Abstract
Diffusion of atoms across the boundary between two bonding layers is the key for achieving excellent thermocompression Wafer on Wafer bonding. In this paper, we demonstrate a novel mechanism to increase the diffusion across the bonding interface and also shows the CMOS in-line process flow compatible Sub 100 °C Cu–Cu bonding which is devoid of Cu surface treatment prior to bonding. The stress in sputtered Cu thin films was engineered by adjusting the Argon in-let pressure in such a way that one film had a compressive stress while the other film had tensile stress. Due to this stress gradient, a nominal pressure (2 kN) and temperature (75 °C) was enough to achieve a good quality thermocompression bonding having a bond strength of 149 MPa and very low specific contact resistance of 1.5 × 10−8 Ω-cm2. These excellent mechanical and electrical properties are resultant of a high quality Cu–Cu bonding having grain growth between the Cu films across the boundary and extended throughout the bonded region as revealed by Cross-sectional Transmission Electron Microscopy. In addition, reliability assessment of Cu–Cu bonding with stress engineering was demonstrated using multiple current stressing and temperature cycling test, suggests excellent reliable bonding without electrical performance degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The ever growing demand for more functionality within a single chip needs a paradigm shift as interconnect delay in planar integration technology is becoming the major bottle neck. 3D integrated circuit (3D IC) technology is regarded as the alternative technology having several advantages such as low RC delay, high speed, improved bandwidth and heterogeneous integration [1,2,3]. Wafer to wafer integration, one of the most sought after 3D IC technologies has received a lot of attention due to its compatibility for precise alignment [4]. Cu–Cu bonding is preferable choice as it not only has excellent electrical and mechanical properties but also has an excellent electromigration resistance [5].
Research in Cu–Cu WoW bonding is focused towards making the process CMOS compatible with minimal thermal budget. Several methodologies that were adapted to achieve the same include room temperature surface activated bonding [6], surface passivation of Cu using Self assembled Monolayers [7, 8], surface passivation using ultrathin passivation layers [9,10,11,12,13,14]. All these surface passivation mechanisms needs either an additional layer or additional process step that are not CMOS compatible, which complicates the entire process flow. The role of these passivation layers is to prevent oxidation of Cu which is the main hindrance for achieving a good quality thermocompression bonding. The native oxide layer and other contaminants act as diffusion barrier and thus necessitate higher temperature and pressure for carrying the bonding.
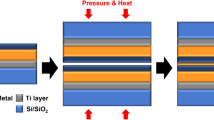
Diffusion of atoms across Cu–Cu layers is one of the key factors for thermocompression bonding. This work focuses towards developing a novel methodology for enhancing this diffusion despite the presence of native oxides. The samples can directly be bonded without any further post processing steps involved. The key idea in enhancing the diffusion across bonding layers is to create a stress gradient in the films. The concept of creating stress gradient is not new and can be achieved by varying in-line Ar pressure that is used to sputter the film. Researchers have demonstrated out of plane inductor coils using this concept [15]. This concept can be utilized to achieve a low temperature and low pressure bonding. Figure 1 shows the schematic of the concept of stress engineering.
2 Bonding Mechanism and Experimental Protocol
When a wafer having a Cu film possessing an inherent compressive stress is brought in contact with a wafer having Cu film engineering with tensile stress, the stress gradient between the Cu films enables diffusion of Cu atoms across the boundary even in the presence of native oxide and contaminants. Stress in these films can be created by varying the Ar pressure [16]. At low Ar pressures, the mean free path of the sputtered atoms tends to be larger and hence the atoms tend to pack closer thereby creating a compressive stress in the film. On the other hand, at high Ar pressures, the mean free path tends to be shorter, the sputter atoms get scattered upon contact with the substrate. Thus it creates a tensile stress in the film. Additionally, Cu surface diffusion coefficient on <111> oriented plane is faster (3–4 orders) as compared to other plane at bonding interface [17]. In order to figure out Cu plane at bonding interface, stressed Cu surfaces were investigated soon after annealing at 75 °C for 50 min under vacuum (10−5 mbar) using Glancing Angle X-ray Diffraction (GIXRD). XRD pattern (Fig. 2) confirms reflections of only pure Cu (111). Also to verify the preferential orientation count at the bonding interface, stressed Cu surfaces were further investigated using Energy Back Scatter Diffraction (EBSD) analysis. Figure 3 clearly depicts the EBSD orientation image map, which indicates extremely high orientation <111>. This is the preferred orientation of Cu plane at the bonding interface [17]. Hence, this enhanced diffusion of Cu through interface would result in lowering the bonding temperature as well as pressure. A systematic study was carried out to figure out the stress in the films as a function of Ar pressure. Then pressures having maximum differential stress were chosen for bonding at a temperature of 75 °C and a constant load of 2 kN for 50 min under vacuum of 10−5 mbar. There was no surface treatment prior to bonding.
The wafers used in this work are n-type, (100) wafers. All experiments were carried out on the 2 in. wafers. Standard RCA cleaning was performed prior to sputtering. 25 nm of Ti was deposited followed by 200 nm of Cu by using sputtering system. The Ti layer acts like an adhesive layer which provides a good adhesion for Copper and Silicon. Before actual deposition started each target was pre-sputtered for at least 20 min. The sputtering system used throughout the work is AJA Int. PHASE II system. While depositing the Copper films Argon pressure was varied for each sample. Before starting the bonding experiments, experiments were carried out to understand the effect of depositing Cu samples at various Argon pressure and to analyze the stress conditions within the Copper film. Samples were sputtered at different Ar pressures keeping the rest of the conditions such as distance between substrate and metal target, and rotation speed of substrate holder while deposition were kept constant. The deposition rate is slightly different for different pressures. Taking this into consideration, the time of deposition was varied so as to ensure that each sample has equal thickness of 200 nm of Cu. Post deposition, stress measurement was performed using wafer bow technique with the help of kSA MOS Ultra (K-Space) ultrascan instruments. Sample pairs with large stress differences within their film were selected for bonding. Samples were stored in desiccator purged with N2 when not in use. Apart from diffusion, the other important consideration is the surface roughness. To understand the impact of different Argon pressure while depositing Cu on the surface roughness of the Cu film Atomic Force Microscopy (AFM) studies were carried out. The bonding operation was performed by using AML wafer bonder. The bonding was carried out at a temperature of 75 °C and a constant load of 2 kN. Post bonding Cross-sectional Transmission Electron Microscopy (XTEM) was performed to analyze the bonded interface up to the atomic level. Mechanical characterization was carried out using Microtester (INSTRON, USA). Electrical characterization was carried out using Keithley source measurement unit. For low temperature measurements CRX-4K Cryogenic Probe Station (Lake Shore Inc.) was deployed and for high temperature measurements Karl Suss probe station was utilized.
3 Results and Discussion
3.1 Measurement of Stress in Sputter Cu Films
Stress measurements were carried using wafer bow measurement technique with the help of kSA MOS Ultra (K-Space) ultrascan instruments. This technique involves measurement of change in radius of curvature of the substrate caused by the deposition of a thin film on the wafer. This is accomplished by first measuring the wafer curvature before the film is deposited and then remeasuring the curvature after the film is deposited. The radius of curvature is measured using a laser scanner. The stress data can be extracted from the difference in the radius of curvature using the material properties of the film. In the present study samples with Cu films deposited at different Ar pressure were analysed. Figure 4a suggests the variation of stress conditions within the Cu film as the Ar pressure increases with equal step. It can be observed that at 3 mTorr of Ar pressure the stress is compressive in nature.
As the Argon pressure increases this compressive stress turns to tensile stress owing to the increase in mean free path. Cu atoms tend to displace from each other on the substrate surface resulting in a tensile stress. Beyond certain pressure, the tensile stress in between Cu atoms on the substrate surface becomes so high that they try to reduce this stress by removing some of the Cu atoms from their position and rearrange themselves by packing themselves closer, resulting in a decreased tensile stress and it traverses towards compressive domain with the Ar pressure [15]. The maximum tensile stress was observed at 12 mTorr and the maximum compressive stress was observed at 3 mTorr.
3.2 Surface Roughness Analysis
One of the most important factors of thermocompression bonding apart from the diffusion is the requirement of smooth Cu surface. Better surface roughness lowers the pressure required for carrying out the bonding. Figure 4b shows the relative surface roughness of Cu samples prepared at different Ar pressures as analysed by Atomic Force Microscopy (Bruker: ScanAsyst). The roughness increases, albeit slightly, with respect to the Ar pressure. This also can be attributed to the decrease in mean path which might result in non-uniform surface morphology.
3.3 Bond Strength Analysis
Based on the above results, two pairs combinations of samples (1) 3–12 mTorr and (2) 12–20 mTorr were chosen and bonded at 75 °C and a constant load of 2 kN for 50 min. These combinations have the maximum stress gradient. Samples were prepared by dicing the 2′′ bonded sample into 1 cm2 pieces then shear test measurement was carried out by Instron microtester, USA. The bond strength of 3–12 mTorr pair is the 149 MPa whereas the bond strength of 12–20 mTorr is only 51 MPa. This results can be explained by taking into account the effect of stress and surface roughness simultaneously. The stress gradient is maximum in the case of 3–12 mTorr pair and at the same time the overall surface roughness taking both samples into consideration, is minimum. An excellent bond strength is a resultant of the same which compared with the reported literatures [11, 12]. The other case of 12–20 mTorr is interesting. Though the stress gradient in this case is more or less the same as that of 3–12 mTorr, increased surface roughness has resulted in a relatively poor bonding. This conclusively proves that both diffusion and surface roughness are critical for achieving low temperature and low pressure bonding. We have carried out multiple iterations of Cu–Cu bonding using this technique and reproducibility of bonding is not a concern at all.
3.4 Interface Qualification
In order to figure out uneven voids at the bonded pair of 3–12 mTorr, C-Mode Scanning Acoustic Microscope (SONOSCAN, UK) analysis was carried out using 100 MHz transducer. The inherent dark region represents reasonable good bonding as shown in Fig. 5 and white spots manifest by tiny dust particles present on the sample surfaces prior to bonding.
3.5 Cross-sectional TEM Analysis
Cross-sectional Transmission Electron Microscopy (XTEM) is an ideal way of studying and understanding the morphology of the bonded interface. Figure 6a depicts the XTEM image of the bonded interface of 3–12 mTorr pair and Fig. 6b represents the high resolution imaging of the marked region of Fig. 6a. The key inferences that can drawn out from XTEM images include the absence of clear bonded interface, the absence of dislocations/voids, the formation and growth of grain boundaries at the interface (Fig. 6b) and the presence of Cu grains that extend throughout the bonded structure are clear indication of excellent bonding.
3.6 Electrical Characterization of the Bonded Structure
In order to carry out electrical characterization, the structure shown in Fig. 7a was fabricated. The bond pad structures were created in the first wafer and the bond pads were opened for probing by selectively etching the other wafer in XeF2 system till the bond pads visible. The metal contact area of the interface is 40 µm × 40 µm. The average specific contact resistance have been found to be 1.5 × 10−8 Ω-cm2 (Fig. 7b), which is superior as compared to other reported literatures are summarized in Table 1. We have carried out multiple iterations of specific contact resistance measurement of Cu–Cu bonded samples with stress variation and results are consistence. Post bonding resistivity measurements were carried out five times in order to ensure the accuracy of the results. There is no distinct change in the specific contact resistance which concludes the Cu–Cu bonding with stress engineering is stable, and high quality.
3.7 Reliability Investigation of the Bonded Structure
Reliability assessments of the bonded structure was confirmed using multiple current stressing, and temperature cycling test. Fabricated bonded structure was tested by applying 1000 cycles of current sweeping between − 0.1 and + 0.1 A at room temperature. The total cycle time was about 65 min. There is no significant degradation in the specific contact resistance (Fig. 8) of the bonded structure using stress engineering, which clearly indicates Cu–Cu bonding is high quality and reliable.
In addition, thermal stability of the bonded structure was investigated using temperature cycling test for 1000 cycles of current sweeping from − 0.1 and + 0.1 A under the temperature range of − 55 to + 125 °C (as per JESD22-A104B standard). Figure 9 clearly suggests, there is no distinct change in specific contact resistance even after 1000 cycles of temperature treatment which confirms Cu–Cu bonding using stress engineering is of high quality, stable and reliable.
4 Conclusion
Stress generated within the Cu thin films prepared for bonding has tremendous impact on the bonding temperature required for thermocompression WoW bonding. Large stress gradient accompanied by surface smoothness can reduce the required bonding temperature and pressure dramatically. In this work concept of stress tailoring was successfully demonstrated which reduces the bonding temperature and pressure by an order of magnitude. Subsequent characterization of bonded interface and very low specific contact resistance of 1.5 × 10−8 Ω-cm2 clearly indicates that the bonding is stable and robust. This process can very well be incorporated with in-line CMOS process flow. This methodology will go a long way in converting visionary 3D IC technology into reality.
References
Im, S., Banerjee, K.: Full chip thermal analysis of planar (2-D) and vertically integrated (3-D) high performance ICs. In: Electron Devices Meeting, pp. 727–730. IEEE (2000)
Xie, Y.: Processor architecture design using 3D integration technology. In: 23rd International Conference on VLSI Design, pp. 446–451. IEEE (2010)
Xu, Z., Lu, J.Q.: High-speed design and broadband modeling of through-strata-vias (TSVs) in 3D integration. IEEE Trans. Compon. Packag. Manuf. Technol. 1(2), 154–162 (2011)
Ko, C.T., Chen, K.N.: Wafer-level bonding/stacking technology for 3D integration. Microelectron. Reliab. 50(4), 481–488 (2010)
Save, D., Braud, F., Torres, J., Binder, F., Muller, C., Weidner, J.O., Hasse, W.: Electromigration resistance of copper interconnects. Microelectron. Eng. 33(1), 75–84 (1997)
Takagi, H., Kikuchi, K., Maeda, R., Chung, T.R., Suga, T.: Surface activated bonding of silicon wafers at room temperature. Appl. Phys. Lett. 68(16), 2222–2224 (1996)
Tan, C.S., Lim, D.F., Singh, S.G., Goulet, S.K., Bergkvist, M.: Cu–Cu diffusion bonding enhancement at low temperature by surface passivation using self-assembled monolayer of alkane-thiol. Appl. Phys. Lett. 95(19), 192108 (2009)
Ghosh, T., Krushnamurthy, K., Panigrahi, A.K., Dutta, A., Subrahmanyam, C., Vanjari, S.R.K., Singh, S.G.: Facile non thermal plasma based desorption of self assembled monolayers for achieving low temperature and low pressure Cu–Cu thermo-compression bonding. RSC Adv. 5(125), 103643–103648 (2015)
Huang, Y.P., Chien, Y.S., Tzeng, R.N., Shy, M.S., Lin, T.H., Chen, K.H., Chiu, C.T., Chiou, J.C., Chuang, C.T., Hwang, W., Tong, H.M., Chen, K.N.: Novel Cu-to-Cu bonding with Ti passivation at 180 °C in 3-D integration. IEEE Electron Device Lett. 34(12), 1551–1553 (2013)
Panigrahi, A.K., Bonam, S., Ghosh, T., Singh, S.G., Vanjari, S.R.K.: Ultra-thin Ti passivation mediated breakthrough in high quality Cu-Cu bonding at low temperature and pressure. Mater. Lett. 169, 269–272 (2016)
Huang, Y.P., Chien, Y.S., Tzeng, R.N., Chen, K.N.: Demonstration and electrical performance of Cu–Cu bonding at 150 °C with Pd passivation. IEEE Trans. Electron Devices 62(8), 2587–2592 (2015)
Panigrahi, A.K., Bonam, S., Ghosh, T., Vanjari, S.R.K., Singh, S.G.: High quality fine-pitch Cu–Cu wafer-on-wafer bonding with optimized Ti passivation at 160 °C. In: IEEE 66th Electronic Components and Technology Conference (ECTC), pp. 1791–1796 (2016)
Panigrahi, A.K., Ghosh, T., Vanjari, S.R.K., Singh, S.G.: Oxidation resistive, CMOS compatible copper based alloy ultrathin films as a superior passivation mechanism for achieving 150 °C Cu–Cu wafer on wafer thermocompression bonding. IEEE Trans. Electron Devices 64, 1239–1245 (2017)
Panigrahi, A.K., Ghosh, T., Vanjari, S.R.K., Singh, S.G.: Demonstration of Sub 150 °C Cu–Cu thermocompression bonding for 3D IC applications, utilizing an ultra-thin layer of Manganin alloy as an effective surface passivation layer. Mater. Lett. 194, 86–89 (2017)
Chua, C.L., Fork, D.K., Van Schuylenbergh, K., Lu, J.P.: Out-of-plane high-Q inductors on low-resistance silicon. J. Microelectromech. Syst. 12(6), 989–995 (2003)
Perry, J.L.: Effects of sputter deposition parameters on stress in tantalum films with applications to chemical mechanical planarization of copper (2014)
Agrawal, P.M., Rice, B.M., Thompson, D.L.: Predicting trends in rate parameters for self-diffusion on FCC metal surfaces. Surf. Sci. 515(1), 21–35 (2002)
Peng, L., Li, H.Y., Lim, D.F., Gao, S., Tan, C.S.: Thermal reliability of fine pitch Cu–Cu bonding with self assembled monolayer (SAM) passivation for Wafer-on-Wafer 3D-Stacking. In: IEEE 61st Electronic Components and Technology Conference (ECTC), pp. 22–26 (2011)
Enquist, P., Fountain, G., Petteway, C., Hollingsworth, A., Grady, H.: Low cost of ownership scalable copper direct bond interconnect 3D IC technology for three dimensional integrated circuit applications. In: IEEE International 3D Systems Integration Conference, pp. 1–6 (2009)
Beilliard, Y., Coudrain, P., Di Cioccio, L., Moreau, S., Sanchez, L., Montmayeul, B., Signamarcheix, T., Estevez, R., Parry, G.: Chip to wafer copper direct bonding electrical characterization and thermal cycling. In: IEEE 3D Systems Integration Conference (3DIC), pp. 1–7 (2013)
Acknowledgements
Authors are grateful for funding support from the Department of Electronics and Information Technology (DietY), Govt. of India and also characterization facilities through Indian Nano electronics User Program (INUP) program from Indian Institute of Technology-Bombay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Asisa Kumar Panigrahi and Tamal Ghosh have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Panigrahi, A.K., Ghosh, T., Kumar, C.H. et al. Direct, CMOS In-Line Process Flow Compatible, Sub 100 °C Cu–Cu Thermocompression Bonding Using Stress Engineering. Electron. Mater. Lett. 14, 328–335 (2018). https://doi.org/10.1007/s13391-018-0037-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-018-0037-y