution was stirred at a room temperature for an additional 30 min. Finally, the solutions with different ratios were drop-cast onto a silicon substrate with a 300 nm thick silica dielectric layer. In particular, in order to reduce the thickness of the crystallized layer, 20 µl of the mixed solution was deposited onto a substrate with a size of approximately 1.3 cm2. The substrate was placed at an angle (3° ~ 5°) to facilitate crystallization, and the crystal growth took place in a solvent rich environment [69,70,71,72].
Upon the completion of crystallization, to minimize the effect of metal electrodes on the crystalline films, we placed the samples in an X-ray diffractometer (XRD) to test the wide-angle physical phases in a bulk sample to obtain source data for XRD. To prepare the metal electrodes, we fabricated OTFTs by thermally evaporating 50 nm gold deposited on the crystals as source and drain. The evaporation rate is 1Å/s at a pressure of 10–7 Torr. The process of drop cast, crystallization and thermal evaporation is illustrated in Fig. 1d.
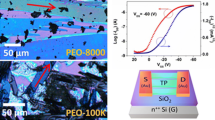
a Principle of operation of p-type OTFTs. b Bottom-gate, top-contact device architecture for OTFTs with p-type semiconductor. c An optical image of pure TIPS pentacene (TP) film. d Fabrication process of OTFTs, which is consisted of drop casting the solution, crystallization, and thermal evaporation of gold as source and drain contact electrodes
Polarized light microscopy captured images of the organic crystals. The electrical properties were characterized by measuring the output curves (IDS-VDS) and transfer curves (IDS-VGS), with a Keithley 2636B Source Meter at room temperature.
Bottom gate, top contact configuration was chosen as the transistor structure type, as shown in Fig. 1b. As illustrated in Fig. 1a, the organic semiconductor layer separates the source and drain. This layout requires that charge carriers, once injected from the electrodes, pass through the organic semiconductor layer to reach the conducting channel. The top-contact electrode, on the other hand, ensures that the organic semiconductor layer, when thin, has a large contact area, thereby reducing contact resistance and simplifying the fabrication process. As pure TIPS pentacene crystallizes, the crystals are randomly oriented and have lower coverage, as shown in Fig. 1c. According to a previous study by Ebagninin et al. [73] PEO with different molecular weights shows significant differences in their rheological properties caused by the entanglement of light and heavy chains when dissolved in solution, which obviously has a great influence on the crystallinity as well as the crystal morphology of TIPS pentacene. In order to further systematically investigate the effect of PEO as a polymer additive on TIPS pentacene, pure TIPS pentacene crystals were set as the control group, while organic crystals with different PEO molecular weights and weight ratios were studied as the experimental group. The optimal molecular weights and optimal weight ratios of PEO were explored and determined based on both crystallinity and crystal morphology.
X-ray diffraction tests were performed on all substrates and obtained 21 sets of X-ray diffractograms. The data curves obtained after testing are shown in Fig. 2a–d. In order to compare the crystallinity, we fitted the curves by using the software MDI Jade 6, measured the relative crystallinity of each set of data, and compared each set of crystallinity data as shown in Fig. 3a–b. Crystallinity is the degree of integrity of the crystal structure in a material, which reflects the percentage of perfect lattices in the crystal [74]. Without considering other influencing factors, a higher relative crystallinity undoubtedly provides OTFTs a stronger charge transport capability.
The relative crystallinity of the pure TIPS pentacene crystals in Fig. 3a is 94.5 ± 5.07%. It was also found from the histograms that the relative crystallinity of the mixed crystals decreases as the weight ratio of PEO in the mixed solution continues to increase. It is noted that the 100K molecular weight PEO at a 1% doping ratio shows a small error in relative crystallinity, indicating stable and reproducible measurements, likely due to uniform film formation and consistent crystallization processes. Conversely, at a 3% doping ratio, the error significantly increases, suggesting greater variability in crystallinity. This larger error may arise from inhomogeneous film formation, increased disorder due to polymer chain entanglement, and higher sensitivity of the X-ray diffraction technique to variations in sample thickness or density at higher concentrations. These factors introduce complexities that amplify measurement inconsistencies, leading to the observed larger error. Therefore, for the OTFTs of TIPS pentacene/PEO mixtures, a lower weight ratio of mixing is favorable for obtaining a higher crystallinity and charge carrier mobility, if the molecular weight of PEO remains unchanged.
As seen in Fig. 3b, when the mixing weight ratio of PEO is constant, PEO with 8K and 100K molecular weights exhibits higher relative crystallinity compared to the counterpart with 300K and 900K molecular weights. It is well known that the molecular weight of a polymer is a quantitative parameter of the molecular chain length. In a previous study by Bunn et al. [75] the molecular chain activity of a polymer was found to decrease if the repeated length of the molecular chain increases (higher molecular weight). This slows down the crystallization of the polymer in solution at any given temperature, leading to decreased crystallinity. Thus, as the molecular weight of a polymer additive increases, the ability decreases of the molecular chains to arrange themselves into single crystals at low concentrations or into dendrites at high concentrations, which reduces the overall crystallinity of organic semiconductor crystals incorporating this polymer additive. It can be seen that the crystals formed when low molecular weight PEO is added as semi-crystalline polymer to TIPS pentacene at lower weight ratios have better relative crystallinity. For example, when 8K PEO was added to TIPS pentacene at 1, 3 and 5% by weight, the relative crystallinity was 92.77 ± 2.46%, 88.66 ± 1.36%, and 85.05 ± 1.19%, respectively. In comparison, the relative crystallinity of TIPS pentacene with 900K molecular weight PEO at 20% by weight was only 56.81 ± 14.53%. Due to the large amount of data, see Table S1 in the Supporting Information for details.
In addition to the relative crystallinity, the crystal morphology also plays a very important role in charge transport. Figure 4 contains the crystal morphology of TIPS pentacene mixed with different weight ratios of PEO (including four molecular weights of 8, 100, 300 and 900K). Since the solutions in this experiment were prepared by heating the PEOs to dissolve in toluene and then gradually cooling them to crystallize in a toluene-rich environment, the mixed solutions crystallized in a manner whereby the molecular chains formed single crystals or dendritic crystals according to the lamellar crystalline structure of the folded chains. From Fig. 4, it is clearly observed that the grain width of the crystals decreases with the increase of PEO mixing ratio, but the coverage of the crystal film increases gradually. In addition, the long-range ordering of the crystals is greatly improved when the PEO mixing ratio is low.
Next, the morphology of the crystals in terms of orientation angle, crystal length, grain width and crystal film coverage were quantitatively investigated. The crystal misorientation angles as shown as θ1 and θ2 in the inset of Fig. 5a. These two angles are formed by a pre-selected baseline crystal with two neighboring crystals on the left and right side, respectively. The mean values of the misorientation angles for the pure TIPS pentacene crystals and the mixture crystals of TIPS pentacene/PEO were obtained based on 10 crystal measurements. For the pure TIPS pentacene crystals without the addition of PEO, the average misorientation angle is 30.81 ± 27.50°. As PEO with molecular weight of 8K is mixed into TIPS pentacene at different weight ratios, the average orientation angle of the obtained crystal films starts to decrease gradually. When the mixing ratio reaches 20%, the average orientation angle is only 10.70 ± 2.52°. By coincidence, when 100K, 300K and 900K molecular weight PEO is mixed in the appropriate weight ratio, the decrease of the crystal misorientation angle was also observed. The reduction of misorientation angle due to polymer additive was also previously confirmed by He et al., who reported the addition of the polymer PαMS led to a significant reduction in the crystal misorientation angle of TIPS pentacene and improved the long-range ordering of the crystal film [76]. However, it is worth noting that the misorientation angle of the crystals increases inversely when the mixing ratio of PEOs of 100K and 300K molecular weights exceeds 10% by weight, and also when the mixing ratio of PEO of 900K molecular weight exceeds 5% by weight. This phenomenon can be attributed to the vertical phase segregation between TIPS pentacene and PEO. It is well known that as a semi-crystalline polymer, its semi-crystalline nature enables PEO to form crystals in a manner similar to TIPS pentacene, while at the same time, its amorphous component plays a very important role in impacting the diffusion coefficient of TIPS pentacene and the surface energy of the faceted evolution in the crystallization process.
In order to compare the morphological differences between the pure TIPS pentacene crystals and the crystals of TIPS pentacene/PEO blends, histograms were plotted based on the PEO molecular weight and mixing weight ratio, including: a misorientation angle (θ), b grain width WG, c crystal coverage, and d crystal length of LG
To visualize the semicrystalline nature of PEO and the role of vertical phase polarization of the amorphous components, three representative experimental groups of thin-film microscope optical images are shown in Fig. 6a–c. When pure TIPS pentacene crystallizes (Fig. 6a), the crystal misorientation angle is large. After the high molecular weight PEO are mixed with TIPS pentacene at lower weight ratios (or low molecular weight PEO were mixed TIPS pentacene at appropriate ratios), the isotropy of the TIPS pentacene is greatly enhanced, and the PEO simultaneously nucleates and crystallizes at the periphery of the TIPS pentacene (as shown in the red boxes in Fig. 6b, c). This phenomenon is related to the physical properties of the PEO itself and the crystallization process of the mixed solution. First of all, from the point of view of physical properties, PEO has a high dielectric constant of about 3.7 ~ 3.9, which is a polar substance. The electrostatic attraction between solute molecules and materials with high dielectric constant is greater, which makes them attract each other and easy to condense. This allows PEO to interact more strongly with polar substrates in mixed solutions, thus preferentially forming an underlying structure. By coincidence, Gu et al. found that poly (vinylidene) fluoride (PVDF), a semi-crystalline polymer similar to PEO, precipitated out of the solution as a substructure, before forming phase separation due to the high dielectric constant of PVDF and increased crystallinity when mixed with the organic substance dimethylphthalate (DMP) [77]. Secondly, the high molecular weight PEO has good thermoplasticity and a high melting point (about 60℃). PEO is completely dissolved in toluene by heating and then crystallizes on the substrate by dropwise addition, and its good thermoplasticity allows PEO to be uniformly distributed on the substrate surface along with the solution. As the toluene evaporates and cools down, PEO with a high melting point precipitates first out of the solution and forms a stable substrate layer on the substrate (as shown in Fig. 4n, o, s, t), which lays the foundation for the TIPS pentacene crystal growth. These two features of PEO have been previously reported in other literatures. For example, Szymczyk et al. utilized the thermoplasticity of PEO to modify the thermoplastic properties of elastomer. In their study, a series of PTT-b-PEO copolymers with different contents of flexible PEO segments were synthesized by two-step melt polycondensation, which resulted in copolymers with good thermoplastic elastomer properties [78]. In contrast, Yang et al. exploited the high melting point property of PEO to improve device stability in exploring its optical properties and self-assembling nanostructures via solvent annealing. They also created a novel intrinsic bifunctional block copolymer electret and investigated the effect of this design and self-assembly morphology on the storage of phototransistors [79]. Finally, in terms of the solution crystallization process, the chain mobility of PEO is limited, and the higher molecular weight of PEO determines its weaker mobility and usually a slower crystallization rate at room temperature, which allows the faster-crystallizing TIPS pentacene to crystallize at the top of the amorphous region of PEO [76]. The layering phenomenon makes PEO lose the ability to guide the crystallization and diffusion of TIPS pentacene, resulting in the reverse increment of misorientation angle as observed in the optical images.
Next, average grain width WG are determined as shown in the inset in Fig. 5b. WG was calculated along the crystal short axis [1 \(\overline{2 }0\)], based on 10 measurements. After summarizing the data, it is found that compared to the TIPS pentacene/PEO hybrid crystals, the crystals of pure TIPS pentacene have an average grain width of 350.37 ± 59.57 μm. Regardless of the influence of any molecular weight of PEO, as the weight ratio of PEO increases, the corresponding grain width appears to be reduced to different extent. This is the inevitable result of the competing crystallization between PEO and TIPS pentacene. Kleemann et al. found that when different polymers are added to TIPS pentacene as additives, the decrease in grain width leads to the loss of polycrystalline structure, and the increase in crystal defects makes it easier to trap carriers and reduces the charge-carrier mobility of the OTFTs [80]. Therefore, from the point of view of grain width, larger TIPS pentacene crystals mixed with a low weight ratio of PEO will show larger charge carrier mobility.
Afterwards, the crystal film coverage and grain length were quantitatively analyzed, as shown in Fig. 5c. Crystals with a certain degree of crystallinity, smaller misalignment angles, and higher crystal film coverage and dimensions (including crystal width and crystal length) can ensure more favorable charge transport properties over a wider range. It can be inferred from Fig. 5c that the crystal film coverage of TIPS pentacene/PEO blended crystal films increases with the increasing weight ratio of PEO mixing, which is influenced by the semicrystalline nature and amorphous components of PEO. When 900K PEO is mixed into TIPS pentacene at 20% weight ratio to form a crystal film, the coverage is as high as 94.58 ± 7.67%. This is a significant increase compared to the coverage of 29.00 ± 9.28% based on pure TIPS pentacene. Unlike the crystal film coverage, the crystal length of TIPS pentacene is affected by multiple factors such as the crystallization of PEO and the guidance of the amorphous component. The thin film crystals formed after the addition of PEO to TIPS pentacene in the appropriate weight ratio show signs of improvement compared to the pure TIPS pentacene crystalline crystals as shown in Fig. 5d. The mean crystal length LG of this study was obtained based on 10 measurements of the crystals along the long axis [210]. The pure TIPS pentacene crystal length was 938.39 ± 454.29 μm, while the TIPS pentacene/PEO blended thin film crystals with a molecular weight of 100 K and a mixing weight ratio of 5% had a crystal length of 4074.20 ± 1474.71 μm. Note that due to the long extension of the crystals on the silicon substrate, multiple microscope optical images are required for the measurements.
To measure the electrical properties of OTFTs, the output curves and transfer curves were tested as shown in Figs. 7, 8. From the transfer curve, the hole mobility of the OTFTs can be calculated based on Eq. 1:
where μ is the hole mobility, IDS is the saturation drain current, Ci is the capacitance of the gate dielectric layer (11.5 nF/cm2 since the SiO2 thickness of the silicon substrate for this study is 300 nm). The length and width of the conductive channel between the gold electrodes are denoted by L and W, respectively, as shown in Fig. 1a. VT and VGS denote the threshold voltage and gate source voltage.
At last, the mobility of pure TIPS pentacene film crystals with those incorporating the PEO additive with four different molecular weights were calculated and compared, as shown in Fig. 9a–d. It is found that the hole mobility of OTFTs was enhanced only for 8 and 100K PEO at mixing weight ratios of 3 and 5%. Among them, the highest value of hole mobility of the TIPS pentacene crystals mixed with 8K PEO at 3% by weight reached 0.73 cm2/Vs. It is worth mentioning that the highest mobility of all four molecular weight PEO was obtained at a weight ratio of 3%. While our experimental results indicate that polymer additives like PEO can sometimes promote nucleation and crystallization of TIPS pentacene, they can also decrease relative crystallinity, especially at higher concentrations or molecular weights. The semi-crystalline nature of PEO reduces molecular chain mobility, competing with TIPS pentacene for crystallization and thus lowering crystallinity. Even with low molecular weight PEO, crystallinity appears lower than pure TIPS pentacene because PEO introduces additional nucleation sites disrupting the perfect lattice structure. However, an appropriate doping ratio of low molecular weight PEO significantly improves crystal morphology, enhancing charge carrier mobility in OTFTs, making PEO a valuable additive despite a slight reduction in crystallinity. For reference, optical images of pure TIPS pentacene crystalline films are provided in Fig. 1c. Therefore, the improved hole mobility is attributed to the combination of the relatively high crystallinity and more superior morphology of the thin-film crystals. This is a 11 times enhancement as compared to that of the pure TIPS pentacene crystalline films as reported from a previous work [81].
In summary, we report to control the crystallization, morphology, and crystallinity of the organic small-molecule semiconductor TIPS pentacene by varying the molecular weight and mixing weight ratio of the semicrystalline polymer PEO as an additive. We found that adding low molecular weight PEO (8K or 100K) at a 3% or 5% weight ratio ensured a relatively high relative crystallinity while maintaining a low misorientation angle and a large crystal width of the TIPS pentacene crystals, resulting in a 11-time enhancement of charge carrier mobility of up to 0.73 cm2/Vs. This is due to that the polymer chains of low molecular weight PEO play an important role in guiding the semiconductor crystallization to form more superior crystal morphologies, while at the same time exerting less influence on the structural integrity of the TIPS pentacene crystals. Unlike the thin film crystals formed by high molecular weight PEO (300K or 900K) as an additive, the low molecular chain activity of high molecular weight PEO leads to a decrease in the crystallinity of the mixed solution. At the same time, when the mixing ratio of PEO increases above a certain percentage, its high dielectric constant, good thermoplasticity, high melting point and other physical properties enable the polymer to preferentially crystallize close to the substrate and vertically phase-separate from the organic semiconductor, eventually forming a two-layer structure. While this increases the coverage of the thin film crystals, the ability of the polymer to control the crystal orientation is gradually weakened, which ultimately reduces the charge transport of the OTFTs. We believe that the present study systematically provides an insight into the laws of how semicrystalline polymers as additives affect the crystallization of organic semiconductors, and also presents a novel pathway for enhancing the charge carrier mobility of organic crystals.
References
Wahab, N.Z.A., Abdulhameed, A., Ismail, A.G., Ramli, M.M., Sidek, R.M., Shafie, S., Mohtar, M.N.: Review-charge carrier mobility of organic thin film transistor: intrinsic and extrinsic influencing factors based on organic semiconducting materials. ECS J. Solid State Sci. Technol. 12(4), 044002 (2023)
Cavallari, M.R., Pastrana, L.M., Sosa, C.D.F., Marquina, A.M.R., Izquierdo, J.E.E., Fonseca, F.J., Amorim, C.A.D., Paterno, L.G., Kymissis, I.: Organic thin-film transistors as gas sensors a review. Materials. 14(1), 3 (2020)
Ma, L.-Y., Soin, N., Aidit, S.N., Rezali, F.A.M., Hatta, S.F.W.M.: Recent advances in flexible solution-processed thin-film transistors for wearable electronics. Mater. Sci. Semiconduct. Process. 165, 107658 (2023)
Nitti, A., Scagliotti, M., Beverina, L., Mariucci, L., Rapisarda, M., Pasini, D.: Solution-processable thin-film transistors from anthradithiophene (ADT) and naphthothiopene (NT) small molecule-based p-type organic semiconductors. Mater. Adv. 4(19), 4590 (2023)
Sun, C., Wang, X., Auwalu, M.A., Cheng, S., Hu, W.: Organic thin film transistors-based biosensors. EcoMat 3(2), e12094 (2021)
Tavasli, A., Gurunlu, B., Gunturkun, D., Isci, R., Faraji, S.: A review on solution-processed organic phototransistors and their recent developments. Electronics 11(3), 316 (2022)
Verma, A., Mishra, V.N., Prakash, R.: A self-aligned, solution-processed low-voltage operated organic thin-film transistor for ammonia gas sensing at room temperature. IEEE Sens. J. 23(6), 5561 (2023)
Wang, N., Yang, A., Fu, Y., Li, Y., Yan, F.: Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 52(2), 277 (2019)
Wu, W., Feng, K., Wang, Y., Wang, J., Huang, E., Li, Y., Jeong, S.Y., Woo, H.Y., Yang, K., Guo, X.: Selenophene substitution enabled high-performance n-type polymeric mixed ionic-electronic conductors for organic electrochemical transistors and glucose sensors. Adv. Mater. (2023). https://doi.org/10.1002/adma.202310503
Yin, X., Yang, J., Wang, H.: Vertical phase separation structure for high-performance organic thin-film transistors: mechanism, optimization strategy, and large-area fabrication toward flexible and stretchable electronics. Adv. Funct. Mater. 32(27), 2202071 (2022)
Yusof, N.S., Mohamed, M.F.P., Ghazali, N.A., Khan, M.F.A.J., Shaari, S., Mohtar, M.N.: Evolution of solution-based organic thin-film transistor for healthcare monitoring- from device to circuit integration: a review. Alex. Eng. J. 61(12), 11405 (2022)
Zhang, C., Zhu, X.Z.: N-type quinoidal oligothiophene-based semiconductors for thin-film transistors and thermoelectrics. Adv. Funct. Mater. 30(31), 2000765 (2020)
Zhu, H., Shin, E.S., Liu, A., Ji, D., Xu, Y., Noh, Y.Y.: Printable semiconductors for backplane TFTs of flexible OLED displays. Adv. Funct. Mater. 30(20), 1904588 (2020)
Almansoori, M.T., Li, X., Zheng, L.: A brief review on e-skin and its multifunctional sensing applications. Curr. Smart Mater. 4(1), 3 (2019)
Cheng, C.Y., Campbell, J.E., Day, G.M.: Evolutionary chemical space exploration for functional materials: computational organic semiconductor discovery. Chem. Sci. 11(19), 4922 (2020)
Degitz, C., Dissertation, Karlsruhe, Karlsruher Institut für Technologie (KIT), 2022. (2022).
Forrest, S.R.: Organic electronics: foundations to applications. Oxford University Press, USA (2020)
He, Z., Chen, J., Li, D.: Crystal alignment for high performance organic electronics devices. J Vacuum Sci Technol A 37(4), 040801 (2019)
Janasz, L., Borkowski, M., Blom, P.W.M., Marszalek, T., Pisula, W.: Organic semiconductor/insulator blends for elastic field-effect transistors and sensors. Adv. Funct. Mater. 32(7), 2105456 (2022)
Liu, K., Ouyang, B., Guo, X.J., Guo, Y.L., Liu, Y.Q.: Advances in flexible organic field-effect transistors and their applications for flexible electronics. Npj Flex. Electron. 6(1), 1–19 (2022)
Zhang, Z.Y., Asare-Yeboah, K., Bi, S., He, Z.R.: Poly(α-methyl styrene) polymer additive for organic thin film transistors. J. Mater. Sci. Mater. Electron. 33(3), 1101 (2022)
Zheng, B., Huo, L.J.: Recent advances of dithienobenzodithiophene-based organic semiconductors for organic electronics. Sci. China Chem. 64(3), 358 (2021)
He, Z., Li, D., Hensley, D.K., Rondinone, A.J., Chen, J.: Switching phase separation mode by varying the hydrophobicity of polymer additives in solution-processed semiconducting small-molecule/polymer blends. Appl. Phys. Lett. 103(11), 113301 (2013)
Kneppe, D., Talnack, F., Boroujeni, B., da Rocha, C.T., Höppner, M., Tahn, A., Mannsfeld, S.C., Ellinger, F., Leo, K., Kleemann, H.: Solution-processed pseudo-vertical organic transistors based on TIPS-pentacene. Mater. Today Energy 21, 100697 (2021)
He, Z., Asare-Yeboah, K., Zhang, Z., Bi, S.: Manipulate organic crystal morphology and charge transport. Org. Electron. 103, 106448 (2022)
Akkerman, H.B., Li, H.Y., Bao, Z.N.: TIPS-pentacene crystalline thin film growth. Org. Electron. 13(10), 2056 (2012)
He, Z., Chen, J., Sun, Z., Szulczewski, G., Li, D.: Air-flow navigated crystal growth for TIPS pentacene-based organic thin-film transistors. Org. Electron. 13(10), 1819 (2012)
Giri, G., Verploegen, E., Mannsfeld, S.C.B., Atahan-Evrenk, S., Kim, D.H., Lee, S.Y., Becerril, H.A., Aspuru-Guzik, A., Toney, M.F., Bao, Z.A.: Tuning charge transport in solution-sheared organic semiconductors using lattice strain. Nature 480(7378), 504 (2011)
He, Z., Xiao, K., Durant, W., Hensley, D.K., Anthony, J.E., Hong, K., Kilbey, S.M., II., Chen, J., Li, D.: Enhanced performance consistency in nanoparticle/TIPS pentacene-based organic thin film transistors. Adv. Funct. Mater. 21(19), 3617 (2011)
Jousselin-Oba, T., Mamada, M., Marrot, J., Maignan, A., Adachi, C., Yassar, A., Frigoli, M.: Excellent semiconductors based on tetracenotetracene and pentacenopentacene: from stable closed-shell to singlet open-shell. J. Am. Chem. Soc. 141(23), 9373 (2019)
Asare-Yeboah, K., Bi, S., He, Z., Li, D.: Temperature gradient controlled crystal growth from TIPS pentacene-poly(alpha-methyl styrene) blends for improving performance of organic thin film transistors. Org. Electron. 32, 195 (2016)
He, Z., Lopez, N., Chi, X., Li, D.: Solution-based 5,6,11,12-tetrachlorotetracene crystal growth for high-performance organic thin film transistors. Org. Electron. 22, 191 (2015)
Chen, J.H., Martin, D.C., Anthony, J.E.: Morphology and molecular orientation of thin-film bis(triisopropylsilylethynyl) pentacene. J. Mater. Res. 22(6), 1701 (2007)
Bi, S., He, Z., Chen, J., Li, D.: Solution-grown small-molecule organic semiconductor with enhanced crystal alignment and areal coverage for organic thin film transistors. AIP Adv. 5(7), 077170 (2015)
Chen, J.H., Anthony, J., Martin, D.C.: Thermally induced solid-state phase transition of bis(triisopropylsilylethynyl) pentacene crystals. J. Phys. Chem. B 110(33), 16397 (2006)
He, Z., Chen, J., Keum, J.K., Szulczewski, G., Li, D.: Improving performance of TIPS pentacene-based organic thin film transistors with small-molecule additives. Org. Electron. 15(1), 150 (2014)
Cho, M.Y., Han, Y.D., Kang, H.S., Kim, K., Kim, K.H., Cho, M.J., Choi, D.H., Joo, J.: Photoresponsive characteristics and hysteresis of soluble 6,13-bis(triisopropyl-silylethynyl)-pentacene-based organic thin film transistors with and without annealing. J. Appl. Phys. 107(3), 6 (2010)
Bier, I. E.: Beyond zeroth order molecular crystal structure prediction: crystal habit prediction, property optimization, and temperature effects (Doctoral dissertation, Carnegie Mellon University), (2022)
Chen, J., Martin, D.C., Anthony, J.E.: Morphology and molecular orientation of thin-film bis (triisopropylsilylethynyl) pentacene. J. Mater. Res. 22(6), 1701 (2007)
Li, Q.-Y., Yao, Z.-F., Wang, J.-Y., Pei, J.: Multi-level aggregation of conjugated small molecules and polymers: from morphology control to physical insights. Rep. Progr. Phys. 84(7), 076601 (2021)
Yavuz, A.: Investigation of perfluoropentacene thin films on substrates with different chemical structures. (2020).
He, Z., Zhang, Z., Bi, S.: Tailoring the molecular weight of polymer additives for organic semiconductors. Mater. Adv. 3(4), 1953 (2022)
Wade, J., Steiner, F., Niedzialek, D., James, D.T., Jung, Y., Yun, D.J., Bradley, D.D.C., Nelson, J., Kim, J.S.: Charge mobility anisotropy of functionalized pentacenes in organic field effect transistors fabricated by solution processing. J Mater. Chem. C 2(47), 10110 (2014)
He, Z., Zhang, Z., Bi, S.: Nanoparticles for organic electronics applications. Mater. Res. Expr. 7, 012004 (2020)
Yamauchi, H., Sakai, M., Kuniyoshi, S., Kudo, K.: Fabrication of n- and p-channel step-edge vertical-channel transistors by electrospray deposition. Japanese J. Appl. Phys. 53(1), 80 (2014)
Alahmed, Z.A., Mansour, S.A., Aydin, M.E., Yakuphanoglu, F.: Hybrid photodiodes based on 6,13-bis(triisopropylsilylethynyl) pentacene: poly 2-methoxy-5-(2-ethyl) hexoxy-phenylenevinylene /p-silicon. Solid State Commun. 163, 23 (2013)
Kim, S.H., Hwang, H.R., Kwon, H.J., Jang, J.: Unipolar depletion-load organic circuits on flexible substrate by self-organized polymer blending with 6, 13-bis(triisopropylsilylethynyl)pentacene using ink-jet printing. Appl. Phys. Lett. 100(5), 4 (2012)
Lee, J., Kwon, J., Lim, J., Lee, C.: An amorphous polythiophene as a binder material for organic thin-film transistor channel applications. Mol. Cryst. Liq. Cryst. 519, 179 (2010)
Park, J.G., Vasic, R., Brooks, J.S., Anthony, J.E.: Characterization of functionalized pentacene field-effect transistors and its logic gate application. J. Appl. Phys. 100(4), 044511 (2006)
Jeong, U., Tarsoly, G., Lee, J., Eun, Y., Do, J., Pyo, S.: Interdigitated ambipolar active layer for organic phototransistor with balanced charge transport. Adv. Electron. Mater. 5, 1800652 (2019)
Kim, K., Nam, K., Li, X., Lee, D.Y., Kim, S.H.: Programmed design of highly crystalline organic semiconductor patterns with uniaxial alignment via blade coating for high-performance organic field-effect. ACS Appl. Mater. & Interfac. 11(45), 42403 (2019)
Ajayan, J., Sreejith, S., Manikandan, M., Sreenivasulu, V.B., Kumari, N.A., Ravindran, A.: An intensive study on organic thin film transistors (OTFTs) for future flexible/wearable electronics applications. Micro Nanostruct. 187, 207766 (2024)
Wang, Z., Huang, L., Zhu, X., Zhou, X., Chi, L.: An ultrasensitive organic semiconductor NO2 sensor based on crystalline TIPS-pentacene films. Adv. Mater. 29(38), 1703192 (2017)
Singh, A.P., Upadhyay, R.K., Jit, S.: High-performance colloidal ZnO quantum dots/TIPS-pentacene Heterojunction-based ultraviolet photodetectors. IEEE Trans. Electron Devices 69(6), 3230 (2022)
Chen, M.N., Ke, C.Y., Nyayachavadi, A., Zhao, H.Y., Ocheje, M.U., Mooney, M., Li, Y.T., Gu, X.D., Liou, G.S., Rondeau-Gagné, S., Chiu, Y.C.: Facile blending strategy for boosting the conjugated polymer semiconductor transistor’s mobility. ACS Appl. Mater. & Interfac. 15(46), 53755 (2023)
Liu, C.F., Li, S.S., Zhang, J., Duan, W.J., Zhang, Y.H., Liu, H.P., Luo, Q., Chen, S.G., Liu, X., Lai, W.Y.: Tailoring crystallization growth of small-molecule organic semiconductors by modification with conjugated polymers for organic field-effect transistors. Adv. Electron. Mater. 9(9), 2201107 (2023)
Zhang, Z., Asare-Yeboah, K., Bi, S., He, Z.: Poly(α-methyl styrene) polymer additive for organic thin film transistors. J. Mater. Sci. Mater. Electron. 33, 1101 (2022)
Zhang, Z., He, Z., Bi, S., Asare-Yeboah, K.: Phase segregation controlled semiconductor crystallization for organic thin film transistors. J. Sci. Adv. Mater. Devices 5(2), 151 (2020)
He, Z., Bi, S., Asare-Yeboah, K., Zhang, Z.: Phase segregation effect on TIPS pentacene crystallization and morphology for organic thin film transistors. J. Mater. Sci. Mater. Electron. 31(6), 4503 (2020)
Ohe, T., Kuribayashi, M., Tsuboi, A., Satori, K., Itabashi, M., Nomoto, K.: Organic thin-film transistors with phase separation of polymer-blend small-molecule semiconductors: Dependence on molecular weight and types of polymer. Appl. Phys. Expr. 2(12), 121502 (2009)
Chae, S., Lee, T.I., Oh, J.Y.: Reduced electrical hysteresis of organic thin-film transistors based on small molecule semiconductor through an insulating polymer binder. Korean J. Chem. Eng. 39(3), 499 (2022)
Bharti, D., Tiwari, S.P.: Phase separation induced high mobility and electrical stability in organic field-effect transistors. Synth. Met. 221, 186 (2016)
Rahi, S., Raghuwanshi, V., Saxena, P., Konwar, G., and Tiwari, S. P.: Effect of annealing on low-voltage organic field-effect transistors with P (VDF-TrFE) gate dielectric. In: 2021 IEEE international conference on flexible and printable sensors and systems (FLEPS), (2021)
Ndikumana, J., Kim, J., Kim, J.Y., Lee, D., An, K.: A review on 2,8-difluoro-5,11-bis(triethylsilylethynyl)anthradithiophene based organic thin film transistor. Flex. Print. Electron. 8(2), 023001 (2023)
Zhao, Y., Wang, W., He, Z., Peng, B., Di, C.-A., Li, H.: High-performance and multifunctional organic field-effect transistors. Chin. Chem. Lett. 34(9), 108094 (2023)
He, Z., Zhang, Z., Asare-Yeboah, K., Bi, S.: Binary solvent engineering for small-molecular organic semiconductor crystallization. Mater. Adv. 4(3), 769 (2023)
Ding, L., Yu, Z.-D., Wang, X.-Y., Yao, Z.-F., Lu, Y., Yang, C.-Y., Wang, J.-Y., Pei, J.: Polymer semiconductors: synthesis, processing, and applications. Chem. Rev. 123(12), 7421 (2023)
Dimitrov, I., Tsvetanov, C.: 4.21—High molecular weight poly (ethylene oxide). In: Matyjaszewski, K., Möller, M. (eds.) Polymer science: a comprehensive reference, pp. 551–569. Elsevier, Amsterdam (2012)
Nakayama, K., Hirose, Y., Soeda, J., Yoshizumi, M., Uemura, T., Uno, M., Li, W., Kang, M.J., Yamagishi, M., Okada, Y.: Patternable solution-crystallized organic transistors with high charge carrier mobility. Adv. Mater. 23(14), 1626 (2011)
He, Z., Zhang, Z., Bi, S., Asare-Yeboah, K., Chen, J.: Ultra-low misorientation angle in small-molecule semiconductor/polyethylene oxide blends for organic thin film transistors. J. Poly. Res. 27(3), 75 (2020)
Bi, S., Li, Y., He, Z., Ouyang, Z., Guo, Q., Jiang, C.: Self-assembly diketopyrrolopyrrole-based materials and polymer blend with enhanced crystal alignment and property for organic field-effect transistors. Org. Electron. 65, 96 (2019)
He, Z., Zhang, Z., Bi, S.: Small-molecule additives for organic thin film transistors. J. Mater. Sci. Mater. Electron. 30, 20899 (2019)
Ebagninin, K.W., Benchabane, A., Bekkour, K.: Rheological characterization of poly (ethylene oxide) solutions of different molecular weights. J. Colloid Interfac. Sci. 336(1), 360 (2009)
Li, S., Qian, G., He, X., Huang, X., Lee, S.-J., Jiang, Z., Yang, Y., Wang, W.-N., Meng, D., Yu, C.: Thermal-healing of lattice defects for high-energy single-crystalline battery cathodes. Nat. Commun. 13(1), 704 (2022)
Bunn, C.: Molecular structure and the crystallinity of long-chain polymers. J. Appl. Phys. 25(7), 820 (1954)
He, Z., Zhang, Z., Asare-Yeboah, K., Bi, S.: Poly (α-methylstyrene) polymer and small-molecule semiconductor blend with reduced crystal misorientation for organic thin film transistors. J. Mater. Sci. Mater. Electron. 30, 14335 (2019)
Gu, M., Zhang, J., Wang, X., Ma, W.: Crystallization behavior of PVDF in PVDF-DMP system via thermally induced phase separation. J. Appl. Poly. Sci. 102(4), 3714 (2006)
Szymczyk, A.: Structure and properties of new polyester elastomers composed of poly (trimethylene terephthalate) and poly (ethylene oxide). Euro. Poly. J. 45(9), 2653 (2009)
Yang, Y.-F., Lin, Y.-C., Ercan, E., Chiang, Y.-C., Lin, B.-H., Chen, W.-C.: Improving the photoresponse of transistor memory using self-assembled nanostructured block copolymers as a photoactive electret. Macromolecules 55(18), 8262 (2022)
Kleemann, H., Schuenemann, C., Zakhidov, A.A., Riede, M., Lüssem, B., Leo, K.: Structural phase transition in pentacene caused by molecular doping and its effect on charge carrier mobility. Org. Electron. 13(1), 58 (2012)
He, Z., Zhang, Z., Bi, S., Asare-Yeboah, K., Chen, J., Li, D.: A facile and novel route to improve TIPS pentacene based organic thin film transistor performance with elastomer. Synth. Met. 262, 116337 (2020)
Funding
This project is financially supported by National Natural Science Foundation of China (NSFC 52374186), Dalian University of Technology (DUT23YG202 and DUT23YG126). A part of the research is conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest or known competing interests associated with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bi, S., Yao, Z., Han, X. et al. Significant Mobility Enhancement by Semicrystalline Polymers Additive for Crystallization and Charge Transport in Organic Field-effect Transistor. Electron. Mater. Lett. (2024). https://doi.org/10.1007/s13391-024-00510-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13391-024-00510-2