Abstract
The present investigation proposes an innovative hybrid energy system based on solar energy equipped with a parabolic trough collector, a supercritical CO2 Brayton cycle (SCBC), a recuperative organic Rankine cycle (RORC), a proton exchange membrane electrolyzer (PEME), and a two-tank direct thermal energy storage system. To ensure the stable operation of the system despite fluctuations in the amount of sunlight received during the day, the mass flow rate (MFR) of the heat transfer fluid in the solar collector is regulated to maintain a consistent inlet temperature for the SCBC and RORC. Additionally, the MFR of the HTF from the hot tank to the bottoming cycles also remains constant. This approach allows the entire system to operate around the clock and under stable conditions. The energy and exergy performance of the entire system is evaluated under varying solar irradiance and ambient temperature over a 24-h period. The results revealed that the system could generate 1.5 MW of net power, 4.47 kg of hydrogen per day, and 18.48 kg/s of hot water with a total energy efficiency of 51.30% and exergy efficiency of 55.7%. The solar collector possessed the lowest exergy efficiency of 33.89% due to a high exergy destruction rate. The PEME possessed maximum energy efficiency of 58.85%, followed by RORC and SCBC with 26.38% and 14.84%, respectively. It is concluded the proposed system can provide a reliable and sustainable supply of electricity, hydrogen, and hot water, demonstrating a promising and viable poly-generation technology for both grid-connected and off-grid applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solar energy is a renewable and sustainable source of energy that has the potential to meet the world’s growing energy demands. It is an abundant and clean source of energy that can be harnessed to produce power, heat, and light. The advantages of solar energy are numerous, reduced greenhouse gas emissions, energy independence, and cost savings [1]. It is an environmentally friendly alternative to traditional energy sources like fossil fuels. An excellent potential to promote energy independence makes it more attractive. By harnessing the power of the sun, individuals and communities can reduce their reliance on traditional sources of energy. This leads to reducing the impact of volatile energy prices and promoting greater energy security [2]. While the initial investment in solar technologies can be high, the long-term savings on electricity bills can be substantial. Furthermore, many governments offer incentives and subsidies to encourage the adoption of solar energy [3].
There are several types of solar technology, including solar photovoltaic (PV) cells, solar thermal systems, and concentrated solar power (CSP) [4,5,6]. CSP systems have emerged as a promising technology for generating electricity from renewable sources. Unlike PV solar panels, which convert sunlight directly into electricity, CSP systems concentrate solar energy to produce high temperatures that can be employed to generate steam to power a turbine and produce electricity [7]. CSP systems have the potential to provide reliable and dispatchable power, making them a beneficial technology for integrating high levels of renewable energy into the grid. Although solar radiation and energy demand tend to peak around the very same time of day, CSP systems are subject to short-term fluctuations on cloudy days. They are incapable of producing electricity at night without the benefit of thermal energy storage (TES) or a backup system (BS) that enables stable operation [8].
There are several different kinds of CSP systems, each with its own unique design and operating principles: parabolic trough collectors (PTC) [9], solar power towers [10], dish/Stirling systems [11], and linear Fresnel reflector [12]. PTCs are the most common type of CSPs that are widely utilized for large-scale power production. A PTC system consists of long, curved mirrors arranged in a trough shape that concentrate sunlight onto a receiver tube running along the focal line. The tube contains a heat transfer fluid (HTF), such as oil or molten salt, which absorbs the concentrated solar energy and carries it to a heat exchanger to produce high-temperature steam. The steam is then used to drive a turbine and generate electricity. PTCs are favored for their relatively low cost, high efficiency, and proven track record. Parabolic trough collectors are also capable of operating at temperatures up to 400 °C, which makes them appropriate for operation in combined heat and power (CHP) coupled systems [13]. Fanaee et al. [14] studied thermal-fluid modeling of a coil filled with alumina nanofluid, examining constant and variable solar heat fluxes using a parabolic concentrator in a specific location during spring. The numerical model, utilizing the finite element method with the LU algorithm and MUMPS solver, shows good agreement with experimental data in porous media. Porosity enhances heat transfer, increasing solar heat transfer to the nanofluid. Variable solar heat flux leads to higher coil temperatures compared to constant fluxes due to enhanced heat exchange with the nanofluid. In a similar study, Rezapour et al. [15] investigated the impact of fluid-thermal parameters, including porosity and permeability, in porous media for solar energy applications. The research demonstrated good agreement between the model and experimental data for normalized temperature and highlights improved heat transfer and solar energy absorbance in porous collectors, albeit with increased pressure drop.
There are various thermodynamic cycles that can be coupled with PTCs, including the Rankine cycle, the Organic Rankine cycle (ORC), the Brayton cycle, and the Kalina cycle. Because of its capability to operate effectively with low-temperature energy sources, plainness, financial appeal, and excellent performance, ORC has gained much attention for small-medium scale power generation systems. Accordingly, peacock et al. [16] studied the possibility of combining spectral-beam-splitting, hybrid photovoltaic-thermal (PVT)-PTC, and ORC technologies. The combined system provided simultaneous power and hot water for buildings. Results indicated that ORC utilization improved the power production of the system by up to 60% and decreased the levelized cost of energy by down to 25%. It was also demonstrated that approximately 24% and 31% of the incoming solar energy is transformed into usable power and heat. Javed et al. [17] conducted the energy and economic analysis of various types of PTC-based ORC configurations, named basic, recuperative, and regenerative. Four different organic working fluids (toluene, nonane, decane, and dodecane) were investigated in the proposed configurations. The results illustrated that the regenerative ORC operating by toluene generated a peak power of 1364 kW, representing an approximate 36% and 25% increase over the basic and recuperative ORC (RORC) systems, respectively. In addition, regenerative with toluene shows the most efficient configuration by 37% efficiency and 211 $/hour operating cost. A novel tri-generation cycle using solar energy and Therminol 66 in parabolic thermal solar collectors was investigated by Fanaee et al. [18]. The collectors transfer solar energy to an ORC with various working fluids. The study explored different fluids' impact on the system's performance. The cycle exhibits a power coefficient of 32.72 and an exergy factor of 23.83%. Economic analysis reveals cost distributions, while increasing solar collector temperature reduces efficiency but marginally affects the total cost.
In order to provide the 1000 kW power demand and 300 kW cold-water demand of a small plant located in Brazil, the implementation of a hybrid energy system comprising a PTC, a regenerative ORC, a biomass burner, and an absorption cooling system was investigated by Morais et al. [19]. The results revealed that the solar field shares the most prominent rate of exergy destruction related to the system, following by the biomass burner and the absorption system. In comparison with others, ORC produces relatively low irreversibility. However, the ORC’s rate of exergy destruction mainly depended on the regenerator and turbine, as well as the working fluid that was selected. A hybrid system driven by PTC combined with ORC and a diffusion absorption refrigeration (DAR) cooling system was proposed by Mousavi et al. [20]. According to the exergy analysis, the PTC field was responsible for the highest rate of exergy destruction in the system, which amounted to 31.80 kW out of a total of 67.89 kW. In addition, the overall system and PTC exergy efficiencies were determined to be around 67% and 55%, respectively. Besides, the power demand of 10 kW and cooling demand of 44 kW were acquired by employing 80 kW and 364 kW of heat duty in the DAR and ORC system, respectively.
Three different configurations of PTC-based ORC, called basic, single-stage regenerative, and double-stage regenerative, were investigated by Kara [21]. These configurations were evaluated in terms of given assumptions and similar parametric studies. The results demonstrated that the total system exergy efficiencies for the basic, the single-stage regenerative, and the double-stage regenerative were computed around 11, 13, and 14%, respectively. Furthermore, concerning the exergy efficiency of the ORC system, it was calculated at around 43, 50, and 52%, respectively. Bellos et al. [22] explored the integration of an ORC with a PTC and wasted heat-driven system to enhance total power production. Toluene, cyclohexane, MDM, and n-pentane were considered as four working fluids of ORC, and the best performance of the system was achieved by Toluene. The outcomes exhibited that the power generated by using Toluene could vary between 479 and 845 kW, with corresponding system efficiencies ranging from around 11 to 20%. Yang et al. [23] proposed a new operation method to ensure consistent power production of 1MW from a round-the-clock operating ORC power system based on PTC technology. The system utilized a thermal storage unit consisting of two tanks for the purpose of heat storage. By implementing a recuperative ORC system using toluene, the system's maximum energy efficiency was obtained around 18%. Taner [24] compared PEM fuel cell systems, specifically focusing on the cathodic plate, between a previous experimental setup and a newly developed nano-design manufacturing for nano/microscale fluids fueled by hydrogen gas. The research aims to showcase the improved efficiency and design of the fuel cell compared to the experimental commercial version. Results indicate energy efficiency of 72.4% and exergy efficiency of 85.22% for the PEMFC under specific pressure and flow rate conditions. The same author investigated the performance of a PEM fuel cell, focusing on pressure and voltage parameters. Using an open cathodic plate fuel cell, the research evaluates the impact on PEM fuel cell performance. The study measured PEM fuel cell efficiency concerning operating pressure and voltage parameters, resulting in energy and exergy efficiencies of 47.6% and 50.4%, respectively.
The Brayton cycle has several advantages over other thermodynamic cycles, such as the capability to operate at high temperatures and pressures, which results in high thermal efficiencies. Additionally, the Brayton cycle has a more straightforward design than other cycles, making it easier to maintain and operate. These advantages have led to the widespread use of the Brayton cycle in many energy applications, such as CSP systems. Accordingly, a thermo-economic analysis regarding a hybrid central tower system combined with the Brayton cycle was performed by Merchán et al. [25]. The results represented that the levelized cost of energy (LCOE) values predicted were around 158 $/MWh. In addition, they concluded that the turbine inlet temperature and solar receiver aperture size were selected as crucial parameters affecting LCOE. Performance comparison of an integrated system including different supercritical CO2 Brayton cycle (SCBC) configurations and the heliostat field, as well as the solar particle receiver system, was conducted by Chen et al. [26]. The proposed system contains two storage tanks, and the effects of hot particles and ambient temperatures on cycle off-design performance were evaluated. They found that variations in the ambient temperature have a more significant impact on the off-design performance of the system compared to the typical variations in the temperature of the HTF. A receiver tower and heliostat field-driven system integrated with thermal energy storage and SCBC was assessed numerically by Khatoon et al. [27]. The findings revealed that the efficiency of the 24-h stable-operated proposed system was around 45%. Moreover, the calculated power generated was about 37 MW and 39 MW, employing regenerative and recompression cycles, respectively. Taner et al. [28] studied the use of Cr-C coated SS304 bipolar plates and a chemical solution (urea, ammonia, methyl alcohol) in the electrolyzer to enhance hydrogen production efficiency and cost-effectiveness. Additionally, super-strong magnets on the electrolysis cells' outer surface were employed to improve performance. As a result, the system achieved a hydrogen production rate of 6 m3 h−1 (1 MW) with a simple payback period of 2.32 years.
Naqvi et al. [29] focused on hydrogen production using an alkaline electrolyzer and includes a techno-economic and enviro-economic analysis. The prototype of the alkaline electrolyzer was developed with optimization for hydrogen production. It utilizes a unique chemical mixture comprising ammonia, ethyl alcohol, urea, and deionized/distilled water. The results showcase a model study that highlights the annual profit and simple payback period of the prototype alkaline electrolyzer system, emphasizing its potential for oxyhydrogen gas production through water electrolysis. The thermodynamic analysis of an integrated solar-based system containing a PTC, a transcritical CO2 cycle, an SCBC, and a CO2-driven cascade refrigeration system to generate hydrogen and poly-generation purposes was accomplished by Bamisile et al. [30]. It was incorporating a steam cycle and a PTC, which operates by potassium as a working fluid, results in an improvement in the overall energy and exergy efficiencies of the comprehensive system up to around 42% and 27%, respectively. Table summarizes the main features and contributions of prior studies, as well as the current study. It offers a concise overview of the critical characteristics of these studies, highlighting what sets them apart from the present paper.
To the best knowledge of authors and the presented literature review, multiple investigators focused on employing CSP systems combined with power cycles for the purpose of useable productions such as electric power, hot water, and hydrogen. Nevertheless, prior studies have given little attention to the possibility of achieving all the beneficial production mentioned earlier through a single hybrid system that combines CSP and power cycles. Hence, the novelty of this study lies in the introduction of a poly-generation system that combines CSP technology with power cycles to simultaneously produce electricity, hydrogen, and hot water. Prior research has not extensively explored this integrated approach, making this study unique and innovative in its contribution to the field. In summary, the study is motivated by the desire to advance the field by introducing a novel and integrated approach to solar energy utilization that can provide multiple forms of valuable output simultaneously. The essential contributions of this paper are as the following: 1 A numerical-based analysis is presented to evaluate the functionality of the solar-based system with around-the-clock production of power, hydrogen, and hot water. 2 The first and second laws of thermodynamics analysis regarding the entire system comprising a PTC, a supercritical CO2 Brayton cycle (SCBC), a recuperative organic Rankine cycle (RORC), and a proton exchange membrane electrolyzer (PEME) are introduced. 3 A parametric study of variables affecting the total system performance is provided. The present article is structured in the following manner: In Sect. 2, there is a detailed explanation of the proposed hybrid system powered by solar energy and utilizing a thermal storage system. The system’s mathematical modeling and thermodynamic analysis are explained in Sect. 3. The main findings and results are scrutinized in Sect. 4. Section 5 summarizes the substantial outcomes and conclusions drawn from this research.
2 System Description
Typically, the hours of maximum solar radiation align with the hours of minimal electricity demand on the power grid. Without the thermal storage unit, a solar-driven integrated system must operate under off-design conditions for a significant portion of the time. As a consequence, the power cycle and the entire system suffer from low energy efficiency. To address this issue, thermal energy storage (TES) systems are often employed. This helps to improve the profitability of solar power plants by allowing them to store excess thermal energy during peak solar hours and use it during periods of high electricity demand. By implementing a TES system, a solar power plant can generate electricity continuously and operate at its optimal design conditions, which helps to maintain high levels of efficiency [31].
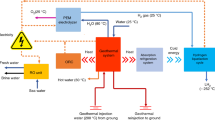
TES technology operates as a buffer between the CSP system and the power generation unit, providing flexibility to the power system. TES technologies can be categorized into different types, including sensible heat storage [32], latent heat storage [33], and thermochemical energy storage [34]. Additionally, they can be classified as either direct or indirect according to the type of heat transfer fluid and storage medium used [35]. The architecture of the proposed hybrid poly-generation system comprising the two-tank energy storage system is demonstrated in Fig. 1. The system employs solar energy via a parabolic trough collector (PTC) for the generation of electricity, hot water, and hydrogen for a residential society. The two-tank TES system, classified as a direct and sensible thermal energy storage, is selected for round-the-clock production.
The present research involves an integrated system that comprises five subsystems: a solar energy collector subsystem, a Brayton power cycle subsystem, a thermal energy storage subsystem, an ORC power cycle subsystem, and an electrolyzer hydrogen production subsystem. A detailed description of each subsystem will be provided in the following. The PTC is selected as the solar energy collector because of its capacity to effectively heat the heat transfer fluid (HTF) to reasonably high temperatures. Accordingly, PTCs have achieved a high degree of commercial development, making them a reliable choice [36].
For the study, it is supposed that the HTF is evenly distributed within the hot or cold storage tanks and that the temperatures in these tanks remain constant at Thot and Tcold, respectively. Consequently, the HTF’s temperatures leaving the hot and cold tanks are maintained at the respective tanks’ temperatures. The HTF leaves the cold tank at a temperature of Tcold and a mass flow rate (MFR) of \({\dot{m}}_{c}\) (state PTC4). The thermal oil then passes through the solar collectors, which take in heat (state PTC5). As the HTF passes through the solar collectors, its temperature rises to Thot, which is equal to the hot tank’s temperature (state PTC1). The HTF leaves the hot tank at a steady temperature of Thot and a constant MFR of \({\dot{m}}_{HTF}\). After exiting the hot tank, it flows into the evaporator 1, transferring energy to the Brayton cycle’s working fluid (state PTC2). At the temperature of Tcold, the HTF leaves evaporator 1 and goes back to the cold tank to finish the cycle of heat exchange (state PTC3). It must be stated that the MFR of the \({\dot{m}}_{HTF}\) is different from the \({\dot{m}}_{c}\). The \({\dot{m}}_{c}\) is adjusted depending on the amount of solar irradiation in order to maintain constant temperatures of the hot and cold tanks. However, the \({\dot{m}}_{HTF}\) remains unchanged to ensure that the bottoming power and hydrogen production units operate at their designed conditions. Pump 1, which circulates the HTF between the hot and cold tanks, is not included in the calculations because its energy requirements are minimal compared to the power generated by the power cycle subsystems.
The hybrid system being proposed employs the combination of two power cycles, namely the supercritical CO2 Brayton cycle (SCBC) and the recuperative ORC (RORC), so that the heat absorbed in evaporator 1 is employed to run them. The SCBC subsystem includes evaporator 1, turbine 1, and compressor. The RORC subsystem contains evaporator 2 (in common by SCBC), turbine 2, recuperator, condenser, and pump 2. CO2 is considered safe and environmentally friendly due to its characteristics of being neither toxic nor flammable. It has an ozone depletion potential (ODP) of 0 and a global warming potential (GWP) of 1, which means it does not contribute to the depletion of the ozone layer or the exacerbation of global warming [37]. While it is not considered as environmentally friendly as some other compounds like carbon dioxide, toluene does have some properties that can be beneficial from an environmental perspective. One of the main advantages of toluene is that it has a relatively low level of toxicity compared to other solvents. This means that it is less harmful to human health and the environment when it is used or released into the air or water. Additionally, toluene has a relatively low level of reactivity, which means that it does not readily react with other chemicals to form harmful byproducts. Another advantage of toluene is that it has a relatively low level of volatility, which means that it does not quickly evaporate into the air. This can help to reduce the amount of emissions that are released into the atmosphere, which can help to improve air quality [38]. Table 1 represents the essential properties of the working fluids being examined, including their critical parameters.
The T-s diagram displays the thermodynamic states in both the SCBC and RORC operational points, as represented in Fig. 2. Through process PTC2-PTC3, the useful heat collected by the solar collector is transferred to the working fluid of SCBC, CO2 (process 2–3). The CO2 stream exiting evaporator 1 enters turbine 1, where it expands and generates electric power (process 3–4). The expanded stream then flows to evaporator 2 to heat the working fluid of RORC, toluene (process 4–1). Then, the pumping operation occurs, and through the process 1–2, the pressure of CO2 is risen. Meanwhile, the heat exploited by CO2 in evaporator 1 is utilized to generate the super-heated toluene at the state of 5 (process 10–5). The super-heated vapor expands in turbine 2 and produces power (process 5–6). Through the process 6–7, the toluene is cooled down, and then, the saturated vapor is condensed in the condenser (process 7–8). Later, during the process 8–9, pump 2 is used to compress the saturated liquid toluene into a high-pressure subcooled liquid. Eventually, in order to finish the cycle, the high-pressure liquid that has been subcooled (state point 9) is heated up by flowing into the recuperator and transforming into high-pressure saturated liquid (process 9–10).
A portion of the electricity produced through the power cycles supplies the proton exchange membrane electrolyzer electrical energy. The condenser inlet water (state 11) is heated by the condensation process of RORC (process 7–8) and then stored in a tank (state 12). After that, a fraction of the hot water flows to the PEM electrolyzer (state 14), and electric power is used with the help of water-splitting technology to electrolyze water and generate hydrogen (state 16) and oxygen (state 15). The rest of the hot water as well as the PEME’s leaving water is utilized by the residential community. The remaining part of the generated power is inverted by an electric power inverter and then used by the residential community.
Overall, the proposed poly-generation system relies on solar energy to drive the SCBC and RORC subsystems. The power cycles operate as sustainable power production units that can meet the power demands of both the proton exchange membrane electrolyzer and residential communities. The condensation process of the RORC produces hot water, which is supplied to the PEME and residential homes. Additionally, the PEME is responsible for producing hydrogen and oxygen, which can be stored in separate tanks or sold as needed.
3 Modeling and Thermodynamic Analysis
3.1 Assumptions and Meteorological Data
The thermodynamic evaluation of the poly-generation hybrid system based on PTC that produces hot water, hydrogen, and electric power is stated in this section. The technical feasibility of the proposed system is assessed by employing energy and exergy analyses. To facilitate the thermodynamic analysis, some reasonable and conceptually accurate assumptions are taken into consideration, as listed below:
-
The analysis is conducted under the steady-state conditions.
-
Both the ambient temperature and DNI remain constant during each hourly interval, and a day is divided into 24-time intervals.
-
The nominal electric power of the integrated system is set to be 1.5 MW, and the proposed system is aimed to kept fix this output for the whole day.
-
The tubes and heat exchangers had no significant pressure drop or heat loss.
-
Since it comprised a tiny proportion of the overall input heat, the thermal storage tanks' heat loss is neglected.
-
Pressure drops through the pipelines are ignored.
-
Heat losses through pumps, compressor, and turbines are not considered.
The solar irradiation data obtained on January 1 in Babolsar, located in the north of Iran at coordinates 36° 42′ 3″ N, 52° 38′ 53″ E, are chosen as a basis for developing the poly-generation system. The solar radiation that reaches the Earth's surface is composed of two types: direct and diffuse radiation. Out of these, only direct radiation, which is also known as beam radiation or direct normal irradiance (DNI), can be utilized by PTCs. In this study, the solar data is provided by the Meteonorm software [39], and Fig. 3 represents the ambient temperature and DNI distributions over the course of the day that is being considered. It should be stated that the maximum DNI and ambient temperature during the day were determined as 953 W/m2 and 294.4 K at 2:00 PM, respectively.
3.2 Parabolic Trough Collector (PTC)
The overall amount of solar energy that is available for the hybrid system can be quantified by measuring the intensity of beam irradiation (Gb) or DNI in (W/m2) that is directed onto the total solar collector area (Ac) in (m2) [40]:
The beneficial thermal energy that the HTF absorbs from the PTC is computed using the following equation:
Here, Tin and Tout are the HTF temperatures at the inlet and outlet of the PTC, respectively, and \({c}_{p}^{HTF}\) is the specific heat capacity of the HTF. Moreover, the variable mass flow rate of the HTF is denoted by \(\dot{{m}_{c}}\), and it is adjusted to maintain a balance between the Thot and Tout, as well as between the Tin and Tcold. In order to investigate the heat loss for the PTC subsystem, an energy balance must be performed. Thus, the energy balance equation of a solar collector is described as [41]:
where \(\dot{{Q}_{\mathrm{L}}}\) is the PTC’s heat loss energy. The PTC’s energy efficiency is calculated by:
There are several different types of PTCs available in the market, each with its own unique design and set of features. Some of the most common types of PTCs include Luz collectors, EuroTrough collectors, Solar Genix collectors, etc. [42]. In the present study, because of the diminished complexity and structural weight of the collector, which leads to a significant cost decrement, EuroTrough ET-150 is considered [43, 44]. As suggested by Blanco et al. [45], the collector’s efficiency is determined by:
Here, the Tamb is specified as the ambient temperature, which is a crucial parameter for the collector’s performance. Since ambient temperature and solar radiation fluctuate in 24 h, the beneficial energy that is taken in by the thermal oil varies over the day. Hourly data for ambient temperature and DNI are gathered, and the collector’s energy conservation equation is formulated as a summation over time:
where the \(\tau \) is the time interval and is equal to 1. A particular mixture of diphenyl oxide and biphenyl is commonly employed as the HTF in the thermal oil loop of PTC and TES subsystems. This mixture has a specific composition, with mass fractions of 73.5% and 26.5% for diphenyl oxide and biphenyl, respectively. The reason for selecting this particular mixture as the HTF is that it can be used for temperatures up to 400 °C, making it suitable for use in high-temperature applications such as PTCs [46]. In order to calculate the thermal oil’s mass flow rate, it is necessary to know its specific heat capacity, cp. Nevertheless, the cp of the thermal oil mixture is unspecified as it varies depending on the temperature. Hence, the specific heat capacity of the HTF is calculated by [47]:
According to [48], the exergy rate of the solar heat radiation absorbed by the collector is assessed by:
where Ts denotes the sun temperature, 5777 (K). The net exergy rate absorbed from the solar collector is determined by [49]:
The exergy rate of the solar collector due to the thermal losses is characterized by Eq. 10:
where Tm is the logistic temperature of the fluid and is computed by Eq. (11):
Ultimately, the solar collector’s exergy balance equation and its exergy efficiency are determined, respectively, as the following [50]:
3.3 Thermal Energy Storage (TES) System
The thermal energy storage (TES) system being considered in this study is a direct storage two-tank unit, which is made of graphite. Graphite is prized for its ability to withstand extremely high temperatures, making it suitable for applications where heat retention and release are critical, such as in advanced energy storage systems. This system is used to separate the hot and cold streams of the HTF in a discrete manner. The temperatures of the Thot and Tcold can be assumed to remain fixed since the thermal oil entering the tanks is at the same temperature throughout the process and is not varying during the day. The HTF temperature entering evaporator 1 (TPTC2) is equal to both Thot and Tout, while the HTF temperature leaving evaporator 1 (TPTC3) is equal to Tcold and Tin. To ensure that the power cycles operate under the desired conditions, it is essential to maintain a constant mass flow rate of the HTF leaving the hot tank. The thermal storage system must supply sufficient HTF for a period of one day. In other words, the system needs to be designed to store enough HTF to meet the thermal energy requirements for a full day. Accordingly, the mass balance in the thermal storage tank is expressed by the following equation [23]:
3.4 Power Cycles
The power cycles considered in this study are the supercritical CO2 Brayton cycle (SCBC) and the recuperator organic Rankine cycle (RORC). In the analysis of these cycles, each individual component is considered a separate control volume and is assumed to be operating under steady-state conditions. Therefore, the equations for energy, exergy, and mass balance can be formulated for each component [51]:
in which, the exergy transferred by heat at a particular temperature, T, is represented by the equation \({\dot{E}}_{Q}=\dot{Q}\cdot (1-\frac{{T}_{0}}{T})\). e and i subscripts refer to the control volume’s outlet and inlet streams. The exergy destruction rate of a component is denoted by \({\dot{E}}_{D}\). In thermodynamics, there are two primary types of exergy: thermo-mechanical exergy and chemical exergy. Thermo-mechanical exergy (exth) comprises three elements: physical exergy (exph), potential exergy (exp), and kinetic exergy (exk):
As the potential and kinetic exergies are disregarded, the overall exergy of a particular stream can be expressed as follows.
By using Eq. (22), the chemical exergy of a gas mixture is described, where X represents the mole fraction. It should be noted that no chemical exergy is considered in the present system except for the PEME. To evaluate the system’s performance, it is necessary to assess its exergy and energy efficiencies. Accordingly, these equations can be stated as follows:
Table 2 provides a comprehensive set of equations that describe the energy and exergy balances related to SCBC and RORC subsystems in detail.
The parameters used for simulation and the operational conditions of the power cycle are listed in Table 3.
The T-Q diagrams of the heat-receiving process, as well as the location of pinch points for all heat exchangers included in the system, are demonstrated in Fig. 4. Considering this figure, Q is a ratio that shows how much heat has been transferred in a heat exchanger at a specific temperature, compared to the total amount of heat that has been transferred overall. In other words, Q is a measure of the efficiency of the heat exchange process at a given temperature. Hence, the corresponding values of Q are considered between 0 and 1. The pinch point location in evaporator 1, evaporator 2, and condenser are located at the maximum heat transfer point of the heat exchanger. It means the pinch point location is placed at the peak temperature of the heat-exchanging process corresponding to the hot and cold streams. Specifically, in evaporator 1, state point 3 experiences the highest temperature during the heat-absorption process that takes place between points 2 and 3 (the cold stream). Similarly, the state point labeled as PTC2 experiences the highest temperature during the heat-release process that occurs between PTC2 and PTC3 (the hot stream). The same pattern is observed in evaporator 2 and the condenser, while in the recuperator, the location of the pinch point is positioned on the opposite side of the heat-exchanging process.
The solution strategy for thermodynamic modeling and analysis of the SCBC and RORC is shown in Fig. 5.
3.5 Proton Exchange Membrane Electrolyzer (PEME)
The electricity generated by the SCBC and RORC systems is consumed by the PEME for hydrogen generation purposes. Accordingly, the initial step in the thermochemical modeling of a PEME is to calculate the theoretical energy required for the hydrogen production through electrolysis. This is essential for understanding the energy requirements of the system and for designing and optimizing the PEME for efficient hydrogen generation. The total energy needed by the PEME is represented by the following equation [52]:
where \(\Delta G\) denotes Gibb’s free energy (the required electrical energy). \(T\Delta S\) represents the thermal energy required for the process in (J/mol H2). It is worth highlighting that the thermodynamic tables are utilized to determine the values of H, S, and G for the electrolysis process of water, hydrogen, and oxygen. Additionally, it should be noted that theoretical energy refers to the energy required for water electrolysis without accounting for any losses. The quantity of hydrogen produced in terms of molar flow rate is estimated based on the recommendation of Esmaili et al. [53] as follows:
In this equation, the PEME current density is denoted by J, and F represents the Faraday constant. Similarly, the quantity of oxygen and water generated in terms of molar flow rate can be computed using the following equation:
The molar flow rate of PEME inlet water is known. Since the required electrical energy (power) of PEME is equal to the inlet electrical exergy, this statement is expressed in the following equation:
in which the PEME voltage, V, is calculated by:
In the above equation, the reversible potential, \({V}_{o}\), is calculated using the Nernst equation. Besides, \({V}_{\mathrm{act},c}\), \({V}_{\mathrm{act}, a}\), and \({V}_{\mathrm{ohm}}\) refer to the cathode activation overpotential, anode activation overpotential, and electrolyte ohmic overpotential, respectively. In an electrolysis unit, the generated hydrogen ions will experience resistance due to the voltage drop caused by the membrane resistance, also known as the ohmic overpotential. This resistance is caused by the electrical current passing through the electrolyte, which creates a voltage drop across the membrane. The voltage drop reduces the efficiency of the electrolysis process, resulting in a decrease in the amount of hydrogen produced. The resistance to ions within the membrane is determined by the membrane temperature, its thickness, and the degree of humidification [54]. The local ionic conductivity of the PEME is expressed by:
In this equation, the function \(\lambda (x)\) describes the water content (moisture level) at a particular location x, which is the depth within the membrane starting from the cathode side. It is calculated by:
\({\lambda }_{\mathrm{c}}\) and \({\lambda }_{\mathrm{a}}\) are the water contents at the cathode-membrane and anode-membrane interfaces, respectively. In addition, D is the membrane thickness. The overall ohmic resistance also can be achieved by [55]:
The remaining equations required for the analysis and modeling of PEME are given in Table 4.
The input data used for modeling the PEM electrolyzer are provided in Table 5.
3.6 Overall System
To evaluate the system's performance, the energy and exergy efficiencies are calculated using the given equations. By determining these efficiencies, it is possible to understand how effectively the system converts input energy into practical work or output energy. This is critical to identify spaces for improvement in the system's design and operation. The energy efficiency of the proposed system is determined by Eq. (34):
In this equation, the lower heating value of hydrogen is represented by \({\overline{LHV} }_{H2}\), and \({\dot{W}}_{\mathrm{system}}\) is calculated as follows:
Furthermore, Eq. (36) provides total solar collector area:
As mentioned before, exergy efficiency is defined as: the ratio of the exergy of the products to the exergy of the fuel input. In other words, it represents the percentage of the exergy provided by the fuel that is actually present in the system's outputs (products). This provides insight into how effectively the system is able to convert the exergy of fuel into usable energy or work:
Here, \({\dot{E}}_{{H}_{2}}\) is the exergy rate of produced hydrogen, and \({\dot{E}}_{\mathrm{in}}\) is the round-the-clock input exergy rate of the system due to the solar radiation. Based on the assumptions and mathematical models described earlier, a simulation program for the entire system is developed using EES software [56]. The purpose of this simulation program is to evaluate the energetic and exergetic performance of the system, as well as conduct a parametric study of variables. EES uses robust and efficient numerical methods to solve complex mathematical equations, including iterative methods, root-finding algorithms, and optimization techniques. It uses a variant of Newton's method to solve systems of nonlinear algebraic equations. The Jacobian matrix needed in Newton's method is evaluated numerically at each iteration. Sparse matrix techniques are employed to improve calculation efficiency and permit rather large problems to be solved in the limited memory of a microcomputer.
4 Results and Discussion
In Babolsar, Iran, the developed system can provide a consistent supply of electricity, hydrogen, and hot water throughout the entire day. The findings of the energy and exergy analyses, as well as the parametric study, are provided below.
4.1 Energy Analysis
Analyzing and optimizing energy efficiency is critical for designing and operating efficiently and warrants thorough examination of energy systems. Therefore, studying energy efficiency is an essential aspect of energy management and conservation that should be done by energy systems investigators. Figure 6 illustrates the changes in the energy efficiency of each subsystem and overall system for a 24-h system operation. As presented before, during 24 h of a day, the DNI and ambient temperature change continuously. This mainly affects the PTC performance and efficiency. In such a way that the higher DNI, the greater the PTC’s energy efficiency. At night, when there is no sunlight, the PTC’s energy efficiency is zero, while other subsystems operate continuously. The key distinction of the proposed system is that it is designed to operate 24 h and is not dependent on the presence of sunlight for its functioning. This contrasts to traditional solar-driven systems, such as the solar-ORC system proposed by Wang et al. [57], which rely on DNI for their energy efficiency. To enable the system to operate in stable operating mode, a two-tank storage system was implemented, as discussed in the previous section. The mass flow rate of the HTF in the PTC is regulated to maintain a stable temperature in the two tanks, while the mass flow rate from the hot tank remains unchanged. In contrast, Wang et al. [57] utilized just a solitary energy storage tank in their operating mode, resulting in fluctuating HTF temperatures and mass flow rates to the power cycle in response to changes in heat input. Consequently, the system operated under diverse circumstances to adapt to the fluctuations in the entering heat. The round-the-clock energy efficiency of the overall system, PEME, RORC, and SCBC is around 51.30%, 58.85%, 26.38%, and 14.84%, respectively, while the maximum energy efficiency of PTC is determined as 74.28%, where achieved at 2:00 PM.
It should be stated that the system’s inlet water temperature at state 11 (see Fig. 1) is considered equal to the ambient temperature and so varies throughout the day. Hence, since the electrolyzer’s functionality is dependent on the inlet water's temperature, the energy efficiency of the PEME changes slightly during the day, as shown in Fig. 7. When the input water temperature increases, the voltage of the PEME decreases, while the thermal energy input increases. However, the effect of increased thermal energy input is not significant. In summary, higher temperatures are beneficial for improving the performance of the electrolyzer. However, it is essential to maintain the operating temperature of PEME below 373 K, as this is the boiling point of water, and any temperature beyond this limit will cause the water to evaporate, leading to a decrease in the ionic conductivity of the electrolyte membrane. Based on the infinitesimal changes (approximately 0.07%) observed in the energy efficiency of the proton exchange membrane electrolyzer over a 24-h period (Fig. 7), it can be concluded that the first law efficiency of this subsystem remains constant, similar to the other subsystems (i.e., SCBC and the RORC).
Compared to other systems reported in the literature, the present system’s overall energy efficiency is relatively high. This could be explained by the fact that the collector has a smaller aperture area (Ac), which is a consequence of a lower mass flow rate determined for the collector (\({\dot{m}}_{\mathrm{c}}\)) [23]. One of the significant benefits of utilizing two power cycles in the system is the distribution of the produced power between the corresponding cycles. This allows for a lower MFR to be required for each power cycle, which leads to a lower mass flow rate for the collector. Figure 8 demonstrates the changes in the mass flow rate during a 24-h period of system operation.
The inlet and outlet mass flow rate of the collector (\({\dot{m}}_{c}\)) is varying throughout the day, which is directly related to the variable amount of DNI in a 24-h period. The higher amount of DNI leads to a greater amount of \({\dot{m}}_{c}\), as represented in Fig. 8. This is arising from the fact that, according to the useful energy equation of the PTC, by keeping the hot and cold tank temperatures at fixed values, the \({\dot{m}}_{\mathrm{c}}\) will be increased. However, the outlet mass flow rate of the hot tank (\({\dot{m}}_{HTF})\) is constant and specified as 2.68 kg/s. The steady amount of \({\dot{m}}_{HTF}\) leads to enduring values of mass flow rate for SCBC and RORC, which are determined as 3.81 kg/s and 8.06 kg/s, respectively (see Fig. 8).
The stable mass flow rate of SCBC and RORC leads to constant power generation of the system, as indicated in Fig. 9. This figure represents the system power generation versus direct normal irradiance for a 24-h system operation. According to the figure, irrespective of the value of DNI, a consistent power amounting to 1.5 MW is generated by the developed system. Approximately 82% of the system’s total generated power is obtained by the RORC, which is equivalent to around 1240 kW. The remaining power output, approximately 270.7 kW, is supplied by the SCBC. The greater power output of RORC compared to the SCBC is attributed to the significantly higher mass flow rate of RORC, which leads to a greater flow energy within the system. Throughout the day and night, approximately 10.7 kW of the system’s power generation will be consumed by PEME, while the remaining net power can be utilized to meet the electricity demands of the residential community.
4.2 Exergy Analysis
The exergy efficiency is a crucial parameter that requires careful investigation. This is because it measures of how efficiently a system can convert available exergy into useful work or output, and it is closely linked to the exergy destruction rate. Therefore, understanding and optimizing exergy efficiency is a fundamental element of designing and operating energy systems. Figure 10 displays the changes in the exergy efficiency relating to the whole system and each subsystem throughout the day. Similar to the explanation of energy efficiency, the variations of ambient temperature and DNI directly affect the exergy efficiency of the PTC. In such a way that, with an increment in the value of DNI and ambient temperature, the exergy efficiency of PTC is increased. The majority of the total input exergy in the PTC is related to the rate of exergy due to losses and destruction. Accordingly, the maximum exergy efficiency of PTC achieved around 33.89%. Typically, the amount of exergy that is destroyed in converting solar energy into thermal energy is high, resulting in the relatively low exergy efficiency of the collector. The collector design, the type of thermal oil used, the operating temperature range, and the level of thermal losses are the main affecting parameters to the PTC exergy efficiency.
The flow exergy of power cycles has a reverse relationship with the ambient temperature; as the ambient temperature rises, the dead state enthalpy and entropy of the working fluid also become greater. Therefore, if the enthalpy and entropy of a given flow are unchanged, but the dead state enthalpy, entropy, and temperature rise, the exergy of the flow will also increase, and conversely, if the dead state parameters decrease, the exergy will decrease as well. Thus, the exergy efficiencies of the RORC and SCBC exhibit a slight increase during the period of 11–16 o’clock when the ambient temperature rises (Fig. 10). The reason for the increase in exergy efficiency is that, despite a decrease in input exergy, the power production of the cycle (exergy of products) remains constant (refer to Eq. 24).
The exergy efficiency of PEME is relatively constant during 24 h of the day and is computed at around 79.56% (see Fig. 10). However, it should be stated that during the period of 11–16 o’clock, it experiences a slight reduction (around 0.4%), which arises from changes in the ambient temperature. These changes affect the rate of flow exergy concerning to the hydrogen, oxygen, and water streams. Yet, since the PEME’s exergy efficiency fluctuations throughout the day are infinitesimal (< 0.5%) can be neglected.
Considering Fig. 10, it is also apparent that the total system’s exergy efficiency remains constant throughout the day. However, given the changes in ambient temperature during the day, it is not possible for the system’s exergy efficiency can’t remain completely constant. As a result, it has been concluded that the entire system’s exergy efficiency undergoes a variation of around 0.4%, during the system's 24-h operation. Based on the presented descriptions, it can be concluded with a high degree of accuracy (with fluctuations of less than 0.5%) that the exergy efficiency of the entire system remains pretty consistent throughout the day, at approximately 55.7%. Comparing the obtained results to those reported in previous studies, it can be concluded that the outcome is entirely satisfactory [58].
Figure 11 shows how the exergy destruction rate of both individual subsystems, as well as the entire system, varies over a 24-h period. The figure demonstrates that the system’s rate of exergy destruction varies throughout 24 h. This is since the exergy destruction rate of the PTC is continually fluctuating, thereby impacting the entire system’s exergy destruction rate. PTCs are not perfectly efficient, and a certain amount of input solar exergy is lost in the process due to various factors such as heat transfer losses, fluid friction, and thermal radiation losses. The RORC’s exergy destruction rate slightly reduces with an increase in the Tamb around the 10–17 o'clock. It comes from the fact that an increase in Tamb results in a decrease in the condenser’s exergy destruction rate, thereby the RORC’s exergy destruction rate reduces in certain hours. Besides, it is consistent with previous observations about exergy efficiency. Around 10–17 o'clock period of the day, the exergy efficiency of RORC was also increased. The impact of Tamb variations against the rate of exergy destruction related to the PEME and SCBC is not significant (< 0.5%) and could almost be neglected (Fig. 11).
The proportion of the exergy destruction rate for each subsystem to the total exergy destruction rate of the system during the 24 h is provided in Fig. 12. As it is displayed, during the midday hours when there is sunlight, the proportion of the PTC exergy destruction rate increases. This increase in the exergy destruction rate for the PTC leads to a decrease in the proportion of the exergy destruction rate for the other subsystems. In other words, during daylight hours, the PTC exergy destruction rate possesses the most influence on the system's overall efficiency. This will be discussed further in the following paragraphs. Moreover, the PEM electrolyzer has the least significant impact on the system’s overall exergy destruction rate.
The maximum total exergy destruction rate calculated for the system, 5057 kW, is observed at 2:00 PM. This particular time was chosen because it corresponds to the moment when the highest values of DNI and Tamb were recorded. At this specific time, the exergy destruction rate of the system is composed of several contributions, with the PTC, RORC, SCBC, and PEME subsystems accounting for around 4223 kW, 628 kW, 195 kW, and 10.65 kW, respectively. It means that at this particular time, the exergy destruction rate of the system is mainly driven by the PTC component, while the other components make smaller contributions. Figure 13 illustrates the proportion of the exergy destruction rate for each subsystem in relation to the overall exergy destruction rate of the system at a specific time, 2:00 PM. In other words, the figure illustrates how much each individual subsystem contributes to the system’s total exergy destruction rate at a particular hour. As discussed previously, the highest share of the exergy destruction rate of the entire system is possessed by PTC, followed by RORC, SCBC, and PEME.
The contribution of the PTC in the system’s overall exergy destruction rate is significantly high (83.51%) compared to other energy subsystems, which means that a significant amount of the available exergy is lost as waste during the conversion process (see Fig. 13). This is due to the fact that PTCs operate at relatively low temperatures, which limits their capacity for generating high-quality energy. Additionally, the curved mirrors used in a PTC can suffer from optical losses, and the heat transfer fluid used in the receiver can experience thermal degradation over time, leading to a decrease in efficiency [59]. In PEME, the exergy destruction rate is directly related to the loss of available exergy that occurs during the conversion of electrical energy to chemical energy in the form of hydrogen. This can be attributed to several factors, such as overpotential losses, activation losses, and ohmic losses [60].
In SCBC and RORC, the exergy destruction rate refers to the loss of available exergy that occurs while transforming energy from one particular form to another. The origins of exergy destruction in power cycles can be attributed to several factors, including thermal losses, mechanical losses, chemical losses, irreversibilities, etc. [61]. Figure 14 demonstrates the proportion of the exergy destruction rate for each component of RORC in relation to the RORC overall exergy destruction rate at 2:00 PM. The exergy destruction rate of the condenser is relatively high compared to other heat exchangers of the RORC. The relatively high-temperature differences between the streams flowing through the condenser result in a substantial amount of energy being required to facilitate the heat transfer process. This, in turn, leads to an increase in the exergy destruction rate of such components. As well, the relatively high share of the exergy destruction rate for turbine 2 comes from irreversibilities and losses such as frictional losses and mechanical losses.
Figure 15 shows how much each individual component of the SCBC contributes to the overall SCBC exergy destruction rate at a given time, 2:00 PM. Based on the information provided, the evaporator 1 component has the highest proportion of the exergy destruction rate compared to the total exergy destruction rate of the SCBC. This is primarily due to the significant temperature differences between the streams passing through the evaporator heat exchanger. These temperature differences result in a substantial amount of energy being required to facilitate the heat transfer process, leading to an increase in exergy destruction. The exergy destruction rate of turbine 1 and compressor is due to the irreversibilities and losses that occur within the turbomachinery process.
Figure 16 demonstrates the hydrogen production and exergy destruction of the system during the 24 h. Although the exergy destruction rate of the system fluctuates over a 24-h period, the process of hydrogen generation is not affected by the irreversibilities and losses of the system. This means that the production of hydrogen can continue uninterrupted around the clock, despite the variations in the system’s performance. The system has the capacity of producing 4.47 kg of hydrogen per day, which is equivalent to approximately 1631 kg of hydrogen per year (8760 h of operation per year). This amount is considered acceptable when compared to other systems that have been reported in the literature [62].
Table 6 offers a comparative analysis between the findings of our current research and the existing literature. This evaluation focuses on two key aspects: the suggested overall system setup and the performance of the system's output. As a result, it is evident that the energy and exergy analyses conducted in our study yield satisfactory results that align well with those reported in previous research.
4.3 Parametric Study
This section concentrates on a parametric study of two variables, namely the condensation temperature of the RORC and the turbine inlet pressure of the SCBC, and their impact on the system’s performance. By analyzing how changes in these variables affect the system outputs, proper insights into the factors that influence the efficiency of the system and subsystems will be provided.
Figure 17 depicts how power generation is affected by the inlet pressure of the SCBC turbine, P3. Increasing the inlet pressure of the SCBC turbine leads to a significant increase in the power production of the SCBC, almost 214 kW. This is because the turbine extracts energy from the working fluid (CO2) by expanding it, and the amount of energy that can be extracted is directly proportional to the pressure of the working fluid at the inlet of the turbine. By increasing the inlet pressure, more energy can be extracted from the working fluid, increasing in the power production of the SCBC. Additionally, increasing the turbine inlet pressure of the SCBC can lead to an increase in the mass flow rate of the SCBC, which results in a reduction in the mass flow rate of the RORC. To maintain a total power production of 1.5 MW, the mass balance of the working fluids in the cycles must remain constant. Therefore, if the SCBC generates more power due to an increase in inlet pressure, the RORC must compensate by reducing its power generation proportionally (by almost 214 kW). In other words, changes to the inlet pressure of the SCBC can affect the power generation of both the SCBC and RORC. They must be carefully managed to maintain the overall efficiency of the system.
Figure 18 demonstrates the effects of SCBC turbine inlet pressure against the energy and exergy efficiencies of SCBC. A rise in the SCBC turbine inlet pressure gradually enhances the first law efficiency of SCBC, from 4.33 to 15.97%. By increasing the inlet pressure of the gas turbine, the temperature of the CO2 at the inlet of the turbine can be increased; thereby, the temperature difference within the evaporator 1 increases. Furthermore, increasing the inlet pressure of the gas turbine can lead to an increase in the power output of the SCBC (see Fig. 18), as more energy can be extracted from the working fluid in the turbine. Moreover, an increment of SCBC turbine inlet pressure improves the SCBC exergy efficiency significantly (from 10.57% to 36.65%).
Figure 19 illustrates how the total system energy and exergy efficiencies are impacted by the inlet pressure of the turbine in the SCBC. Enhancing the pressure in the range of 10,000–30,000 kPa results in a slight enhancement of the overall system's energy efficiency, from 49.74 to 51.45%. As well, an increment in the SCBC turbine pressure slightly raises the exergy efficiency of the system from 54.48 to 55.58%. This indicates that the SCBC turbine inlet pressure has a relatively minor effect versus the ηsys and ψsys. In other words, variations made to the inlet pressure SCBC turbine are unlikely to result in a substantial effect on the system's performance.
Figure 20 demonstrates how the energy and exergy efficiencies of the RORC are affected by the Tcond of RORC. With the rise in temperature from 342.2 to 372.2 K, the energy efficiency of the RORC gradually reduces from 27.14 to 23.32%. This is due to the fact that boosting the Tcond decreases the temperature gap between the heat source (SCBC) and RORC, leading to a decrease in the energy efficiency of the RORC. The same explanation is valid for the variation of RORC exergy efficiency versus RORC condensation temperature. Maximum possible work output is determined by the Carnot efficiency. Accordingly, by increasing the condensation temperature, the Carnot efficiency is reduced, leading to a decrease in the exergy efficiency of the cycle. Thus, the RORC exergy efficiency is reduced from 59.5 down to 50.19%.
The trend observed for the variation of RORC energy and exergy efficiencies concerning the condensation temperature is also applicable to the overall system efficiencies, as depicted in Fig. 21. In summary, raising the condensation temperature of the RORC adversely affects both the ηsys and ψsys, as it reduces the temperature difference between the topping heat source and the working fluid of the bottoming cycle. Consequently, an increase in the condensation temperature of RORC results in a decrease in the energy and exergy efficiencies of the entire system. Specifically, the system's energy efficiency decreases from 53.27 to 53.89%, and its exergy efficiency decreases from 58.31 to 48.44%. These changes reflect a reduction in the system's ability to convert thermal energy into practical work and denote that the performance of the RORC has a significant impact on the overall efficiency of the system. Hence, optimizing the design and operation of the RORC is critical to achieving maximum efficiency for the entire system.
Figure 22 illustrates how the energy and exergy efficiencies of the PEM electrolyzer are affected by the condensation temperature of the RORC. Increasing the condensation temperature results in an increase in the temperature of the water leaving the condenser. This temperature is identical to the temperature of the water entering the PEME. Put differently, elevating the condensation temperature of the RORC results in a rise in the temperature of the water fed to the PEM electrolyzer subsystem. Accordingly, when the PEME inlet water temperature grows, the energy efficiency of PEME is slightly enhanced. This is because by increasing the operational temperature, the voltage of the PEME decreases while the thermal energy input increases. However, the effect of increased thermal energy input is not significant. As well, as discussed earlier, the inlet water temperature of the condenser is equal to the ambient temperature, which varies throughout the day. Consequently, the inlet water temperature of the PEME also fluctuates during the day in proportion to the ambient temperature. This leads to a fluctuation in the energy efficiency of the PEME, which is reflected in the bulge observed in the distribution of the PEME energy efficiency over the day (see Fig. 22). Stated differently, changes in the Tamb have an impact on the effectiveness of both the condenser and the PEM electrolyzer.
Increasing the condensation temperature of RORC from 342.2 up to 372.2 K leads to an enhancement of its exergy efficiency from 78.8 to 83.11% (see Fig. 22). This is because electrolysis is a highly endothermic process that requires the input of thermal energy to drive the reaction. Raising the temperature of the water supplied to the PEM electrolyzer decreases the quantity of thermal energy necessary to facilitate the reaction, leading to an increase in exergy efficiency. Additionally, increasing the inlet water temperature can also reduce the overpotential of the electrolysis reaction. Lowering the overpotential can lead to a reduction in the quantity of electrical energy required to drive the reaction, leading to a higher exergy efficiency.
5 Conclusions
This research put forward an innovative operational approach for a stable output solar thermal power system. The proposed poly-generation system is equipped with a supercritical CO2 Brayton cycle (SCBC), a recuperative organic Rankine cycle (RORC), a two-tank direct thermal energy storage, and a proton exchange membrane electrolyzer (PEME). The energy and exergy analyses of the proposed system around the clock and under various solar irradiations and ambient temperatures on a particular day are conducted. The considered method aimed to uphold the system performance under the prescribed conditions and ensure a steady output. The temperature of the HTF in thermal storage tanks is fixed by adjusting the MFR of thermal oil in the solar collector. As a result, the SCBC and RORC operation was stabilized, leading to a consistent output of power, hydrogen, and hot water for the residential house. Significant outcomes of this investigation are provided below:
-
The performance of the parabolic trough collector (PTC) system is influenced by changes in ambient temperature and solar irradiance, with its highest energy efficiency observed at 2:00 PM, 74.28%. The energy efficiency of the PEME, RORC, and SCBC subsystems throughout a 24-h operation is around 58.85%, 26.38%, and 14.84%, respectively. Moreover, the entire system has an energy efficiency of approximately 51.30%.
-
The proposed hybrid system, which combines two power cycles, offers advantages such as more efficient power distribution, potentially reducing collector size and overall system costs.
-
The proposed integrated system can generate a consistent net power output of 1.5 MW, regardless of the solar irradiance level. The majority of the electric power, approximately 82%, is produced by the RORC subsystem. The SCBC subsystem supplies the remaining power output of 270.7 kW.
-
Most of the total input exergy in the PTC is lost or destroyed, resulting in a maximum exergy efficiency of around 33.89%. The exergy efficiencies of the RORC and SCBC subsystems show a slight increase during 11–16 o'clock when the ambient temperature rises. This is because the power generation of the cycle remains constant despite the decrease in input exergy. The exergy efficiency of the PEME subsystem remains relatively stable throughout the day, with a value of around 79.56%. Likewise, the entire system’s total exergy efficiency remains almost consistent, at approximately 55.7%, despite fluctuations in solar irradiance and ambient temperature.
-
At 2:00 PM, the exergy destruction rate of the system is a result of several contributions, with the PTC, RORC, SCBC, and PEME subsystems accounting for almost 4223 kW, 628 kW, 195 kW, and 10.65 kW, respectively. At 2:00 PM, the system experiences its highest possible rate of exergy destruction, which amounts to 5057 kW. Despite fluctuations in the \({\dot{E}}_{dest}\) of the system over a 24-h period, the hydrogen production process is not affected by the system's irreversibilities and losses. Therefore, the proposed system can produce 4.47 kg of hydrogen per day.
-
The ranking of each subsystem's contribution to the \({\dot{E}}_{dest}\) of the total system is as follows: PTC (83.51%), RORC (12.4%), SCBC (3.85%), and PEME (0.21%). It is discovered that a significant amount of the available exergy is lost as waste during the conversion process in the PTC.
-
The power generation of both the SCBC and RORC subsystems can be affected by changes in the inlet pressure of the SCBC. Gradually raising the inlet pressure of the SCBC turbine, 10,000–30,000 kPa, leads to a progressive improvement in the energy efficiency of the SCBC. This increment also significantly improves the exergy efficiency of the SCBC because more energy can be extracted from the CO2 in the gas turbine at high pressures. However, changes in the turbine inlet pressure of the SCBC do not possess a considerable influence on the overall system efficiency.
-
Increasing the condensation temperature of the RORC from 342.2 K to 372.2 K results in a gradual reduction in both the energy efficiency and exergy efficiency of the RORC. This is because the higher condensation temperature reduces the temperature differences between the SCBC and the RORC, leading to decreased efficiency. Similarly, raising the condensation temperature of RORC has an adverse effect on both ηsys and ψsys. The entire system's energy efficiency decreased from 53.27% to 53.89%, and its exergy efficiency decreased from 58.31% to 48.44%. Hence, it can be concluded that optimizing the design and operation of the RORC is critical to achieving maximum efficiency for the entire system.
-
Increasing the condensation temperature of the RORC results in a corresponding increase in the temperature of the water supplied to the PEME subsystem. The rise in temperature results in a slight improvement in the energy efficiency of the PEME. Additionally, increasing the RORC condensation temperature from 342.2 K to 372.2 K improves the RORC's exergy efficiency from 78.8% to 83.11%. It has also been found that changes in Tamb affect the efficiency of both the RORC condenser and the PEME subsystem.
6 Future works
In future investigations, it is suggested to conduct exergo-economic, exergo-environmental, and advanced exergy analyses. Furthermore, multi-objective optimization of the critical parameters regarding the performance of the proposed system could be considered. Additionally, the effectiveness of the proposed system can be evaluated in various regions worldwide while investigating the impact of financial parameters like electricity price, inflation rate, and discount rate through parametric analyses. Ultimately, the present study's mathematical modeling and methodologies can also be considered in experimental investigations.
Abbreviations
- A c :
-
Collector aperture area (m2)
- c p :
-
Specific heat capacity (J kg−1 K−1)
- \({E}_{\mathrm{act},i}\) :
-
Activation energy
- ex :
-
Specific exergy (kJ kg−1)
- \(\dot{Ex}\) :
-
Exergy flow rate (kW)
- F :
-
Faraday constant (C mol−1)
- G :
-
Gibbs free energy (J mol−1)
- G b :
-
Solar beam radiation (W m−2)
- h :
-
Specific enthalpy (kJ kg−1)
- J :
-
Current density (A m−2)
- J 0 :
-
Exchange current density (A m−2)
- \({J}_{i}^{\mathrm{ref}}\) :
-
Pre-exponential factor (A m−2)
- \(\dot{m}\) :
-
Mass flow rate (kg s−1)
- \(\dot{N}\) :
-
Molar flow rate (mol s−1)
- P :
-
Pressure (kPa)
- \(\dot{Q}\) :
-
Heat transfer rate (kW)
- R :
-
Gas constant (J kg−1 K−1)
- R PEME :
-
Ohmic resistance (Ω)
- s :
-
Specific entropy (J kg−1 K−1)
- T :
-
Temperature (K)
- \(V\) :
-
Voltage (V)
- \({V}_{\mathrm{o}}\) :
-
Reversible potential (V)
- \({V}_{\mathrm{act},c}\) :
-
Activation overpotential (V)
- \({V}_{\mathrm{act}, a}\) :
-
Anode activation overpotential (V)
- \({V}_{\mathrm{ohm}}\) :
-
Cathode activation overpotential (V)
- \(\dot{W}\) :
-
Power (kW)
- \(\eta\) :
-
Energy efficiency (−)
- ψ :
-
Exergy efficiency (−)
- τ :
-
Time interval (1 h)
- \(\sigma (x)\) :
-
PEME local ionic conductivity (s m−1)
- λ(x) :
-
Water content at location x in the membrane (Ω−1)
- \({\lambda }_{a}\) :
-
Water content at the anode-membrane interface (Ω−1)
- \({\lambda }_{c}\) :
-
Water content at the cathode-membrane interface (Ω−1)
- CSP:
-
Concentrated solar power
- DNI:
-
Direct normal irradiance
- GWP:
-
Global warming potential
- HTF:
-
Heat transfer fluid
- LHV:
-
Lower heating value
- ODP:
-
Ozone depletion potential
- PEME:
-
Proton exchange membrane electrolyzer
- PTC:
-
Parabolic trough collector
- PVT:
-
Photovoltaic-thermal
- RORC:
-
Recuperative organic Rankine cycle
- SCBC:
-
Supercritical CO2 Brayton cycle
- TES:
-
Thermal energy storage
- 0:
-
Dead state
- 1–17:
-
State points (see Fig. 1)
- amb:
-
Ambient
- c :
-
Collector
- cold:
-
Cold tank
- cond:
-
Condenser or condensation
- ch:
-
Chemical
- cr:
-
Critical state
- D :
-
Destruction
- e :
-
Exit
- eva1:
-
Evaporator 1
- eva2:
-
Evaporator 2
- hot:
-
Hot tank
- i or in:
-
Inlet
- k :
-
Kinetic
- L :
-
Loss
- m :
-
Logistic temperature
- p :
-
Potential
- ph:
-
Physical
- PP :
-
Pinch point
- Q :
-
Heat
- rec:
-
Recuperator
- s :
-
Solar
- th :
-
Thermal
- u :
-
Useful
References
Güney, T.: Solar energy, governance and CO2 emissions. Renew. Energy 184, 791–798 (2022)
Axon, C.J.; Darton, R.C.: Sustainability and risk–a review of energy security. Sustain. Prod. Consum. 27, 1195–1204 (2021)
Kılıç, U.; Kekezoğlu, B.: A review of solar photovoltaic incentives and policy: selected countries and Turkey. Ain Shams Eng. J. 13(5), 101669 (2022)
Mekhilef, S., et al.: The application of solar technologies for sustainable development of agricultural sector. Renew. Sustain. Energy Rev. 18, 583–594 (2013)
Suresh, B.V., et al.: Natural energy materials and storage systems for solar dryers: State of the art. Sol. Energy Mater. Sol. Cells 255, 112276 (2023)
Rathore, N., et al.: A comprehensive review of different types of solar photovoltaic cells and their applications. Int. J. Ambient Energy 42(10), 1200–1217 (2021)
Islam, M.T., et al.: A comprehensive review of state-of-the-art concentrating solar power (CSP) technologies: Current status and research trends. Renew. Sustain. Energy Rev. 91, 987–1018 (2018)
Zhang, H.L., et al.: Concentrated solar power plants: Review and design methodology. Renew. Sustain. Energy Rev. 22, 466–481 (2013)
Fuqiang, W., et al.: Progress in concentrated solar power technology with parabolic trough collector system: A comprehensive review. Renew. Sustain. Energy Rev. 79, 1314–1328 (2017)
Luo, Y.; Lu, T.; Du, X.: Novel optimization design strategy for solar power tower plants. Energy Convers. Manage. 177, 682–692 (2018)
Zayed, M.E., et al.: Performance prediction and techno-economic analysis of solar dish/stirling system for electricity generation. Appl. Therm. Eng. 164, 114427 (2020)
Bellos, E.: Progress in the design and the applications of linear Fresnel reflectors–a critical review. Thermal Sci. Eng. Prog. 10, 112–137 (2019)
Jamshidian, F.J.; Gorjian, S.; Shafieefar, M.: Techno-economic assessment of a hybrid RO-MED desalination plant integrated with a solar CHP system. Energy Convers. Manage. 251, 114985 (2022)
Fanaee, S.A.; Rezapour, M.: The modeling of constant/variable solar heat flux into a porous coil with concentrator. J. Solar Energy Eng. 142(1), 011004 (2019)
Rezapour, M.; Fanaee, S.A.; Ghodrat, M.: Developed brinkman model into a porous collector for solar energy applications with a single-phase flow. Energies (2022). https://doi.org/10.3390/en15249499
Peacock, J., et al.: Techno-economic assessment of integrated spectral-beam-splitting photovoltaic-thermal (PV-T) and organic Rankine cycle (ORC) systems. Energy Convers. Manage. 269, 116071 (2022)
Javed, S.; Tiwari, A.K.: Performance assessment of different Organic Rankine cycle (ORC) configurations driven by solar energy. Process. Saf. Environ. Prot. 171, 655–666 (2023)
Fanaee, S.A., et al.: Novel design for tri-generation cycle with parabolic trough collector: an exergy-economic analysis. Thermal Sci. Eng. Progress 24, 100871 (2021)
Morais, P.H.D.S., et al.: Energy, exergetic and economic analyses of a combined solar-biomass-ORC cooling cogeneration systems for a Brazilian small plant. Renew. Energy 157, 1131–1147 (2020)
Mousavi, S.A.; Mehrpooya, M.; Delpisheh, M.: Development and life cycle assessment of a novel solar-based cogeneration configuration comprised of diffusion-absorption refrigeration and organic Rankine cycle in remote areas. Process. Saf. Environ. Prot. 159, 1019–1038 (2022)
Kara, O.: Evaluation of solar source and ground cooling performance in three different organic rankine cycle (ORC) configurations. J. Therm. Anal. Calorim. 148(10), 4401–4425 (2023)
Bellos, E.; Tzivanidis, C.: Investigation of a hybrid ORC driven by waste heat and solar energy. Energy Convers. Manage. 156, 427–439 (2018)
Yang, J., et al.: Thermodynamic analysis and optimization of a solar organic Rankine cycle operating with stable output. Energy Convers. Manage. 187, 459–471 (2019)
Taner, T.: The novel and innovative design with using H2 fuel of PEM fuel cell: efficiency of thermodynamic analyze. Fuel 302, 121109 (2021)
Merchán, R.P., et al.: Thermo-economic and sensitivity analysis of a central tower hybrid Brayton solar power plant. Appl. Therm. Eng. 186, 116454 (2021)
Chen, R., et al.: Design and off-design performance comparison of supercritical carbon dioxide Brayton cycles for particle-based high temperature concentrating solar power plants. Energy Convers. Manage. 232, 113870 (2021)
Khatoon, S.; Kim, M.-H.: Preliminary design and assessment of concentrated solar power plant using supercritical carbon dioxide Brayton cycles. Energy Convers. Manage. 252, 115066 (2022)
Taner, T.; Naqvi, S.A.H.; Ozkaymak, M.: Techno-economic analysis of a more efficient hydrogen generation system prototype: a case study of PEM electrolyzer with Cr-C coated SS304 bipolar plates. Fuel Cells 19(1), 19–26 (2019)
Naqvi, S.A.H., et al.: Hydrogen production through alkaline electrolyzers: a techno-economic and enviro-economic analysis. Chem. Eng. Technol. 46(3), 474–481 (2023)
Bamisile, O., et al.: Comparative performance analysis of solar powered supercritical-transcritical CO2 based systems for hydrogen production and multigeneration. Int. J. Hydrog. Energy 46(52), 26272–26288 (2021)
Liu, T., et al.: Techno-economic feasibility of solar power plants considering PV/CSP with electrical/thermal energy storage system. Energy Convers. Manage. 255, 115308 (2022)
Olivkar, P.R., et al.: Effect of sensible heat storage materials on the thermal performance of solar air heaters: state-of-the-art review. Renew. Sustain. Energy Rev. 157, 112085 (2022)
Sharma, A.; Chauhan, R.: Integrated and separate collector storage type low-temperature solar water heating systems with latent heat storage: a review. Sustain. Energy Technol. Assess. 51, 101935 (2022)
Zheng, H., et al.: Efficient direct solar-driven thermochemical energy storage of (AlMgFeMn)OxCaCO3 pellets in a fluidized bed reactor. Energy Convers. Manage. 285, 116990 (2023)
Zhang, H., et al.: Technical and economic analysis of Brayton-cycle-based pumped thermal electricity storage systems with direct and indirect thermal energy storage. Energy 239, 121966 (2022)
Hassan, M.A., et al.: Internally shielded receivers for parabolic trough solar concentrators operating with supercritical carbon dioxide: analytical assessment. Energy Convers. Manage. 280, 116789 (2023)
Fimbres Weihs, G.A., et al.: Life cycle assessment of co-firing coal and wood waste for bio-energy with carbon capture and storage–new South Wales study. Energy Convers. Manage. 273, 116406 (2022)
Gonçalves, A.D.; Martins, T.G.; Cassella, R.J.: Passive sampling of toluene (and benzene) in indoor air using a semipermeable membrane device. Ecotoxicol. Environ. Saf. 208, 111707 (2021)
Remund, J., et al.: Meteonorm version 8. METEOTEST (www. meteotest. com), 2020.
Zhang, S., et al.: Energy and exergy analyses of a parabolic trough concentrated solar power plant using molten salt during the start-up process. Energy 254, 124480 (2022)
Duffie, J.A. and W.A.: Beckman, Solar engineering of thermal processes. 2013: John Wiley & Sons.
Fernández-García, A., et al.: Parabolic-trough solar collectors and their applications. Renew. Sustain. Energy Rev. 14(7), 1695–1721 (2010)
Tzivanidis, C.; Bellos, E.; Antonopoulos, K.A.: Energetic and financial investigation of a stand-alone solar-thermal organic Rankine cycle power plant. Energy Convers. Manage. 126, 421–433 (2016)
Fredriksson, J., et al.: A comparison and evaluation of innovative parabolic trough collector concepts for large-scale application. Sol. Energy 215, 266–310 (2021)
Blanco, J. et al.: Technical comparison of different solar-assisted heat supply systems for a multi-effect seawater distillation unit. in ISES solar world congress. (2003).
Lang, C.; Lee, B.: Heat transfer fluid life time analysis of diphenyl oxide/biphenyl grades for concentrated solar power plants. Energy Procedia 69, 672–680 (2015)
Yu, H., et al.: Optimal design and operation of an organic Rankine Cycle (ORC) system driven by solar energy with sensible thermal energy storage. Energy Convers. Manage. 244, 114494 (2021)
Petela, R.: Exergy of heat radiation. J. Heat Transf. 86(2), 187–192 (1964)
Akbarzadeh, S.; Valipour, M.S.: Energy and exergy analysis of a parabolic trough collector using helically corrugated absorber tube. Renew. Energy 155, 735–747 (2020)
AlZahrani, A.A.; Dincer, I.: Energy and exergy analyses of a parabolic trough solar power plant using carbon dioxide power cycle. Energy Convers. Manage. 158, 476–488 (2018)
Boodaghi, H.; Etghani, M.M.; Sedighi, K.: Performance analysis of a dual-loop bottoming organic Rankine cycle (ORC) for waste heat recovery of a heavy-duty diesel engine, part I: thermodynamic analysis. Energy Convers. Manage. 241, 113830 (2021)
Ni, M.; Leung, M.K.H.; Leung, D.Y.C.: Energy and exergy analysis of hydrogen production by a proton exchange membrane (PEM) electrolyzer plant. Energy Convers. Manage. 49(10), 2748–2756 (2008)
Esmaili, P.; Dincer, I.; Naterer, G.F.: Energy and exergy analyses of electrolytic hydrogen production with molybdenum-oxo catalysts. Int. J. Hydrog. Energy 37(9), 7365–7372 (2012)
Nami, H.; Akrami, E.: Analysis of a gas turbine based hybrid system by utilizing energy, exergy and exergoeconomic methodologies for steam, power and hydrogen production. Energy Convers. Manage. 143, 326–337 (2017)
Liu, S., et al.: Multi-objective optimization of proton exchange membrane fuel cell geometry and operating parameters based on three new performance evaluation indexes. Energy Convers. Manage. 277, 116642 (2023)
Klein, S.A. and S.F.: Alvarda, Engineering equation solver (EES). 2007, F-chart software, WI.
Wang, J., et al.: Off-design performance analysis of a solar-powered organic Rankine cycle. Energy Convers. Manage. 80, 150–157 (2014)
Khalid, F. and Kumar, R.: Thermodynamic assessment of a new PTC operated polygeneration system for fresh water, cooling, electricity and hydrogen production for a residential community. Int. J. Hydrog. Energy, (2023).
Kumar, A., et al.: A review on exergy analysis of solar parabolic collectors. Sol. Energy 197, 411–432 (2020)
Hai, T. et al.: Applying energy-exergy, environmental, sustainability, and exergoeconomic metrics and bi-objective optimization for assessment of an innovative tri-generation system. Int. J. Hydrog. Energy, (2023).
Wang, Y., et al.: Thermodynamic and exergy analysis of a novel PEMFC-ORC-MH combined integrated energy system. Energy Convers. Manage. 264, 115709 (2022)
Mohammadi, A.; Mehrpooya, M.: A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 158, 632–655 (2018)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Etghani, M.M., Boodaghi, H. Design and Performance Assessment of a Novel Poly-generation System with Stable Production of Electricity, Hydrogen, and Hot Water: Energy and Exergy Analyses. Arab J Sci Eng 49, 10471–10499 (2024). https://doi.org/10.1007/s13369-023-08410-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08410-7