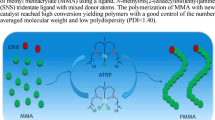
Abstract
Atom transfer radical polymerization (ATRP) has achieved widespread use in living polymerization. However, until now there has been little report that macroinitiators initiate polymerization in different catalytic systems. The preparation of bromine-terminated polymethyl methacrylate (PMMA-Br) and chlorine-terminated PMMA (PMMA-Cl) were carried out via reverse atom transfer radical polymerization (RATRP). The PMMA with halogen termination and narrow polydispersity (Mn = 12,000–15,000 g/mol, Mw/Mn = 1.1–1.2) were used as macroinitiators. The block copolymer of polymethyl methacrylate and polyacrylonitrile (PMMA-b-PAN) was prepared in different catalytic systems through normal ATRP. The analyses of the 1H NMR showed that the PMMA prepared by RATRP were end-functionalized by halogen atoms, demonstrated the activities of the PMMA macroinitiators. The molecular weight and polydispersity index (PDI) of the polymers were analyzed using gel permeation chromatography (GPC). The results indicated that the block polymers that the molecular weight of the block copolymer after chain extension has increased significantly and the molecular weight distribution is narrow (Mn = 17,000–25,000 g/mol, Mw/Mn = 1.1–1.3). The kinetics of these polymerization processes were studied as a function of monomers to the macroinitiator molar ratio. It was found that the polymerizations in different catalytic systems coincidence first-order kinetics with respect to monomers.
Graphic Abstract
In this paper, we reported that the chain extension in different catalytic systems is practicable. The PMMA-Cl and PMMA-Br were obtained in initiating systems of AIBN/FeCl3·6H2O/triphenylphosphine (PPh3) and AIBN/CuBr2/PMDETA, respectively. As the macroinitiators, the PMMA-Cl and PMMA-Br initiate the polymerization in FeCl2/PPh3 and CuBr/PMDETA catalytic systems, respectively. The molecular weight and PDI of the polymers were analyzed using GPC.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
ATRP as a new type of controlled/living polymerization has been developed rapidly, and has been widely used in the polymer molecular structure designed and the synthesis of the functional polymer materials. ATRP is a good polymerization method for preparing block copolymers [1,2,3]. The block copolymer obtained by ATRP could improve the performance, such as, thermal properties [4], mechanical properties [5], chemical resistance [6], blending performance [7], and so on.
ATRP is to be the research hotspot in the polymer field due to the advantages that extensive applicable monomers and flexible reactions [8,9,10,11,12,13]. However, in normal ATRP, the catalyst of the transition metal complex in a low oxidation state is unstable. After Matyjaszewski and Xia [14] first reported the reverse ATRP which initiated by the common radical polymerization initiators with higher oxidation state transition metal complex. The reverse ATRP reaction is carried out with the transition metal in oxidatively stable and more active [15, 16]. There are many literatures showed that the product could only be the linear homopolymers with the reverse ATRP process [17,18,19].
However, Hou et al. [20] reported that the polyacrylonitrile obtained was end-functionalized by chlorine atoms and can act as a macro-initiator for the extension polymerization. Rattanathamwat et al. [21] prepared the poly(3-hexyl thiophene)-b-poly(styrene-co-chloromethylstyrene) [P3HT-b-P(S-co-CMS)] with the CuBr/pentamethyldl-ethylenetrlamlne (PMDETA) catalytic system via ATRP. In addition, the chlorine atom of CMS has reactive functionality for ATRP and can act as an initiator or the chain transfer agent.
In this paper, we reported that the chain extension in different catalytic systems is practicable. The PMMA-Cl and PMMA-Br were obtained in initiating systems of AIBN/FeCl3·6H2O/triphenylphosphine (PPh3) and AIBN/CuBr2/PMDETA, respectively. As the macroinitiators, the PMMA-Cl and PMMA-Br initiate the polymerization in FeCl2/PPh3 and CuBr/PMDETA catalytic systems, respectively. The molecular weight and PDI of the polymers were analyzed using GPC (Scheme 1).
2 Experiment
2.1 Materials
Methyl methacrylate (MMA, Tianjin Damao Chemical Reagent Factory) and acrylonitrile (AN, Tianjin Fuchen Chemical Reagent Factory) were purified by distillation. N,N-Dimethylformamide (DMF, Tianjin Branch Miou Chemical Reagent Co., Ltd.), 2, 2′-azobis (isob-butylronitrile) (AIBN, Aldrich), triphenylphosphine (PPh3, Aldrich), N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA, Aldrich), FeCl3·6H2O (Aldrich), FeCl2 (Aldrich), CuBr (Aldrich), CuBr2 (Aldrich), and Dimethyl sulfoxide (DMSO, Aldrich) were used as received.
2.2 Synthesis of the Macroinitiators
In a typical run, the reaction flask with DMF was charged with MMA (0.1 mol) and catalyst/ligand (FeCl3·6H2O/PPh3 or CuBr2/PMDETA) (0.001 mol/0.001 mol), equipped with a magnetic stirring bar. After thorough deoxygenation by several vacuum-nitrogen cycles. Subsequently, the AIBN (0.001 mol) dissolved in the DMF was added the reaction flask. The reaction flask was placed in oil bath preheated to 90℃. After 7 h, the polymerization mixture was cooled down. Afterward, the mixture solution was precipitated in 10-fold excess of the anhydrous ethanol. After filtered, the precipitates were dried in vacuum for 24 h. Table 1 lists the results of the synthesis of macroinitiators.
2.3 The Chain Extension
In a typical polymerization, the macroinitiators, catalyst/ligand (FeCl2/PPh3 or CuBr/PMDETA) (0.001 mol/0.001 mol) and AN (0.2 mol) were added to the flask with DMF. The solutions were protected by the nitrogen. The mixture was stirred at 78 °C for 7 h under nitrogen atmosphere. Afterward, the mixture solution was precipitated in 10-fold excess of the anhydrous ethanol. After filtered, the precipitates were dried in vacuum for 24 h. Table 2 showed the specific details of the reaction condition.
2.4 Characterization
Fourier transform infrared (FTIR) spectra analysis was performed via a Fourier infrared spectroscopy Spectrum One B type (US PerkinElmer Co, Ltd). 1H NMR spectrum was conducted in DMSO at room temperature on a Bruker AV600 NMR spectrometer. Tetramethylsilane was used as an internal standard. The polymers were dissolved in DMSO as a solvent. The molecular weight and PDI of polymers were analyzed by Wyatt GPC/SEC-MALS, USA. The ratio of monomer to n-butanol was measured by Gas Chromatograph (Agilent Technologies inc, USA) to calculate monomer conversion.
3 Result and Discussion
The 1H NMR spectra of the macromolecular initiators, which included halogen atoms at end of the chain, were shown in the Fig. 2. As we all know, the RATRP is a reversible equilibrium between the radical (reactive species) and the organic macromolecular halide (dormant species). As shown in the Fig. 1a, the signals appeared at 0.74 ppm (b), 1.82 ppm (c) and 3.56 ppm (d) were assigned to –CH3, –CH2 and –CH3 proton resonance in the PMMA, respectively. The weak peak located at 1.20 (a) was attributed to the proton resonance (–CH3) in the radical initiators (AIBN). It is easy to see that there are two peaks at 4.38 ppm (e) and 0.98 ppm (f), which deviated from the chemical shift of other protons of AN unit for the electronic-attracting function of Br at the end of the chain. The Fig. 1b shows the 1H NMR spectra of the PMMA-Cl macroinitiators. There also was a weak peak at 4.45 ppm that belongs to the proton of AN at the end of the chain. The results proved that the “living” feature of the macroinitiators.
To demonstrate the presence of an active end group, the PMMA macroinitiators containing different halogen atoms at the chain-termination initiated the polymerization of AN through the normal ATRP. Figure 2a shows the GPC trace of PMMA-Br macroinitiators (Mn = 14,640, Mw/Mn = 1.122) and the corresponding block copolymer in different catalytic systems. PMMA-Br initiated the polymerization of AN in the CuBr/PMDETA catalytic system, and the molecular weight of the block copolymer obtained increases, and the polydispersity is narrow (Mn = 22,720 g/mol, Mw/Mn = 1.301). The PMMA-Br initiated the polymerization of AN in the FeCl2/PPh3 catalytic system. The molecular weight of the block copolymer also increases, and the polydispersity is narrow (Mn = 17,610 g/mol, Mw/Mn = 1.085), which indicated macroinitiators still have the polymerization activity in the different catalytic system.
The GPC trace of the PMMA-Cl macroinitiators (Mn = 14,810, Mw/Mn = 1.119) and the corresponding block copolymer in different catalytic systems were shown in the Fig. 2b. The molecular weight of block copolymer initiated by PMMA-Cl in the FeCl2/PPh3 catalytic system increased to 21,820 g/mol. However, the molecular weight of block copolymer initiated by PMMA-Cl in the CuBr/PMDETA catalytic system also increased (Mn = 25,640 g/mol, Mw/Mn = 1.166). A small amount of copper bromide in the polymerization system was regarded as a Lewis acid and increased the activity of the macroinitiators, thereby replacing the chlorine-terminated macroinitiators with bromine atoms.
The retention of the terminal groups in the PMMA-b-PAN synthesized from PMMA-Br/FeCl2/PPh3 was further analyzed by 1H NMR spectroscopy. As shown in the Fig. 3, the characteristic signals at 0.78 ppm (b), 1.90 ppm (c) and 3.58 ppm (d) were assigned to –CH3, –CH2 and –CH3 proton resonance in the main-chain, respectively. The signal at 0.97 ppm (a) was attributed to –CH3 proton resonance of the AIBN initiators. The assignments of these peaks are similar to those reported by Li et al. [17] and Khan et al. [22] In addition, the small signal (f) at 4.30 ppm was assigned to the methylene protons adjacent to the bromine atom at the chain end.
The chain end of the PMMA macroinitiators and the corresponding block copolymer in different catalytic systems were further demonstrated by the transmittance of FTIR. In the Fig. 4, the intensity of peak at 1737 cm− 1 and 1152 cm− 1 were attributed to the stretching vibration of C =O and the stretching vibration of C–O–C in the PMMA macroinitiators. The peak could be seen at 2951 cm− 1 corresponded to the stretching vibration of C–H. After the chain extension, the new peak at 2243 cn− 1 was attributed to the stretching vibration of C≡N, which demonstrate the appearance of PAN chain. Merits attention, there is an apparent peak at 710 cm− 1–650 cm− 1 which is due to the stretching vibration of C–X (X = Br, Cl), it indicated that the polymer chain is terminated with halogen atom.
The first-order kinetic curve of the ATRP reaction is regarded as the most important characteristics of a living polymerization. The kinetic plots of ln([M]0/[M]t) versus reaction time was displayed in Fig. 5. Clearly, the liner relationship of ln([M]0/[M]t) versus time in all reactions were observed, which indicated the polymerization was first order with respect to monomer concentration and the concentration of propagation radicals was constant. According to the slope, the apparent rate constant (kpapp) of the PMMA-Br and PMMA-Cl polymerization process were calculated to be 4.186 × 10− 5 s− 1 and 3.456 × 10− 5 s− 1, respectively [23]. And the rate of the polymerization catalyzed by CuBr/PMDTA and FeCl2/PPh3 decreased a lot, which is much smaller than the PMMA polymerization process. In the Fig. 5b, the kpapp for the polymerization processes of the block copolymer that catalyzed by CuBr/PMDTA were 1.958 × 10− 5 s− 1, with the PMMA-Cl as the macroinitiator, that is higher than the polymerization catalyzed by FeCl2/PPh3. The result indicated that when PMMA-Cl is used as the initiator, the rate of the polymerization catalyzed by CuBr/PMDTA is higher than the polymerization catalyzed by FeCl2/PPh3, which is in agreement with the GPC results.
4 Conclusions
The different catalytic systems were successfully used in the chain extension. Polymerization proceeded according to the mechanism of living/controlled radical polymerization, was confirmed by the analysis of the chain end. The results of FTIR and 1H NMR indicate the “living” feature. The GPC trace of the polymers and the first-order kinetic plot show that the chain extensions in different catalytic systems are successful.
References
Chen, L., Jianhua, H., Yanhui, L., Dajun, L., Jian, L., Xu, C., Qian, D.: Synthesis and self-assembly of brushshapedblock copolymer structure via ATRP and ROP. Opt. Mater. 11, 110590 (2020)
Gnanaseelan, M., Kalita, U., Janke, A., Pionteck, J., Singha, N.K.: All methacrylate block copolymer/TiO2 nanocomposite via ATRP and In-situ sol-gel process. Mater. Today Commun. 22, 100728 (2019)
Wang, Y.Z., Liu, L., Jiang, S.Y., Li, S., Lan, T.Y., Zu, L.W., Dong, S.D.: Synthesis of Modified TiO2 Nanoparticles with Polyacrylonitrile and Poly(hydroxyethyl acrylate) via ATRP. Chemistryselect. 5, 4695–4700 (2020)
Altintas, O., Josse, T., Abbasi, M., De Winter, J., Trouillet, V., Gerbaux, P., Wilhelm, M., Barner-Kowollik, C.: ATRP-based polymers with modular ligation points under thermal and thermomechanical stress. Polym. Chem. 6, 2854–2868 (2015)
Fleet, R., Dungen, E.T.A.V.D., Klumperman, B.: Novel glycopolymer brushes via ATRP: 2. Thermal and mechanical properties. Macromol. Chem. Phys. 212, 2209–2216 (2011)
Sun, L., Zhou, Y., Zhou, X., Ma, L., Wei, H.: Synthesis of a triple-responsive double hydrophilic block copolymer prodrug using a reducible RAFT–ATRP double-head agent. ACS Appl. Polym. Mater. 2, 2126–2133 (2020)
Liu, L., Chen, H., Yang, F.: Enhancing membrane performance by blending ATRP grafted PMMA–TiO or PMMA–PSBMA–TiO in PVDF. Sep. Purif. Technol. 133, 22–31 (2014)
Yamago, S., Nakamura, Y.: Recent progress in the use of photoirradiation in living radical polymerization. Polymer 54, 981–994 (2013)
Siegwart, D.J., Oh, J.K., Matyjaszewski, K.: ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 37, 18–37 (2012)
Sumerlin, B.S., Tsarevsky, N.V., Louche, G., Lee, R.Y., Matyjaszewski, K.: Highly efficient “click” functionalization of poly(3-azidopropyl methacrylate) prepared by ATRP. Macromolecules 38, 7540–7545 (2005)
Coessens, V., Matyjaszewski, K.: End group transformation of polymers prepared by ATRP, substitution to azides. J. Macromol. Sci. Part A. 36, 667–679 (1999)
Kamigaito, M., Ando, T.T., Sawamoto, M.: Metal-catalyzed living radical polymerization. Chem. Rec. 101, 3689–3746 (2001)
Ran, J., Wu, L., Zhang, Z., Xu, T.: Atom transfer radical polymerization (ATRP): a versatile and forceful tool for functional membranes. Prog. Polym. Sci. 39, 124–144 (2014)
Matyjaszewski, K., Xia, J.: Fundamentals of Atom Transfer Radical Polymerization. Wiley, Hoboken (2003)
Zheng, Y., Pan, K., Jiang, L., Zhang, J., Dan, Y.: Copper-based reverse ATRP process of styrene in mixed solvents. 43, 2557–2563 (2007)
Jianhui Xia, K.M.: Controlled/“living” radical polymerization. Homogeneous reverse atom transfer radical polymerization using AIBN as the initiator. Macromolecules 30, 7692–7696 (1997)
Li, S., Wang, Y.Z., Ma, L.Q., Zhang, X.Z., Dong, S.B., Liu, L., Zhou, X.L., Wang, C.L., Shi, Z.: Synthesis of PAN with adjustable molecular weight and low polydispersity index (PDI) value via reverse atom transfer radical polymerization. Des. Monomers Polym. 22, 180–186 (2019)
Ma, J., Chen, H., Zong, G., Wang, C., Liu, D.: FeCl3/acetic acid-mediated reverse atom transfer radical polymerization of acrylonitrile. J. Macromol. Sci. Part A. 47, 1075–1079 (2010)
Cai, J., Chen, T., Wang, G.Z., Gao, J., Ma, R., Yan, C.J.: The study on the reverse atom transfer radical polymerization of MMA catalyzed by acetylacetonate cobolt(II) complex supported by ionic liquid. Adv. Mater. Res. 476–478, 2188–2192 (2012)
Hou, C., Qu, R., Ji, C., Wang, C., Wang, C.: Synthesis of polyacrylonitrile via reverse atom transfer radial polymerization (ATRP) initiated by diethyl 2,3-dicyano-2,3-diphenylsuccinate, FeCl, and triphenylphosphine. Polym. Int. 55, 326–329 (2006)
Rattanathamwat, N., Wootthikanokkhan, J., Nimitsiriwat, N., Thanachayanont, C., Asawapirom, U.: Kinetic studies of atom transfer radical polymerisations of styrene and chloromethylstyrene with poly(3-hexyl thiophene) macroinitiator. Adv. Mater. Sci. Eng. 2015, 1–13 (2015)
Khan, M.Y., Zhou, J., Chen, X., Khan, A., Mudassir, H., Xue, Z.G., Lee, S.W., Noh, S.K.: Exploration of highly active bidentate ligands for iron (III)-catalyzed ATRP. Polymer. 90, 309–316 (2016)
Nanda, A.K., Hong, S.C., Matyjaszewski, K.: Concurrent initiation by air in the atom transfer radical polymerization of methyl methacrylate. Macromol. Chem. Phys. 204, 1151–1159 (2003)
Acknowledgements
The research was funded by the National Natural Science Foundation of China (Grant No. 21376127), the Science, Technology Department of Heilongjiang Province and the Graduate Innovation Research Project of Qiqihar University (Grant No. YJSCX2019059), and the help of the Key Laboratory of Polymeric Composite Materials of Qiqihar University in testing. Fundamental Research Funds in Heilongjiang Provincial Universities (Grant Nos. 135309110, 135409406).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, L., Dong, S. et al. The “Living” Feature of the ATRP Macroinitiators in Different Catalytic Systems. Electron. Mater. Lett. 17, 136–141 (2021). https://doi.org/10.1007/s13391-021-00268-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-021-00268-x