Abstract
Treatment of brackish water from pathogenic microbes is crucial for sustainable aquaculture production and preventing the spread of infectious diseases. However, the treatment of brackish water is still challenging due to the high salinity and the high antimicrobial resistance. Here, we exploit a facile and effective approach to synthesize silica gel embedded with silver nanoparticles (7–48 nm) for broad-spectrum antimicrobial activity. The incorporation of silver nanoparticles into silica gel (AgNPs@SG) is confirmed by flame atomic absorption spectrometry, X-ray diffraction, N2 physisorption, and transmission electron microscopy. The AgNPs@SG material exhibits wide-spectrum antimicrobial activity against the studied microorganisms (Pseudomonas aeruginosa, Escherichia coli, and Candida albicans) due to preventing the aggregation of silver nanoparticles and their effective contact with the microorganisms. Most importantly, the applicability of the synthesized AgNPs@SG for the microbial treatment of brackish water is investigated on different water samples collected from Manzala Lake. Remarkably, the amount of viable bacteria in the brackish water decreases by about 93% using AgNPs@SG material that not only combats antibiotic-resistant strains but also works under harsh conditions such as multiple-source contamination, high eutrophic state, and salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aquaculture, the farming of fish and seafood, is an indispensable source of animal protein for human consumption [1, 2]. Remarkably, it is a fast-growing food production industry to meet the huge demand of the world’s growing population and to achieve a sustainable food supply. However, aquaculture production has to be improved by an additional 46.4 million metric tons by 2030 to ensure a food-secure future [3, 4]. In this regard, brackish water aquaculture and also coastal aquaculture have a crucial role in the overall fisheries improvement since it supplies demanded fish as food on regular basis [5]. Unfortunately, the untreated and partially treated effluents are usually released into the freshwater system especially in developing countries, resulting in microbial proliferation, and consequently contamination with pathogenic bacteria, viruses, and parasites [6,7,8]. Besides the severe impacts on ecosystems, microbial contamination influences not only the health of fish but also raises serious concerns regarding fish used for human consumption [9]. The appearance of diseases, however, hinders the growth of the aquaculture and fisheries industry. One model example that highlights the aforementioned aspects is Lake Manzala in northern Egypt.
Lake Manzala is one of the largest and most important northern coastal lakes in Egypt and a target of many different research studies [10,11,12,13,14]. It is a valuable natural brackish water resource for the fish caught, wildlife, and the hydrologic and biologic regime. Furthermore, it is recognized as the most productive fishery ground of the country’s lakes, since it produces about 50% of the fish caught in the northern lakes and freshwater fisheries [15]. However, Manzala Lake suffers from large contents of pollutants discharged from various sources, for example, industrial, agricultural, and domestic untreated waste. As a result, it is heavily polluted by heavy metals and high nutrients released from Bahr El Baqar, Faraskour, El Inaniya, El Serw, El Gamaliya, El Matariya, Hadus, and Ramsis drains [16]. The bacterial counts such as total viable bacterial and fecal coliform of lake water and fish indicate high contamination in Manzala Lake, where 90 isolates were identified including Escherichia coli, Citrobacter freundii, Proteus mirabilis, and Sphingomonas paucimobilis [17]. Different antibiotics such as penicillin, ampicillin, cefotaxime, chloramphenicol, rifampin, tetracycline, streptomycin, and gentamicin were tested against the isolates. The results showed a high resistance pattern among the different species comprising plasmid DNA, indicating that these bacterial pathogens have risk factors in the communities around Lake Manzala. Generally, a potential risk to humans and the environment is revealed by the appearance of antibiotic-resistant bacteria in aquaculture ponds [18]. Consequently, there is a pressing need to develop robust and selective antimicrobial materials to combat a wide range of microbes without harmful effects on the aquatic species and the environment.
On that front, silver nanoparticles (AgNPs) are of outstanding importance because of their distinctive physicochemical and biological properties. AgNPs have been recognized as an effective antimicrobial agent against a wide spectrum of pathogens even those having antibiotic resistance [19, 20]. Several physical and chemical approaches such as physiochemical, photochemical, chemical reduction, and microwave irradiation are typically utilized for the synthesis of these nanoparticles [21,22,23,24]. Most of these methods necessitate labor-intensive equipment and are expensive and hazardous because of the usage of toxic chemicals. Alternatively, biological routes for the synthesis of AgNPs are eco-friendly, non-toxic, affordable, and simple. In this regard, natural compounds, plant extracts, bacteria, fungi, algae, etc., can be effectively employed as reducing, capping, and stabilizing agents for the synthesis of AgNPs instead of expensive toxic chemicals [21,22,23,24]. For example, the biologically synthesized AgNPs using different plant extracts exhibited a good zone of inhibition against both bacterial and fungal strains [25]. Moreover, AgNPs were synthesized using leaf extracts of Hibiscus cannabinus as a template and stabilizing agent [34]. The influence of silver ion concentrations (5.0, 8.0, and 10 mM) on the antibacterial activity of these AgNPs against different bacteria (E. coli, Proteus mirabilis, and Shigella flexneri) was investigated. Notably, AgNPs synthesized at a low Ag+ ion concentration (5.0 mM) showed enhanced antibacterial activity relative to those synthesized at higher Ag+ ion concentrations [26].
The effectiveness of AgNPs antibiotic resistance is due to their different ways of interaction such as interaction with sulfhydryl groups of enzymes, attachment to the cell membrane surface, or generating reactive oxygen species (ROS), resulting in the microorganism cell death [27,28,29,30].
One should emphasize that the antimicrobial efficiency of AgNPs is strongly influenced by their size, shape, stability, and surface functionalization [31, 32]. Normally, AgNPs have a high affinity to agglomerate due to the high surface energy, leading to a substantial decrease in their antimicrobial ability as a function of time. To prevent agglomeration, various strategies have been developed including capping, organic caging, and encapsulation [33]. Among them, encapsulation is an effective approach for stabilizing nanoparticles due to several merits such as simplicity, providing extremely long-term stability, shelf-storing ability, and maintaining high activity even at a low concentration of nanoparticles [34,35,36]. The encapsulating materials may include various inorganic oxides, carbon, clay, silica, and metal–organic frameworks, which endows the metal nanoparticles with additional functionality [34, 36,37,38,39]. For instance, the antibacterial properties of Ag NPs encapsulated in chitosan-4-((E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl) benzoic acid were evaluated against S. aureus and E. coli [41]. Obviously, functionalized Ag NPs strongly inhibited S. aureus and E. coli bacterial growth with diameters of 20 ± 0.53 and 20 ± 0.45 nm, respectively [40]. Additionally, biosynthesized AgNPs were encapsulated in silica composites during the gel formation of MCM-41. Interestingly, the results show an excellent antimicrobial effect of these materials against S. aureus, E. coli, and Candida albicans applying either static (cup–plate) or dynamic (CFU) techniques [36]. In this sense, the use of silica-based materials for stabilizing nanoparticles for biological applications is of special interest due to their biocompatibility, high chemical and thermal stability, and hydrophilic nature [36, 39, 41]. Furthermore, the surface area of silica-based materials is typically large, allowing better contact between the microorganisms and the functionalized AuNPs, consequently achieving much higher antimicrobial activity than conventional AgNPs.
So far, most of the reported antimicrobial materials are more suitable for biomedical applications (e.g., wound-healing) than water disinfection purposes due to their limited capability to disinfect only small volumes of water [8, 34, 36]. Furthermore, the salinity of brackish water might be another significant challenge for many antimicrobial materials. In this contribution, we report on the development of novel antimicrobial material based on the encapsulation of biosynthesized AgNPs into silica gel (AgNPs@SG) via a facile one-pot synthesis approach. The AgNPs@SG is characterized by flame atomic absorption spectrometry, Fourier-transform infrared spectroscopy, X-ray diffraction, N2 physisorption, scanning electron microscopy, and transmission electron microscopy. The antimicrobial activity of the AgNPs@SG is investigated against Pseudomonas aeruginosa, E. coli, and C. albicans as representative microorganisms for Gram-positive, Gram-negative bacteria, and yeast, respectively, using the cup–plate method. Most importantly, the applicability of the synthesized AgNPs@SG for the disinfection of brackish water is evaluated on different water samples collected from Manzala Lake. The developed material might be applicable for other antimicrobial purposes, not only for water disinfection.
2 Experimental
2.1 Materials and Methods
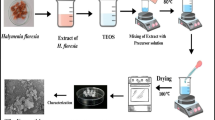
All chemicals were of analytical reagent grade and used as received. Double-distilled water was used during the experiments. Sodium trisilicate (Na2Si3O7, 10% H2O) was obtained from Fluka, Switzerland. Silver nitrate (AgNO3) was supplied by Merck, Germany. Macrococcus bovicus was isolated locally from the soil and was used for the biosynthesis of AgNPs from AgNO3 with a size range of about 7–48 nm, as previously described [27]. Natural water samples were collected from the middle of Manzala Lake (Alhomra) and at the Bahr El Baqar drain inflows (Elbashtir).
2.2 Synthesis of AgNPs@SG
Silica gel was synthesized using trisodium silicate as a source of silicon. Typically, 46 mL of 1.23 M Na2Si3O7 was diluted by double-distilled water to 100 mL before mixing with 60 mL of 1.5 mmol L−1 AgNPs. After stirring for 30 min, the pH of the mixture was adjusted to 2.0 ± 0.1 using concentrated HCl. The formed gel was allowed to stand for 24 h at room temperature (around 25 °C), centrifuged, washed three times with double-distilled water, and dried at 50 °C for 72 h. Approximately 3.67 g of faint reddish-brown silica gel containing AgNPs was formed (AgNPs @SG).
2.3 Characterization
Infrared spectra were recorded in the regime of 4000–400 cm−1 by a Perkin-Elmer 2000 FTIR spectrophotometer using KBr disks. The powder XRD pattern of AgNPs @SG was recorded by a Bruker AXS D8 ADVANCE using CuKα radiation (λ = 1.54 Å) under 40 kV and 30 mA. Specific surface area, pore volume, and average pore diameter were measured by the N2 physisorption method. Surface area measurements were conducted on a Quantachrome USA NOVA 22 system by gaseous N2 adsorption at -196 °C. The AgNPs@SG sample (0.2 g) was degassed at 300 °C for 3 h. The TEM characterization was carried out using a JEOL model 1200 EX electron microscope at an operating voltage of 120 kV.
2.4 Antimicrobial Activity Using the Cup–Plate Method
The bacteria strains (Gram-positive, P. aeruginosa, and Gram-positive, E. coli) and yeast (C. albicans) were inoculated in a 20 ml of sterile nutrient broth and incubated at 37 °C for 16–18 h. The nutrient broth cultures were wiped onto the surface of sterile nutrient agar plates by a sterile cotton swab. Agar wells were prepared using a sterile 10-mm-diameter cork drill (Srinivasan et al., 2001). Using a micropipette, 100 μl of AgNPs@SG or control suspensions were added to different wells in the plate. The plates were incubated in an upright position at 37 °C for 24 h. The diameter of the damping zones was measured in millimeters and the results were recorded.
3 Results and Discussion
3.1 Characterization
The FTIR spectrum of AgNPs@SG is presented in Fig. 1a. The bands at around 3470 and 1642 cm−1 are attributed to the stretching and bending vibrations of silanol groups (Si–OH) and water molecules, respectively. The bands observed at 1083 and 796 cm−1 are typical for the anti-symmetric and symmetric stretching modes (Si–O–Si) of [SiO4] moieties, respectively. Moreover, the peak noticed at 965 cm−1 is corresponding to the Si–OH stretching mode, while the band at 466 cm−1 is ascribed to Si–O–Si bending vibrations [42, 43]. These findings reveal that the addition of AgNPs during the synthesis does not affect the formation of silica gel.
Figure 1b shows the XRD pattern of the synthesized AgNPs@SG material. The amorphous hump of silica is detected at Braggs’ angle of 2θ = 26.65° which is characteristic of amorphous silica. The weak peaks at 2θ = 39.14° and 2θ = 44.34° are matching to the (111) and (200) reflections of Ag, respectively (JCPDS 04-0873). However, the (220) diffraction is barely noticeable, which might be due to the small size of AgNPs. These observations indicate the encapsulation of AgNPs into silica gel [42].
Flame AAS analysis of AgNPs@SG after acid digestion confirms the presence of Ag in the synthesized sample at a concentration of 3.40%.
The N2 physisorption analysis was carried out to investigate the textural characterization of the AgNPs@SG material. The N2 adsorption–desorption isotherm indicates that the curve is attributed to type IV(a) isotherm and H2 hysteresis loop, indicating a uniform pore diameter distribution (Fig. 2a). The textural properties of the AgNPs@SG material are shown in the inset of Fig. 2a. The AgNPs@SG material has a large surface area of (SBET = 537.2 m2/g), as calculated according to Brunauer, Emmett, and Teller [44]. The total surface area value is comparable to those reported in the literature, revealing that the presence of AgNPs hardly influences the surface area [45]. Figure 2b displays the pore size distribution calculated according to the Barrett, Joyner, and Halenda (BJH) method for the synthesized sample [46]. The narrow distribution with a mean pore diameter of r = 1.6 nm indicates the uniform mesoporous structure of AgNPs@SG.
Figure 3a presents the TEM images of the synthesized AgNPs@SG material. As observed, AgNPs appeared as black nanoparticles with particle sizes of about 10–50 nm. They are homogeneously embedded into the branches of the flower-like structure of silica gel.
Figure 3b–d displays the SEM micrographs of the AgNPs@SG recorded at different magnifications. A rough surface characterized by agglomerated irregular and dense particles is observed for the AgNPs@SG. These observations are in good agreement with N2 physisorption analysis which shows a high surface area of the AgNPs@SG. Furthermore, AgNPs are homogeneously distributed over the surface of silica gel as highlighted in red circles (Fig. 3b, c).
3.2 Antimicrobial Activity
The antimicrobial activity of the AgNPs@SG material was investigated against S. aureus, E. coli, and C. albicans using the cup–plate method based on the zone of inhibition. The diameters of the inhibition zones obtained by the synthesized AgNPs@SG, and silica gel compared to those reported in the literature are shown in Table 1. As expected, silica gel is inactive toward the tested microbes. On the other hand, the silica gel encapsulating AgNPs exhibits significant broad-spectrum antimicrobial activity with comparable inhibition zone diameters of about 12 mm against all the investigated microbes. This antimicrobial activity might be due to the adsorption of the microorganism from the bulk to the hydrophilic silanol surface of SG in close proximity to the AgNPs surface, which is driven by the hydrophilicity of the mesoporous silica surface structure [36]. The plasmonic effect of AgNPs on the surface of the microorganism allows the fatal effect of AdNPs on the microbes at this close contact between them. Still, the mechanism of AgNPs as antimicrobial agents is not fully understood. AgNPs can inhibit bacterial growth by promoting DNA damage, membrane destruction, ROS generation, protein denaturation, and enzyme inactivation [27,28,29, 47]. Furthermore, AgNPs can adhere to fungal hyphae and generate insoluble compounds to inactivate sulfhydryl groups of the fungal cell wall, disrupt the membrane, and bind lipids and enzymes, inducing cell lysis [48]. As presented in Table 1, the antimicrobial functionality of the AgNPs@SG material is comparable and even higher than that of many reported modified materials in the literature. The higher antimicrobial activity of AgNPs@SG material could be explained by the large surface area of the silica gel offering an active adsorbing hydrophilic surface for the microorganism in such a trap to contact AgNPs, facilitating the interaction between the microbes and AgNPs. These results indicate the potential of the proposed material with affinity to stabilize AgNPs without affecting their activity.
3.3 Broad-Spectrum Antimicrobial Disinfection of Contaminated Brackish Water
Brackish water samples were collected from the middle of Manzala Lake (Alhomra) and near the Bahr El Baqar drain inflows (Elbashtir) to represent low and high biological oxygen demand (BOD) values (6.3–10.9 and 34.2–42.5 mg L−1) while the state of eutrophication was low and very high, respectively [10]. The antimicrobial activity of the proposed AgNPs@SG was assessed using brackish water samples. For this purpose, three different mass concentrations of AgNPs@SG were investigated and the colony-forming units (CFU) analysis was performed. As shown in Table 2 and Fig. 4, the rate of microbial reduction sustainably increases with the mass of AgNPs@SG due to the higher content of AgNPs. Interestingly, the amount of viable bacteria in the brackish water decreases by about 93%, using 0.10 g of the proposed material. Most importantly, the synthesized AgNPs@SG has not only the ability to combat antibiotic-resistant strains but also serves under harsh conditions such as high BOD, eutrophication, and salinity.
4 Conclusions
A green and a facile approach is presented for the decoration of silica-gel with AgNPs (AgNPs@SG) for the antimicrobial treatment of brackish water. The embedding of AgNPs into silica gel prevents the aggregation of AgNP and hence sustains their dominant antimicrobial efficacy. The AgNPs@SG material shows a wide-spectrum and high antimicrobial activity against the investigated microorganisms (P. aeruginosa, E. coli, and C. albicans). Interestingly, the amount of viable bacteria in the brackish water reduces by about 93% using AgNPs@SG material that combats antibiotic-resistant strains. However, the toxicity of immobilized AgNPs of different concentrations on living organisms has not been investigated yet. Such investigations are crucial for drawing a consistent picture of the practical application of AgNPs for the microbial treatment of water. The present study provides a proof of concept for the validity of AgNPs@SG to work as an antimicrobial material under harsh conditions such as multiple-source contamination, high eutrophic state, and salinity, thus we selected brackish water for the antimicrobial investigations. Therefore, AgNPs@SG might be auspicious candidates as filling agents for column filters for sterilizing water supplies, and for combating biomedical pathogens.
References
The State of World Fisheries and Aquaculture 2020: Sustainability in Action (FAO) (2020).
Napier, J.A.; Haslam, R.P.; Olsen, R.-E.; Tocher, D.R.; Betancor, M.B.: Agriculture can help aquaculture become greener. Nat. Food 1, 680–683 (2020). https://doi.org/10.1038/s43016-020-00182-9
Bank, W.: Fish to 2030: prospects for fisheries and aquaculture (English) (2013)
Thiang, E.L.; Lee, C.W.; Takada, H.; Seki, K.; Takei, A.; Suzuki, S.; Wang, A.; Bong, C.W.: Antibiotic residues from aquaculture farms and their ecological risks in Southeast Asia: a case study from Malaysia. Ecosyst. Health Sustain. 7, 1926337 (2021). https://doi.org/10.1080/20964129.2021.1926337
Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M.: A 20-year retrospective review of global aquaculture. Nature 591, 551–563 (2021). https://doi.org/10.1038/s41586-021-03308-6
Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B.: The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006). https://doi.org/10.1126/science.1127291
Lai, W.W.-P.; Lin, Y.-C.; Wang, Y.-H.; Guo, Y.L.; Lin, A.Y.-C.: Occurrence of emerging contaminants in aquaculture waters: cross-contamination between aquaculture systems and surrounding waters. Water Air Soil Pollut. 229, 249 (2018). https://doi.org/10.1007/s11270-018-3901-3
Kumar, A.; Boyer, C.; Nebhani, L.; Wong, E.H.H.: Highly bactericidal macroporous antimicrobial polymeric gel for point-of-use water disinfection. Sci. Rep. 8, 7965 (2018). https://doi.org/10.1038/s41598-018-26202-0
Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valciņa, O.; Bartkevičs, V.; Bērziņš, A.: Major foodborne pathogens in fish and fish products: a review. Ann. Microbiol. 66, 1–15 (2016). https://doi.org/10.1007/s13213-015-1102-5
Elmorsi, R.; Abou-El-Sherbini, K.; Hamed, M.: Physicochemical properties of Manzala Lake, Egypt. Egypt. J. Chem. 0, 519–535 (2017). https://doi.org/10.21608/ejchem.2017.776.1025
Zahran, E.; Elbahnaswy, S.; Mamdouh, A.; El-Matbouli, M.: Xenosteroids in aquaculture with special consideration to Lake Manzala (Northern Delta Lake, Egypt): types, sources and mechanism of action. Aquac. Res. 52, 1–16 (2021). https://doi.org/10.1111/are.15504
Elmorsi, R.R.; Abou-El-Sherbini, K.S.; Abdel-Hafiz Mostafa, G.; Hamed, M.A.: Distribution of essential heavy metals in the aquatic ecosystem of Lake Manzala, Egypt. Heliyon 5, e02276 (2019). https://doi.org/10.1016/j.heliyon.2019.e02276
Abdel-Hamid, H.; Gaber, H.S.; El-Khayat, H.M.M.; Mahmoud, K.M.A.; Abu Taleb, H.M.A.: Studies on the effect of pollution on Lake Manzala ecosystem in Port-Said, Damietta and Dakahlia governorates, Egypt. J. Egypt. Soc. Parasitol. 45, 153–166 (2015). https://doi.org/10.12816/0010861
Elmorsi, R.R.; Mostafa, G.A.-H.; Abou-El-Sherbini, K.S.: Homoionic soda-activated bentonite for batch-mode removal of Pb(II) from polluted brackish water. J. Environ. Chem. Eng. 9, 104606 (2021). https://doi.org/10.1016/j.jece.2020.104606
Nasr, M.; Elbeih, S.F.; Negm, A.M.; Kostianoy, A.: Overview for recent applications of remote sensing in Egypt. In: Environmental Remote Sensing in Egypt. Springer, Geophysics, pp. 13–22. Springer, Cham (2020). https://doi.org/10.1007/978-3-030-39593-3_2
Hamed, Y.A.; Abdelmoneim, T.S.; ElKiki, M.H.; Hassan, M.A.; Berndtsson, R.: Assessment of heavy metals pollution and microbial contamination in water, sediments and fish of Lake Manzala, Egypt. Life Sci. J. 10, 86–99 (2013)
Zaky, M.M.M.; Salem, M.A.M.: Environmental factors influencing antibiotic resistant bacterial pathogens in polluted Lake Manzala, Egypt. J. Bacteriol. Parasitol. (2015). https://doi.org/10.4172/2155-9597.1000249
Nadella, R.K.; Panda, S.K.; Madhusudana Rao, B.; Pani Prasad, K.; Raman, R.P.; Mothadaka, M.P.: Antibiotic resistance of culturable heterotrophic bacteria isolated from shrimp (Penaeus vannamei) aquaculture ponds. Mar. Pollut. Bull. 172, 112887 (2021). https://doi.org/10.1016/j.marpolbul.2021.112887
Oćwieja, M.; Adamczyk, Z.; Morga, M.; Kubiak, K.: Silver particle monolayers: formation, stability, applications. Adv. Colloid Interface Sci. 222, 530–563 (2015). https://doi.org/10.1016/j.cis.2014.07.001
Islam, M.A.; Jacob, M.V.; Antunes, E.: A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manage 281, 111918 (2021). https://doi.org/10.1016/j.jenvman.2020.111918
Abass Sofi, M.; Sunitha, S.; Ashaq Sofi, M.; Khadheer Pasha, S.K.; Choi, D.: An overview of antimicrobial and anticancer potential of silver nanoparticles. J. King Saud Univ. Sci. 34, 101791 (2022). https://doi.org/10.1016/j.jksus.2021.101791
Huq, Md.A.; Ashrafudoulla, Md.; Rahman, M.M.; Balusamy, S.R.; Akter, S.: Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: a review. Polymers (Basel) 14, 742 (2022). https://doi.org/10.3390/polym14040742
Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z.: A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 19, 674 (2022). https://doi.org/10.3390/ijerph19020674
Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K.: Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: a review. Adv. Colloid Interface Sci. 256, 326–339 (2018). https://doi.org/10.1016/j.cis.2018.03.001
Vinodhini, S.; Vithiya, B.S.M.; Prasad, T.A.A.: Green synthesis of silver nanoparticles by employing the Allium fistulosum, Tabernaemontana divaricate and Basella alba leaf extracts for antimicrobial applications. J. King Saud Univ. Sci. 34, 101939 (2022). https://doi.org/10.1016/j.jksus.2022.101939
Bindhu, M.R.; Umadevi, M.: Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 101, 184–190 (2013). https://doi.org/10.1016/j.saa.2012.09.031
Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V.: Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 7, 1831 (2016). https://doi.org/10.3389/fmicb.2016.01831
Mikhailova, E.O.: Silver nanoparticles: mechanism of action and probable bio-application. J. Funct. Biomater. 11, 84 (2020). https://doi.org/10.3390/jfb11040084
Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J.: Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 12, 4271–4275 (2012). https://doi.org/10.1021/nl301934w
Athreya, A.G.; Shareef, M.I.; Gopinath, S.M.: Antibacterial activity of silver nanoparticles isolated from cow’s milk, hen’s egg white and lysozyme: a comparative study. Arab. J. Sci. Eng. 44, 6231–6240 (2019). https://doi.org/10.1007/s13369-018-3685-1
Agnihotri, S.; Mukherji, S.; Mukherji, S.: Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 4, 3974–3983 (2014). https://doi.org/10.1039/C3RA44507K
Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; Pohl, P.; Krolicka, A.; Piosik, J.: The impact of surface functionalization on the biophysical properties of silver nanoparticles. Nanomaterials 9, 973 (2019). https://doi.org/10.3390/nano9070973
Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L.: Stabilization of silver and gold nanoparticles: preservation and improvement of plasmonic functionalities. Chem. Rev. 119, 664–699 (2019). https://doi.org/10.1021/acs.chemrev.8b00341
Abdel-Aziz, M.S.; Abou-El-Sherbini, K.S.; Hamzawy, E.M.A.; Amr, M.H.A.; El-Dafrawy, S.: Green synthesis of silver nano-particles by Macrococcus bovicus and its immobilization onto Montmorillonite clay for antimicrobial functionality. Appl. Biochem. Biotechnol. 176, 2225–2241 (2015). https://doi.org/10.1007/s12010-015-1710-3
Khan, Z.: Encapsulation of silver nanoparticles into the helix of water soluble starch and their sensing properties. Int. J. Biol. Macromol. 136, 165–176 (2019). https://doi.org/10.1016/j.ijbiomac.2019.05.012
Abou-El-Sherbini, K.S.; Amer, M.H.A.; Abdel-Aziz, M.S.; Hamzawy, E.M.A.; Sharmoukh, W.; Elnagar, M.M.: Encapsulation of biosynthesized nanosilver in silica composites for sustainable antimicrobial functionality. Global Chall. 2, 1800048 (2018). https://doi.org/10.1002/gch2.201800048
Fan, C.; Lv, X.; Liu, F.; Feng, L.; Liu, M.; Cai, Y.; Liu, H.; Wang, J.; Yang, Y.; Wang, H.: Silver nanoclusters encapsulated into metal-organic frameworks with enhanced fluorescence and specific ion accumulation toward the microdot array-based fluorimetric analysis of copper in blood. ACS Sens. 3, 441–450 (2018). https://doi.org/10.1021/acssensors.7b00874
Zhuang, P.; Zhang, P.; Li, K.; Kumari, B.; Li, D.; Mei, X.: Silver nanoclusters encapsulated into metal-organic frameworks for rapid removal of heavy metal ions from water. Molecules 24, 2442 (2019). https://doi.org/10.3390/molecules24132442
Tian, Y.; Qi, J.; Zhang, W.; Cai, Q.; Jiang, X.: Facile, one-pot synthesis, and antibacterial activity of mesoporous silica nanoparticles decorated with well-dispersed silver nanoparticles. ACS Appl. Mater. Interfaces 6, 12038–12045 (2014). https://doi.org/10.1021/am5026424
Mathew, T.V.; Kuriakose, S.: Photochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groups. Mater. Sci. Eng. C 33, 4409–4415 (2013). https://doi.org/10.1016/j.msec.2013.06.037
Parandhaman, T.; Das, A.; Ramalingam, B.; Samanta, D.; Sastry, T.P.; Mandal, A.B.; Das, S.K.: Antimicrobial behavior of biosynthesized silica–silver nanocomposite for water disinfection: a mechanistic perspective. J. Hazard Mater. 290, 117–126 (2015). https://doi.org/10.1016/j.jhazmat.2015.02.061
Antony, R.; David Manickam, S.T.; Kollu, P.; Chandrasekar, P.V.; Karuppasamy, K.; Balakumar, S.: Highly dispersed Cu(ii), Co(ii) and Ni(ii) catalysts covalently immobilized on imine-modified silica for cyclohexane oxidation with hydrogen peroxide. RSC Adv. 4, 24820–24830 (2014). https://doi.org/10.1039/C4RA01960A
Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Zhang, X.; Xia, C.: Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method: a simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem. Mater. 20, 5090–5099 (2008). https://doi.org/10.1021/cm8006233
Brunauer, S.; Emmett, P.H.; Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938). https://doi.org/10.1021/ja01269a023
Brahmi, D.; Merabet, D.; Belkacemi, H.; Mostefaoui, T.A.; Ouakli, N.A.: Preparation of amorphous silica gel from Algerian siliceous by-product of kaolin and its physico chemical properties. Ceram. Int. 40, 10499–10503 (2014). https://doi.org/10.1016/j.ceramint.2014.03.021
Barrett, E.P.; Joyner, L.G.; Halenda, P.P.: The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 73, 373–380 (1951). https://doi.org/10.1021/ja01145a126
Venkatesan, J.; Kim, S.-K.; Shim, M.: Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 6, 235 (2016). https://doi.org/10.3390/nano6120235
Hamedi, S.; Ghaseminezhad, M.; Shokrollahzadeh, S.; Shojaosadati, S.A.: Controlled biosynthesis of silver nanoparticles using nitrate reductase enzyme induction of filamentous fungus and their antibacterial evaluation. Artif. Cells Nanomed. Biotechnol. 45, 1588–1596 (2017). https://doi.org/10.1080/21691401.2016.1267011
Shameli, K.; Ahmad, M.M.B.; Mohsen, Z.; Yunis, W.Z.; Ibrahim, N.A.: Fabrication of silver nanoparticles doped in the zeolite framework and antibacterial activity. Int. J. Nanomed. 6, 331–341 (2011). https://doi.org/10.2147/IJN.S16964
Zahran, M.K.; Ahmed, H.B.; El-Rafie, M.H.: Surface modification of cotton fabrics for antibacterial application by coating with AgNPs-alginate composite. Carbohydr. Polym. 108, 145–152 (2014). https://doi.org/10.1016/j.carbpol.2014.03.005
Kora, A.J.; Beedu, S.R.; Jayaraman, A.: Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2, 17 (2012). https://doi.org/10.1186/2191-2858-2-17
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no known competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abou-El-Sherbini, K.S., Elmorsi, R.R., Elnagar, M.M. et al. Silica-Gel Incorporated Biosynthesized-Silver Nanoparticles for Sustainable Antimicrobial Treatment of Brackish Water Aquaculture. Arab J Sci Eng 48, 7387–7394 (2023). https://doi.org/10.1007/s13369-022-07395-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07395-z