Abstract
This study reports the synthesis of 2D leaf-like ZIF-L and its modification using [BMIM][BF4] ionic liquid (IL). The nanosheet was then embedded up to 5 wt% into the polyethersulfone (PES) to form mixed matrix hollow fiber membrane (MMHFM) to separate CO2/N2 gases. IL altered the surface charge of the ZIF-L which results in a more uniform dispersion of ZIF-L in the polymer matrix and minimizes the ZIF-L agglomeration on the lumen side. The filler–polymer interaction was improved as suggested by the TGA data. An evaluation of the performance of the MMHFM indicated that the synergistic effect between the filler modification and its loading is crucial in improving the performance of the membrane. The incorporation of unmodified ZIF-L resulted in a significant loss of selectivity at low loading (≥ 1 wt%). Meanwhile, permeance and selectivity trade-off are absent up until 2.5 wt% IL@ZIF-L. Besides, better ZIF-L dispersion and enhanced interaction between the filler and polymer are the factors that improved the separation performance of the MMHFM, hence highlighting the vital role of IL in the system. At the feed pressure of 2 bar, PES/IL@Z0.5 improved the CO2 permeance and ideal CO2/N2 selectivity by 106% and 86%, respectively, relative to the pristine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Article search using keywords of polymeric membrane and gas separation on Scopus database revealed that the number of articles published in the last decade has doubled. It suggests that the technology showed promising effectiveness and reliability. On top of that, the technology garnered attention due to its low cost, ease of operation and being highly flexible. A mixed matrix membrane (MMM) is developed by embedding a filler into the polymer matrix. As a result, the effectiveness of the system was boosted as compared to the pristine polymeric membrane. Such a strategy is prevalent among researchers due to the high permeability offered by the system, although the selectivity is typically compromised. Nevertheless, several approaches solved the trade-off between permeability and selectivity, and the upper limit has been surpassed in many cases.
Metal–organic frameworks (MOFs) are suitable to be used as fillers in MMMs. They are typically highly selective due to their pore aperture size and able to enhance the permeability of gas molecules due to their large surface area and pore volume. To date, there are several shapes of MOFs that have been incorporated in the development of MMM and this factor was found to significantly affect the performance of the membrane [1]. This study proposes the usage of zeolitic imidazole framework in nanosheet form (known as ZIF-L) as a filler to develop a mixed matrix hollow fiber membrane (MMHFM). The self-synthesized ZIF-L undergoes a facile synthesis method to control the critical radius of the crystal during the nucleation and growth process. Since ZIF-L is produced using the same precursors as ZIF-8, they both have a structure consisting of Zn tetrahedral and 1-methylimidazole linker. Consequently, identical pore opening can be anticipated, although BET surface area and pore volume differ. It was claimed that ZIF-L has a similar aperture of 3.4 Å in diameter perpendicular to the 2D crystal layer, identical to the ZIF-8 and comparable if ZIF-L layers are dispersed parallel to the membrane surface [2]. Performance improvement by nanosheet MOFs were reported by several works that used MoS2 [3] and Cu(BDC) [4]. These MOFs were not modified and the performance of the MMM peaked at lower filler content than the typical particle shapes. For instance, Zhu et al. [5], Liu et al. [3] and Rodenas et al. [4] utilized 5 wt%, 4.67 wt% and 4 wt%, respectively. Such promising results were reported due to the nanosheet’s large aspect ratio and highly tortuous path in the polymer matrix. However, ionic liquid modified nanosheet was never reported as filler in a MMM and will be explored in detail in this study.
One of the main aims of filler modification is to improve the dispersion of filler, especially at high loading. Although in many cases, the ideal nanosheet loading is relatively lower compared to the MOFs in particle shape, but from our point of view, the modification of nanosheet MOFs is indeed necessary. Modifying nanosheet will introduce multiple intermolecular forces of interaction between the components in MMM. As a result, interfacial defects could be avoided which caused by poor polymer-filler adhesion and repulsive forces between the components [6]. Although these facts are known, the information available is restricted to the particle shaped MOFs. This factor motivates the current work. To prove the importance of nanosheet modification on the filler dispersion, component interaction and membrane’s performance, ionic liquid [BMIM][BF4] (which will be known as IL hereafter) will be used. It is hypothesized that the performance of the membrane could peak at a much lower loading due to the absence of interfacial defects and improved affinity toward CO2.
The schematic of hypothetical interaction between the components is proposed in Fig. 1. In theory, an unmodified ZIF-L interacted with the polymer matrix through hydrogen bonding. Such bond is possible due to the participation of O = S = O part in polyethersulfone (PES). Unfortunately, this type of molecular interaction is not necessarily strong and is dependent on the distance of the methyl group to the proton acceptor as well as the angle between the electron acceptor and the donor [7]. Besides, the IL is attached to the ZIF-L structure through its anion. The anion bridged the cation of the IL and ZIF-L. In this case, the B–F bonding became stronger due to the presence of an imidazole ring in the system [8]. Also, a stack of IL layers is another configuration to consider. The [BMIM]+ ions from the IL attached to the ZIF-L could interact with another IL layer via electrostatic interaction [9, 10] before approaching the polymer matrix. Either way, the ZIF-L is surrounded by an ion layer, resulting in better dispersion of the ZIF-L due to the stronger repulsive charge than the unmodified form. Additionally, the π–π stacking between the imidazole in both ZIF-L and IL will strengthen the interaction between these two components in the system. These possible interactions between the components shall reduce the risk of interfacial defect and agglomeration of filler, hence improving the performance of the system. Furthermore, the IL@ZIF-L increases the affinity of the system toward CO2 due to the strong electrostatic interaction between the modified ZIF-L and the gas molecules. The affinity toward CO2 is enhanced via hydrogen bond formation between; (i) oxygen in CO2 molecules with the hydrogen atom of the alkyl chain in the cation of IL (C–H⋅⋅⋅O), (ii) CO2 molecules with BMIM+ and (iii) CO2 molecules with fluorinated anions [11]. In the absence of IL, the nanosheet and CO2 interacted by the van der Waals [12].
ZIF in the form of nanosheet will be synthesized, and its properties will be altered in this work. Both ZIF-L and IL@ZIF-L will be used in the formation of the asymmetric MMHFM. It is believed that the configuration of the membrane reported in this work is unique and the performance evaluation of nanosheet embedded asymmetric hollow fiber membrane is intriguing. Ideal filler loading, filler dispersion’s pattern and thermal stability will differ significantly, and such information is not available for nanosheet and IL-modified nanosheet as filler in MMM. A detailed and specific report on the pattern of nanosheet dispersion, improvement of filler–polymer interaction as well as its influence in enhancing the separation performance of MMHFM will be revealed in this study.
2 Experimental
2.1 Chemicals
Polyethersulfone (PES) was purchased from BASF (Ultrason E6020P, MW of 58,000) and was dried to remove moisture before its use. 1-Methyl-2-pyrrolidone (NMP), triethylamine, n-hexane and acetone were supplied by Merck. Methylimidazole, zinc nitrate hexahydrate, 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) were purchased from Sigma-Aldrich. Sylgard 184 from Dow Corning was also used in this work. All chemicals were used as received.
2.2 ZIF-L and IL@ZIF-L Synthesis
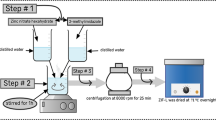
ZIF-L was prepared via the bottom-up approach at room temperature. The method was adopted based on the report by Khan et al. [13]. Firstly, 1.475 g of zinc nitrate hexahydrate and 3.25 g of methylimidazole were weighed and dissolved in 100 mL of deionized water; 250 μL of triethylamine was added to the methylimidazole before mixing with the zinc solution. The mixture was then stirred rapidly for 1 h. The reaction product was centrifuged and washed with deionized water at least three times before drying at 65 °C for 24 h.
To modify ZIF-L, it was first heated to 150 °C. The desired amount of IL was dissolved in 30 mL acetone [8]. In this study, the ratio between the IL and ZIF-L was kept constant at 5:95 by mass. ZIF-L was then added to the IL solution and stirred at room temperature for 15 min in a sealed container. Next, the mixture was then placed in a stirring mantle containing a water bath at 35 °C to allow the acetone to evaporate. Once the acetone was removed entirely, the ZIF-L was dried at 105 °C in vacuum condition overnight. The product is named IL@ZIF-L.
2.3 Membrane Fabrication
The dope solution for the pristine membrane was prepared by dissolving 29 wt% PES in NMP. The solution was prepared at 60 °C where it was stirred overnight at 250 rpm. On the other hand, the dope solution for MMHFM was prepared by the priming protocol. This method mixes the low concentration polymer solution with the filler suspension in NMP. In this work, 5 wt% of PES was firstly prepared and stirred at 60 °C for 4 h. Concurrently, the filler was dispersed in a portion of NMP. The suspension was stirred gently for 45 min at room temperature and sonicated for another 45 min. These steps were repeated 3 times. Mixing the suspension and the polymer solution was carried out for an hour at 60 °C. The remaining PES and NMP were then added to form the final dope solution. All dope solutions were degassed before the spinning process for at least 3 h. The process to fabricate MMHFM using modified and unmodified ZIF-L is illustrated in Fig. 2.
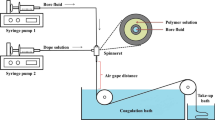
The hollow fiber membranes were fabricated by the dry-jet wet phase inversion method. All membranes were fabricated at identical spinning conditions as specified in Table 1. The spinneret internal/external diameter ratio used in this study was 0.5:1. A gear pump and nitrogen gas were used to assist the extrusion of dope solution through the spinneret. The collected fiber was immersed in distilled water, which was refreshed daily. After 3 days, the fibers were air-dried before characterization and performance tests were conducted. The membranes in this work are known according to PES, PES/Zx and PES/IL@Zx, representing the pristine hollow fiber PES membrane and the others are the mixed matrix. The subscript x refers to ZIF-L loading in the membrane which was calculated according to the following equation:
2.4 Filler Characterization
Firstly, the formation of ZIF-L was confirmed via field emission scanning electron microscope (FESEM, SUPRA TM 35vp Zeiss, Germany) imaging at the accelerating voltage of 5 kV. The nanosheet was viewed at 50,000 times magnification to observe its shape and dimension. The zeta potential of ZIF-L and IL@ZIF-L was determined by the Malvern Zetasizer Nano Z where samples were inserted in a disposable folded capillary cell. Rigaku X-ray diffractometer with CuKα as the radiation source was used to verify the crystal structure at 2θ angle range of 3–75° (40 kV, 30 mA). Meanwhile, the chemical structure of the nanosheet was characterized using fourier transform infrared spectroscopy (FT-IR) at a wavelength range of 600 to 3600 cm−1, while its pore textural properties were determined using Micromeritics ASAP 2020. Similarly, the modified ZIF-L was characterized using the aforementioned analytical equipment and conditions. Thermogravimetric analysis (TGA) was conducted using PerkinElmer TGA7 to identify the decomposition temperature (Td) of ZIF-L and IL@ZIF-L. Samples were subjected to heating under nitrogen, up to 800 °C where the heating rate was fixed to 10 °C/min. Additionally, an x-ray photoelectron spectroscopy (XPS) analysis was carried out by using KRATOS Axis Ultra DLD (Al Kα).
2.5 Membrane Characterization
The cross-sectional and surface morphologies of all membranes fabricated in this study were viewed using a scanning electron microscope (SEM). HITACHI Tabletop Microscope instrument (TM-3000-Japan) operated at 15 kV was used for this purpose. All samples were freeze-fractured using liquid nitrogen, and they were coated with gold before viewing. Additionally, the presence of Zn, B and F in the chosen MMHFM was confirmed using energy-dispersive X-ray spectroscopy (EDX) connected with the FESEM. Meanwhile, the XRD and TGA analyses of the membranes were conducted according to the procedure applied for filler characterization as can be found in the previous subsection.
The tensile test on the membranes was carried out using Instron 3366, according to the ASTM D3379. To perform the test, a 70-mm hollow fiber membrane was glued on support using rapid setting epoxy. The gage length was fixed to 20 mm, and the sample was subjected to 10N load cell at a crosshead speed of 5 mm/s. The test was conducted at room temperature, with a relative humidity of 50%.
2.6 Gas Permeation Test
Before the permeation test, the hollow fiber membrane was dip-coated with Sylgard 184. The coating solution was prepared by first dissolving 5 wt% of the elastomer in n-hexane before adding the curing agent at 10:1 ratio by mass. The curing process took place at room temperature for 24 h. A stainless-steel hollow fiber module was prepared by inserting a membrane fiber with an effective length of 10 cm. The end of the hollow fiber membrane was potted using Araldite two parts epoxy resin in the module holder. After complete curing of the epoxy resin, the module was installed in the pilot unit to carry out the permeation test. All samples were subjected to pure gases of at least 99.99% purity. In this study, N2 and CO2 were fed at a pressure between 2 and 5 bar. The temperature during the permeation test was kept at 25 °C. The pressure on the permeate side was allowed to stabilize before any readings were taken, and a soap bubble flowmeter was used to measure the permeate flow rate. The pilot unit was installed with a pressure sensors and transmitters in the feed and permeate streams. The gas permeance was calculated by using the following equation:
where \(\frac{P}{l}\) is the permeance of the gas in GPU (1 GPU = 1 × 10−6 cm3 (STP)/(cm2⋅s⋅cmHg). QSTP is the corrected volumetric flow rate to standard temperature and pressure (273 K, 1 atm), Δp is the trans-membrane pressure difference (cmHg) and A is the effective membrane surface area (cm2). Meanwhile, selectivity was calculated by simply taking the ratio between the permeance of CO2 to N2.
3 Results and Discussion
3.1 ZIF-L and IL@ZIF-L Characteristics
The images of ZIF-L and IL@ZIF-L synthesized in this work are shown in Fig. 3. It can be seen that the ZIF-L has a 2D leaf-like shape, consistent with many studies [12, 13]. The average dimension of the nanosheet is 1.4 μm × 3.3 μm × 108 nm (width × length × thickness). More importantly, modification of the ZIF-L with IL does not change its size and shape [14].
On the other hand, the surface charge of ZIF-L and its modified form were determined at pH 7. Table 2 summarizes the properties of ZIF-L and IL@ZIF-L synthesized in this study. It was found that ZIF-L is positively charged (+ 4.46 mV) at the condition mentioned. The surface charge obtained is close to the surface charge of ZIF-8, as reported by Abdelhamid et al. [15]. In contrast, the presence of IL in the framework causes the ZIF-L to have a negative surface charge (− 1.46 mV). Such value was recorded due to the presence of an anionic group in the IL, hence proving its immobilization in the ZIF-L.
The N2 adsorption–desorption isotherms of ZIF-L and IL@ZIF-L are presented in Fig. 4. All samples exhibited the Type I isotherm where the unmodified ZIF-L has a higher BET surface area and pore volume at 91.85 m2/g and 0.07 cm3/g, respectively. Furthermore, the gate opening phenomenon in ZIF-L structure can be observed in the semi-log plot, as indicated in the red squares. Modifying the nanosheet with IL has further reduced the BET surface area and pore volume to 24.43 m2/g and 0.05 cm3/g, respectively. The reduction of the surface area and pore volume indicates the success of immobilization of IL in ZIF-L [8].
Unlike Khan et al. [13] that heated the nanosheet to 200 °C to achieve 135 m2/g, the ZIF-L in this work was heated at 150 °C. The heating condition was adhered to during the analysis to keep the consistency of the data and procedure of ZIF-L activation. ZIF-L was reported to decompose at 200 °C, and it breaks into smaller pieces at 150 °C in vacuum conditions [12]. The heating condition used in this study is sufficient to allow solvent removal and activation of ZIF-L [16]. Also, the presence of IL in the ZIF-L structure could cause the nanosheet to decompose at a much lower temperature. Data from TGA analysis as shown in Fig. 5 validated this hypothesis. ZIF-L recorded significant weight loss at the following temperatures; (i) starting at < 100 °C due to the removal of the solvent and (ii) around 270 °C due to the decomposition of the imidazole [5]. Furthermore, the declining thermal stability of ZIF-L at around 450 °C is caused by the structural collapse of the ZIF-L. On the other hand, the IL completely decomposes at around 500 °C while IL@ZIF-L starts to decompose at a lower temperature (around 220 °C) due to the presence of imidazole in the structure of IL. As the IL decomposes from the IL@ZIF-L structure, it removes some parts of ZIF-L as well. Such a condition proves the existence of interaction between ZIF-L and IL.
Next, the crystallinity of ZIF-L and IL@ZIF-L were confirmed by comparing their XRD peaks with the simulated, as shown in Fig. 6. As demonstrated in the figure, intense diffraction peaks (2θ) can be observed at around 7.4°, 10.4°, 12.8°, 15.6°, 16.8° and 18.0°. The significant peaks of the samples matched to the simulated by Kim et al. [17]. Our calculations suggested that the lattice constants a, b and c for the sample are approximately 23.644 Å, 16.947 Å and 19.756 Å, respectively. On the other hand, IL@ZIF-L showed an identical pattern of diffraction peaks to the ZIF-L but less intense. This is due to the amorphous property of the IL [16]. Moreover, it reveals that the composite of IL and ZIF-L has been formed successfully. It is hypothesized that the modification process did not significantly affect the crystallinity of the nanosheet at the ratio of IL:ZIF-L used in this work.
The XPS spectra of ZIF-L and IL@ZIF-L can be found in Fig. 7. The analysis was carried out to confirm the interaction between IL and ZIF-L. It is clear from Fig. 7a that the ZIF-L is free from F 1s (682 eV) peaks [18]. Based on the high-resolutions Zn 2p spectra, the ZIF-L is characterized by the presence of intense Zn 2p peaks at 1019 eV and 1042 eV. The first peak is designated to the Zn 2p3/2 and the latter is Zn 2p1/2, hence suggesting that the Zn is in tetrahedral coordination [19]. Other than that, the C 1s showed a peak at around 282 eV while N 1s is determined by the peak at 395 eV. These peaks are associated with the imidazole group present in the ZIF-L structure. Attention should be paid to the slight shift of the binding energy of N 1s, C 1s and Zn 2p in IL@ZIF-L sample. The change in the binding energy of the electron is caused by the interaction of the ZIF-L with the anion of the IL.
Meanwhile, Fig. 8 shows the FT-IR spectra of ZIF-L, [BMIM][BF4] and their composite. The peak at 690 cm−1 characterizes Zn-N bond in ZIF-L [20]. Meanwhile, the peaks between 1250 and 4120 are assigned to the bond of C–N and C=N [21, 22]. As for [BMIM][BF4], the most intense peak is in the range of 1600–900 cm−1, where the butyl is characterized by the peak at around 987, 1017 and 1037 cm−1 [8].
The FT-IR spectra of the composites showed an almost identical pattern to the ZIF-L, although different intensities and some spectra shifts were observed. Characteristics of both components are apparent, indicating that they are intact. For example, the peak at 753 cm−1, which refers to the out-of-plane bending of the imidazole ring intensifies in the composites due to the presence of the imidazole group in the ZIF-L and IL. The presence of the butyl group can be observed in the composite as the peak around this region intensifies. Similarly, the peak at 1178 cm−1 corresponds to the C-H bending intensified in the composite. At the higher spectra region, a broad peak in the range around 3120 to 3200 cm−1 is assigned to the quaternary amine salt formation with tetrafluoroborate [23]. According to Koyuturk et al. [8], significant peak shifts from 2939, 3113 and 3121 cm−1 are sufficient to indicate the interaction between the components, where anion of IL interacts with ZIF-L and receives electrons, leading to stronger bond in the anion.
3.2 Membrane Characteristics
Figures 9, 10, 11 and 12 provide the morphology images of all membranes produced in this work. Specifically, Fig. 9 provides the morphology of pristine PES while the morphology of the MMHFM can be found in Figs. 10, 11 and 12. All membranes fabricated in this study have a similar structure; dense outer skin with a sponge-like substructure in the middle. Also, teardrop structure can be observed on the lumen as water was used as the bore fluid during the fabrication process. The influence of IL on the phase separation is not observed, unlike the report by Lessan and Foudazi [24]. This is because the IL is immobilized in the ZIF-L and the rheological behavior of the polymer solution is not impacted. Based on Fig. 10, it is obvious that the inclusion of non-modified ZIF-L in the dope solution resulted in poor dispersion of the nanosheet at all loading and it evidently agglomerates (forming a petal-like structure) on the lumen, as pointed out in the figure. This phenomenon is obvious as the loading reaches 1 wt% ZIF-L. According to Fig. 10e–h, the ZIF-L is not uniformly dispersed in the polymer matrix and the nanosheet’s presence toward the outer edge of the membrane is very minimal, and agglomerated. It is believed that the aspect ratio of the nanosheet and the high density of large ZIF-L clusters contributed to such observation.
On the contrary, IL modification was proven to improve the dispersion of the filler in the MMHFM as shown in Fig. 11. The improvement of ZIF-L’s dispersion can be observed clearly by comparing Figs. 10h and 11h. Agglomeration of the filler on the lumen side was clearly reduced. This is due to the surface charge of the modified ZIF-L and the established interaction between the filler and polymer matrix. Besides the absence of ZIF-L’s accumulation on the lumen, the dispersion of ZIF-L is more uniform across the membrane layer. Filler agglomeration was not observed and very minimal at higher loadings. Agglomeration of filler results in interfacial defect and accumulation of ZIF-L on the lumen could negatively impact the separation performance.
Additionally, the surface morphology of the MMHFM in this work (Fig. 12) reveals that the membrane fabricated using non-modified ZIF-L resulted in a very minimal presence of ZIF-L on the membrane’s surface. This is due to the poor dispersion of the filler and the nanosheet is mainly located on the lumen side. Meanwhile, the ZIF-L is visible on the surface as they were modified using IL. In fact, at low loading (≤ 1 wt%), the ZIF-L exists as an individual nanosheet piece and does not agglomerate on the surface. Uniform distribution of filler in an asymmetric membrane is highly desirable as the separation mainly took place on the dense layer of the membrane.
The membrane PES/IL@Z5 was used to demonstrate the presence of IL in the MMHFM through the EDX mapping. The distribution of the elements in the membrane is as shown in Fig. 13. According to this figure, a portion of the IL remains in the membrane’s structure and leaching of IL is limited. This is because the IL is caged in the framework of ZIF, which was also proven by the nitrogen adsorption analysis. In fact, the high viscosity of dope used in this work that increases the degree of chain entanglement of the polymer could hinder the leaching of IL [25]. Similarly, Kamble et al. [26] and Chen et al. [27] claimed that the IL is highly likely to be entrapped between the nanosheets, resulting in a minimal loss. On top of that, the element mapping provided in Fig. 13 supported the hypothesis that the ZIF-L is dispersed across the membrane structure and not accumulated on the lumen side as mentioned previously.
Furthermore, Fig. 14a, b provides the TGA data of membranes used in this work. All samples showed a similar profile of thermal stability at two primary stages. The first significant weight loss recorded at around 100 °C is attributed to the release of water and solvent from the membrane [28]. The second stage of considerable weight loss differs between the mixed matrix and pristine membrane. The inclusion of 0.5 wt% ZIF-L in the polymer matrix retained a similar decomposition temperature to the pristine (around 539 °C) due to very low loading. At higher ZIF-L loading, the decomposition temperature of unmodified PES/ZIF-L is appreciably lower than the pristine. For instance, the Td for PES/ZIF1 was found to be 535 °C. The trend continues for higher loading where PES/Z2.5 and PES/Z5 recorded Td at 510 °C and 482 °C, respectively. Evidently, this is because ZIF-L decomposes at a lower temperature than the PES. Also, the low thermal stability of the MMHFM using unmodified ZIF-L is due to the interrupted polymer chain and such a trend was also observed for ZIF-8 and ZIF-67 [29].
On the contrary, the modification of ZIF-L resulted in better thermal stability of the membrane. The decomposition profile in Fig. 14b clearly shows that MMHFM made using modified ZIF-L resulted in the shift of the profile to a higher temperature before significant decomposition of the sample took place. This situation indicates that the interaction between the modified filler and the polymer is stronger that decomposition of the MMM requires more energy. While the Td for PES/IL@ZIF0.5, PES/IL@ZIF1 and PES/IL@ZIF2.5 improved by 1 to 10 °C relative to the pristine, PES/IL@ZIF5 recorded lower Td (at around 518 °C). Similar to the unmodified ZIF-L, such a phenomenon was observed for PES/IL@ZIF5 which is highly possible due to very high filler loading that causes strong disruption of polymer chains and void formation [30].
Other than significant weight loss due to water and solvent at around 100 °C, there is another significant step of weight loss in IL-modified membrane. Basically, the trend obeys the PES thermal stability profile as this is the major component in the sample and only the onset of degradation temperature is affected by the presence of IL which improves the thermal stability of the membrane. Nevertheless, significant weight loss starts at around 539–549 °C because of the degradation of PES. According to Li et al. [31], the significant weight loss is a result of degradation reactions including complete loss of sulfone, ether group, as well as the hydrogen on the benzene rings in the form of sulfur dioxide, water, and hydrogen. Upon the completion of such reaction, the sample turns to char (36 wt%).
Additionally, identifying the crystallinity of a MMM is crucial as it affects its mechanical properties as well as the permeability of the gas molecules [32]. Figure 15 provides the XRD peaks of PES/Z5 and PES/IL@Z5. These two samples were chosen for this analysis due to the high amount of ZIF-L loading in the membrane. In this regard, the significant peaks can be observed clearly, and proper data analysis can be made. Based on the figure, broader peaks were obtained for PES/Z5 and PES/IL@Z5, compared to the ZIF-L and IL@ZIF-L (refer Fig. 6) due to the amorphous property of the main component in the sample, which is the PES. The most significant peak in Fig. 15 (around 18°) is typically found in pure PES samples [33]. Nevertheless, the significant peaks associated with ZIF-L (for instance, at around 7.4° and 10.4°) are obvious in PES/IL@Z5. Such peak verifies that the IL has neither changed the shape nor the size of the nanosheet, as also supported by its FESEM and XRD analysis (Sect. 3.1). Note that the presence of IL in PES/IL@Z5 causes the peaks to be more intense compared to PES/Z5. Such observation is due to improved filler dispersion in the membrane where it can be easily found on the membrane’s surface.
Figure 16 shows the tensile stress data of the membranes fabricated in this work before they deform. Interestingly, the incorporation of ZIF-L enhances the mechanical strength of the membrane at 0.5 wt% (8.11 MPa) before showing a significant declining trend at higher loading. Clearly, increasing the loading of ZIF-L in the polymer phase influences the integrity of the membrane. This is because the polymer chain is interrupted due to the presence of the inorganic materials. The agglomeration of ZIF-L and interfacial voids at higher loading worsens the scenario. In other words, interfacial interaction is highly crucial in determining the mechanical strength of a MMM [34] and enhancing the interaction between the components is necessary.
The membrane fabricated using modified ZIF-L can withstand higher force. For instance, the tensile stress of PES/IL@Z1 was improved by 26% compared to PES/Z1. This trend is applicable to all ZIF-L loading in this study. As mentioned, the presence of IL improves the interaction between the filler and polymer. It was proven in the earlier subsections that the agglomeration and interfacial defects are minimal while thermal stability improved compared to the unmodified ZIF-L. These factors contributed to the improved strength of the membrane. Meanwhile, Li et al. [35] agree that stiffer interphase resulted in the enhancement of the mechanical strength of a MMM.
3.3 Membrane Performance Analysis
The data of gas permeance and ideal CO2/N2 selectivity of the membranes developed in this study can be found in Fig. 17. Clearly, the inclusion of the 2D filler in the polymer matrix has enhanced the permeance of the gases, especially CO2. As a result, the selectivity of the system improved. This is due to the presence of highly selective ZIF-L that only allows CO2 gas molecules to permeate. On top of that, the tortuous path was created for the gas to permeate due to the shape of ZIF-L. The presence of filler in glassy polymer affects the polymer chain movement [36]. As more free volume is available, the gas can easily permeate across the membrane. However, among the MMHFM fabricated using unmodified ZIF-L, only PES/Z0.5 demonstrated the most remarkable increment of selectivity relative to the pristine PES at all feed pressure tested. Meanwhile, increasing the loading of unmodified ZIF-L turns out to be counterproductive.
At higher ZIF-L loading, the permeance of N2 gas increases greatly. This situation has negatively impacted the membrane’s ideal selectivity toward CO2. The trade-off between permeance and selectivity has clearly occurred. It is mainly due to the membrane defects and the increment of fractional free volume because of polymer chain disruption [37]. Interfacial defects allow gas with a larger size to permeate through the membrane. As pictured by the SEM images, the agglomeration of ZIF-L on the lumen side is also a major factor that diminishes the thin dense layer and forms surface defects. A steady decline of the selectivity was observed starting at 1 wt% of ZIF-L while the selectivity of the system further declines up to 62.1% at 5 wt%, relative to the pristine membrane. In fact, the selectivity is approaching the Knudsen diffusion’s selectivity at the pressure of 5 bar. Hence, loading higher than the 5 wt% was not pursued. Based on the available data, parallel improvement of permeance and selectivity was obtained at the lowest unmodified ZIF-L loading where the defects, voids and agglomeration are minimal.
On the contrary, alteration of the nanosheet with IL has resulted in significant improvement of the membrane’s separation performance. At the feed pressure of 2 bar, PES/IL@Z0.5, PES/IL@Z1 and PES/IL@Z2.5 demonstrated superior performance relative to the pristine PES membrane and any other MMHFM in this study. The simultaneous improvement of permeance and selectivity by these membranes is evident. The permeance of N2 was suppressed, which resulted in the improvement of the selectivity of the membrane. This is primarily due to the presence of IL which improved the polymer-filler interaction. Besides, it was proven from SEM images that the agglomeration of the filler in the membrane is minimal with the presence of IL. It is widely known that the separation process took place based on the solution diffusion mechanism and for asymmetric hollow fiber membrane, the largest resistance for the gas separation is mainly on the dense layer formed on the outer skin of the membrane. Uniform dispersion throughout the membrane structure allows more filler to be located on the dense layer of the membrane instead on the lumen or porous substructure.
Improvement of the membrane’s performance using IL@ZIF-L relative to the unmodified ZIF-L is particularly significant at the high loading. Although all membranes did not surpass the Robeson’s upper limit, but PES/IL@Z2.5 showed the greatest permeance and selectivity improvement relative to the unmodified ZIF-L loaded at similar loading by about 60.2% and 61.7%, respectively. Due to improved affinity, the immobilization of IL on the ZIF-L framework allows faster transportation of CO2 for the gas to permeate across the membrane [27]. Besides, Atash Jameh et al. [38] claimed that the filler has a unique ability for selective adsorption improvement and permeability increment due to their high affinity for CO2 rather than their molecular sieving mechanism. While such a condition is favorable for CO2 to travel, greater resistance is presented for N2 due to the configuration of the nanosheet and affinity of IL. This trend aligns with Shete et al. [39] and Kamble et al. [26].
In general, the trade-off effect remains a major concern despite the incorporation of IL@ZIF-L. Although such phenomenon is highly anticipated for a non-modified filler, our findings suggested that performance enhancement by IL is still limited by the amount of filler. The performance data of PES/IL@Z5 are taken as an example. It suffers from very low selectivity although it was proven that agglomeration is minimal due to IL. Herein, it is inferred that the membrane suffers from major defects at either modified or non-modified 5 wt% of ZIF-L. As the TGA data suggest, the poor thermal stability of MMHFM at highest filler loading is a result of polymer chain interruption. Such a situation allows gas of any size to permeate based on the Knudsen diffusion mechanism easily. It is hypothesized that the use of ZIF-L at loading 2.5 wt% is relevant or else, the system will experience a significant downturn of performance, despite filler modification. Nevertheless, the usage of IL allows exceptional performance to be recorded at much higher loading than its unmodified form. More importantly, this study reveals that improving interaction between filler and polymer; hence, uniform dispersion of ZIF-L in the polymer matrix is not a singular approach to enhance the performance of the mixed matrix. Optimum filler loading is also prominent in this study in addition to the pressure influence.
Figures 17 demonstrates the dependency of the membrane’s performance on the feed pressure too. Increasing the feed pressure has resulted in the improved permeance of both gases for all membranes. Except for PES/Z0.5, PES/IL@Z0.5 and PES/IL@Z1, all other MMHFMs experienced a steady decline of selectivity as the feed pressure increased. Interestingly, the presence of IL in the membrane allows the membrane to retain the upward trend of permeance and selectivity although such a case is only applicable for filler loading ≤ 1 wt%. As an example, the selectivity of PES/IL@Z0.5 increased from 4.21 to 5.88 as the feed pressure increases from 2 to 5 bar. Apparently, the presence of IL improves the pressure threshold before the performance of the membrane deteriorates. Such a claim apparent by comparing the performance of PES/Z2.5 and PES/IL@Z2.5. This is due to the saturation of CO2 as the pressure increases, and hence it reduces its permeation rate [40].
More importantly, this study found that the membrane’s performance was enhanced by using minimal filler loading (within the range studied in this work). This is due to the high aspect ratio of the nanosheet, where Kamble et al. [26] concluded similarly using 2D MoS2 as filler. Such observation was not observed in the 3D shape filler, specifically ZIF-8, proving the role of the filler’s geometry on the separation performance of a MMM. A membrane fabricated using ZIF-8 as the filler typically recorded performance improvement at much higher filler loading and bounded by a certain threshold before showing counterproductive results [16, 35]. For comparison purposes, Table 3 provides the performance data of MMHFM developed using a few types of MOFs of different shapes. Based on the current study and literature provided in the table, it is evident that nanosheet requires much lower loading to enhance the membrane’s selectivity compared to the typical 3D shape of ZIF-8. The inclusion of high filler loading incurs the cost and could compromise the mechanical strength of the membrane. On top of that, the presence of IL in the structure of 0.5 wt% filler has doubled the permeance of CO2 and the selectivity was improved by 86% at feed pressure of 2 bar.
4 Conclusions
The synthesis of ZIF-L and its incorporation as a filler in a MMHFM was discussed. To enhance the performance of the membrane, it was found that two key factors must be critically looked at. They are filler modification and optimum filler loading. The first resulted in much better dispersion of ZIF-L, especially at high loading, while the latter reduces polymer chain disruption and membrane defects. Overall, the presence of IL in the structure of ZIF-L has altered its properties such as surface area and surface charge. Both conditions have resulted in improved filler dispersion and enhancement of interaction between the filler and polymer matrix which elevates the permeance and selectivity of the membrane toward CO2.
References
Sarfraz, M.; Arshad, A.; Ba-Shammakh, M.: Predicting gas permeability through mixed-matrix membranes filled with nanofillers of different shapes. AJSE (2021). https://doi.org/10.1007/s13369-021-05996-8
Zhong, Z.; Yao, J.; Chen, R.; Low, Z.; He, M.; Liu, J.Z.; Wang, H.: Oriented two-dimensional zeolitic imidazolate framework-L membranes and their gas permeation properties. J. Mater. Chem. A. (2015). https://doi.org/10.1039/C5TA03707G
Liu, Y.-C.; Chen, C.-Y.; Lin, G.-S.; Chen, C.-H.; Wu, K.C.W.; Lin, C.-H.; Tung, K.-L.: Characterization and molecular simulation of Pebax-1657-based mixed matrix membranes incorporating MoS2 nanosheets for carbon dioxide capture enhancement. J. Membr. Sci. (2019). https://doi.org/10.1016/j.memsci.2019.04.025
Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X., and Gascon, J.: Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. (2015). https://doi.org/10.1038/nmat4113
Zhu, W.; Li, X.; Sun, Y.; Guo, R.; Ding, S.: Introducing hydrophilic ultra-thin ZIF-L into mixed matrix membranes for CO2/CH4 separation. RSC Adv. (2019). https://doi.org/10.1039/C9RA04147H
Vinh-Thang, H.; Kaliaguine, S.: Predictive models for mixed-matrix membrane performance: a review. Chem. Rev. (2013). https://doi.org/10.1021/cr3003888
Knak Jensen, S.J.; Tang, T.-H.; Csizmadia, I.G.: Hydrogen-bonding ability of a methyl group. J. Phys. Chem. A (2003). https://doi.org/10.1021/jp035024h
Koyuturk, B.; Altintas, C.; Kinik, F.P.; Keskin, S.; Uzun, A.: Improving gas separation performance of ZIF-8 by [BMIM][BF4] incorporation: interactions and their consequences on performance. J. Phys. Chem. C (2017). https://doi.org/10.1021/acs.jpcc.7b00848
Hu, Z.; Vatamanu, J.; Borodin, O.; Bedrov, D.: A molecular dynamics simulation study of the electric double layer and capacitance of [BMIM][PF6] and [BMIM][BF4] room temperature ionic liquids near charged surfaces. Phys. Chem. Chem. Phys. (2013). https://doi.org/10.1039/C3CP51218E
He, Z.; Alexandridis, P.: Nanoparticles in ionic liquids: interactions and organization. Phys. Chem. Chem. Phys. (2015). https://doi.org/10.1039/C5CP01620G
Thomas, A.; Ahamed, R.; Prakash, M.: Selection of a suitable ZIF-8/ionic liquid (IL) based composite for selective CO2 capture: the role of anions at the interface. RSC Adv. (2020). https://doi.org/10.1039/D0RA07927H
Chen, R.; Yao, J.; Gu, Q.; Smeets, S.; Baerlocher, C.; Gu, H.; Zhu, D.; Morris, W.; Yaghi, O.M.; Wang, H.: A two-dimensional zeolitic imidazolate framework with a cushion-shaped cavity for CO2 adsorption. Chem. Commun. (2013). https://doi.org/10.1039/C3CC44342F
Khan, I.U.; Othman, M.H.D.; Ismail, A.F.; Ismail, N.; Jaafar, J.; Hashim, H.; Rahman, M.A.; Jilani, A.: Structural transition from two-dimensional ZIF-L to three-dimensional ZIF-8 nanoparticles in aqueous room temperature synthesis with improved CO2 adsorption. Mater. Charact. (2018). https://doi.org/10.1016/j.matchar.2018.01.003
Md Nordin, N.A.H.; Racha, S.M.; Matsuura, T.; Misdan, N.; Abdullah Sani, N.A.; Ismail, A.F.; Mustafa, A.: Facile modification of ZIF-8 mixed matrix membrane for CO2/CH4 separation: synthesis and preparation. RSC Adv (2015). https://doi.org/10.1039/C5RA02230D
Abdelhamid, H.N.; Dowaidar, M.; Hällbrink, M.; Langel, Ü.: Gene delivery using cell penetrating peptides-zeolitic imidazolate frameworks. Microporous Mesoporous Mater. (2020). https://doi.org/10.1016/j.micromeso.2020.110173
Guo, Z.; Zheng, W.; Yan, X.; Dai, Y.; Ruan, X.; Yang, X.; Li, X.; Zhang, N.; He, G.: Ionic liquid tuning nanocage size of MOFs through a two-step adsorption/infiltration strategy for enhanced gas screening of mixed-matrix membranes. J. Membr. Sci. (2020). https://doi.org/10.1016/j.memsci.2020.118101
Kim, S.; Shamsaei, E.; Lin, X.; Hu, Y.; Simon, G.P.; Seong, J.G.; Kim, J.S.; Lee, W.H.; Lee, Y.M.; Wang, H.: The enhanced hydrogen separation performance of mixed matrix membranes by incorporation of two-dimensional ZIF-L into polyimide containing hydroxyl group. J. Membr. Sci. (2018). https://doi.org/10.1016/j.memsci.2017.12.022
Yan, Y.; Zhu, Y.; Zhang, L.; Zou, C.; Hu, Z.; Zhou, H.; Cai, L.: Study on the anodic behavior of AISI E52100 steel in two fluorine-containing ionic liquids. Res. Chem. Intermed. (2021). https://doi.org/10.1007/s11164-020-04386-3
Dangwal, S.; Ronte, A.; Lin, H.; Liu, R.; Zhu, J.; Lee, J.S.; Gappa-Fahlenkamp, H.; Kim, S.-J.: ZIF-8 membranes supported on silicalite-seeded substrates for propylene/propane separation. J. Membr. Sci. (2021). https://doi.org/10.1016/j.memsci.2021.119165
Huang, D.; Xin, Q.; Ni, Y.; Shuai, Y.; Wang, S.; Li, Y.; Ye, H.; Lin, L.; Ding, X.; Zhang, Y.: Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. (2018). https://doi.org/10.1039/C7RA09794H
Amedi, H.R.; Aghajani, M.: Aminosilane-functionalized ZIF-8/PEBA mixed matrix membrane for gas separation application. Microporous Mesoporous Mater. (2017). https://doi.org/10.1016/j.micromeso.2017.04.001
Liu, J.; He, J.; Wang, L.; Li, R.; Chen, P.; Rao, X.; Deng, L.; Rong, L.; Lei, J.: NiO-PTA supported on ZIF-8 as a highly effective catalyst for hydrocracking of Jatropha oil. Sci. Rep. (2016). https://doi.org/10.1038/srep23667
Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C.: Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab. J. Chem. (2016). https://doi.org/10.1016/j.arabjc.2013.09.034
Lessan, F.; Foudazi, R.: Effect of [EMIM][BF4] ionic liquid on the properties of ultrafiltration membranes. Polymer (2020). https://doi.org/10.1016/j.polymer.2020.122977
Hołda, A.K.; Vankelecom, I.F.J.: Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. (2015). https://doi.org/10.1002/app.42130
Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P.: Polyethersulfone based MMMs with 2D materials and ionic liquid for CO2, N2 and CH4 separation. J. Environ. Manage. (2020). https://doi.org/10.1016/j.jenvman.2020.110256
Chen, D.; Ying, W.; Guo, Y.; Ying, Y.; Peng, X.: Enhanced Gas Separation through Nanoconfined Ionic Liquid in Laminated MoS2 Membrane. ACS Appl. Mater. Interfaces. (2017). https://doi.org/10.1021/acsami.7b15762
Jamil, N.; Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Roslan, R.A.; Lau, W.J.; Ismail, A.F.: Mixed matrix membranes incorporated with reduced graphene oxide (rGO) and zeolitic imidazole framework-8 (ZIF-8) nanofillers for gas separation. J. Solid State Chem. (2019). https://doi.org/10.1016/j.jssc.2018.11.028
Meshkat, S.; Kaliaguine, S.; Rodrigue, D.: Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 mixed matrix membranes for CO2 separation. Sep. Purif. Technol. (2020). https://doi.org/10.1016/j.seppur.2019.116150
Ehsani, A.; Pakizeh, M.: Synthesis, characterization and gas permeation study of ZIF-11/Pebax® 2533 mixed matrix membranes. J. Taiwan Inst. Chem. Eng. (2016). https://doi.org/10.1016/j.jtice.2016.07.005
Li, X.-G.; Shao, H.-T.; Bai, H.; Huang, M.-R.; Zhang, W.: High-resolution thermogravimetry of polyethersulfone chips in four atmospheres. J. Appl. Polym. Sci. (2003). https://doi.org/10.1002/app.13091
Alqaheem, Y.; Alomair, A.A.: Microscopy and spectroscopy techniques for characterization of polymeric membranes. Membranes (2020). https://doi.org/10.3390/membranes10020033
Velu, S.; Arthanareeswaran, G.; Lade, H.: Removal of organic and inorganic substances from industry wastewaters using modified aluminosilicate-based polyethersulfone ultrafiltration membranes. Environ. Prog. Sustain. Energy (2017). https://doi.org/10.1002/ep.12614
Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y.: Metal-organic framework-graphene oxide composites: a facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Membr. Sci. (2016). https://doi.org/10.1016/j.memsci.2016.08.043
Li, H.; Tuo, L.; Yang, K.; Jeong, H.-K.; Dai, Y.; He, G.; Zhao, W.: Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: interfacial toughening effect of ionic liquid. J. Membr. Sci. (2016). https://doi.org/10.1016/j.memsci.2016.03.050
Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J.: Sorption, transport, and structural evidence for enhanced free volume in poly(4-methyl-2-pentyne)/fumed silica nanocomposite membranes. Chem. Mater. (2003). https://doi.org/10.1021/cm020672j
Shah Buddin, M.M.H.; Ahmad, A.L.: A review on metal-organic frameworks as filler in mixed matrix membrane: recent strategies to surpass upper bound for CO2 separation. J. CO2 Util. (2021). https://doi.org/10.1016/j.jcou.2021.101616
Atash Jameh, A.; Mohammadi, T.; Bakhtiari, O.: Preparation of PEBAX-1074/modified ZIF-8 nanoparticles mixed matrix membranes for CO2 removal from natural gas. Sep. Purif. Technol. (2020). https://doi.org/10.1016/j.seppur.2019.115900
Shete, M.; Kumar, P.; Bachman, J.E.; Ma, X.; Smith, Z.P.; Xu, W.; Mkhoyan, K.A.; Long, J.R.; Tsapatsis, M.: On the direct synthesis of Cu(BDC) MOF nanosheets and their performance in mixed matrix membranes. J. Membr. Sci. (2018). https://doi.org/10.1016/j.memsci.2017.12.002
Liu, B.; Li, D.; Yao, J.; Sun, H.: Improved CO2 separation performance and interfacial affinity of mixed matrix membrane by incorporating UiO-66-PEI@[bmim][Tf2N] particles. Sep. Purif. Technol. (2020). https://doi.org/10.1016/j.seppur.2020.116519
Dai, Y.; Johnson, J.R.; Karvan, O.; Sholl, D.S.; Koros, W.J.: Ultem®/ZIF-8 mixed matrix hollow fiber membranes for CO2/N2 separations. J. Membr. Sci. (2012). https://doi.org/10.1016/j.memsci.2012.01.044
Zhu, H.; Jie, X.; Cao, Y.: Fabrication of functionalized MOFs incorporated mixed matrix hollow fiber membrane for gas separation. J. Chem. (2017). https://doi.org/10.1155/2017/2548957
Khan, I.U.; Othman, M.H.D.; Jilani, A.; Ismail, A.F.; Hashim, H.; Jaafar, J.; Zulhairun, A.K.; Rahman, M.A.; Rehman, G.U.: ZIF-8 based polysulfone hollow fiber membranes for natural gas purification. Polym. Test. (2020). https://doi.org/10.1016/j.polymertesting.2020.106415
Acknowledgements
The authors acknowledged the financial assistance and facilities provided by Universiti Sains Malaysia, Universiti Teknologi MARA and Fundamental Research Grant Scheme by Ministry of Higher Education Malaysia (FRGS/1/2020/TK0/USM/01/4, 203.PJKIMIA.6071484).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah Buddin, M.M.H., Ahmad, A.L. Synergistic Effect of 2D ZIF Loading and Ionic Liquid Modification on Improving Gas Separation Performance of PES-Based Mixed Matrix Hollow Fiber Membrane. Arab J Sci Eng 48, 16243–16261 (2023). https://doi.org/10.1007/s13369-023-07948-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-07948-w