Abstract
To enhance the ability and practicality of chitosan used for wastewater treatment, this study aimed to modify chitosan through cross-linking and addition of magnetic properties. Cross-linked magnetic chitosan microspheres were prepared using the inverse phase dispersion method and then applied for Cd2+ removal from water. Fe3O4 nanoparticles prepared from local iron sand were embedded with chitosan cross-linked by polyethylene glycol diglycidyl ether (PEDGE) to produce PEDGE cross-linked magnetic chitosan (PEDGE-MCh) microspheres. PEDGE-MCh microspheres were characterized by Fourier transform infrared, scanning electron microscopy energy-dispersive X-ray spectroscopy, and X-ray diffraction analyses. Altogether, the characterization confirmed the formation of cross-linking and the interaction among components in the adsorbent. Additionally, the characterization also revealed the additional features of functional groups, rougher surface, and more amorphous properties beneficial in pollutant removal. Further, the batch adsorption experiments suggest that the cross-linking and addition of Fe3O4 improved the adsorption capacity. The highest adsorption capacity was obtained at pH 5 with a contact time of 40 min. Adsorption isotherm studies indicated that the Cd2+ adsorption onto PEDGE-MCh microsphere was Redlich-Peterson dependent (R2 = 0.9996 and root-mean-square errors = 0.064). The regeneration remained the primary challenge of PEDGE-MCh application in the wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chitosan is a biopolymer produced from deacetylation process of chitin using alkaline [1, 2]. Chitosan is widely used in various industries such as health, cosmetics, agriculture, livestock and paper industries [3,4,5,6]. Recently, chitosan has gained a spotlight as an emerging adsorbent material for industrial wastewater treatment [7], including in removing notorious heavy metal from the water [8, 9]. Chitosan posseses both N- and O-containing functional groups which are useful in forming interaction with the heavy metal, either through complexation or electratostatic interaction [10, 11]. As an adsorbent, chitosan has some advantages, such as non-toxic, biodegradable, and abundant availability. However, chitosan in pure form has certain limitations, including high solubility at low pH levels and weak mechanical properties. In addition, chitosan is usually used as a powder adsorbent and its size makes it very difficult to separate from the solution after the adsorption process. Therefore, this study aimed to improve the chitosan through modifications to enhance its usage as an adsorbent.
Some researchers have reported chitosan modifications using a cross-linking agent to improve chitosan stability in acidic solutions [12]. The most common cross-linking agent of chitosan is glutaraldehyde [13,14,15,16]. However, glutaraldehyde is toxic and consumes the NH2 groups of chitosan during the cross-linking (aldehyde reacts with an amine). The lost of NH2 could contribute to low adsorption efficiency, since it is the primary binding site for metal ions. Therefore, a non-toxic cross-linking agent, namely polyethylene glycol diglycidyl ether (PEDGE), was used in this work. PEDGE forms a cross-linking network of chitosan by reacting with hydroxyl group (OH).
Herein, we embedded the PEDGE-cross-linked chitosan with magnetic iron oxide (Fe3O4) particles to ease the separation of adsorbent from the reactor, as conducted by multiple studies [8, 17,18,19]. Most studies, however, used commercial Fe3O4 for magnetic chitosan preparation [20, 21]. The commercial iron oxide is expensive, resulting in a high-cost adsorbent. Whereas, in this research, the Fe3O4 separated from local iron sand and readily available in Aceh, Indonesia, was used. This study also performed characterization using X-ray diffraction (XRD), Fourier transform infrared (FT-IR), and scanning electron microscopy—energy-dispersive X-ray spectroscopy (SEM-EDS) analysis. The adsorption capacity of PEDGE-MCh microspheres was examined for Cd2+ removal from water with various contact times, pH, and initial concentrations of Cd2+. The novelty of this study is derived from the modification of chitosan beads using PEDGE and natural Fe3O4, in which to the best of our knowledge, it has never been reported before.
2 Materials and Methods
2.1 Materials
Chemicals used in this study were PEGDE, HCl, NH4OH, NaOH, acetic acid, and Cd(NO3)2. Otherwise stated, all used chemicals were analytical grade and purchased from Sigma-Aldrich (Selangor, Malaysia). Iron sand was collected from a local beach in Aceh, Indonesia. Chitosan (C0831 with deacetylation degree of 75.0–85.0%) was procured from Tokyo Chemical Industry co. Ltd. Japan.
2.2 Fe 3 O 4 Isolation from iron Sand
Local iron sand was washed with hot water, dried and 15 g was added to a glass beaker containing 100 mL of HCl (12 M). The mixture was stirred at 70 °C for 30 min, cooled to room temperature, and filtered using a filter paper. NH4OH (± 2 mL) was dropped into the solution while stirring at 70 °C for 30 min until a black precipitate was formed. The black precipitate (Fe3O4) was dried in an oven at 70 °C for 2 h.
2.3 Preparation of PEDGE-MCh Microspheres
Chitosan (0.35 g) was added to 20 mL of acetic acid (2%) and stirred for 2h at room temperature. PEDGE (0.07 g) was added to the acidic chitosan solution and stirred for a further 2h. To the cross-linked chitosan solution, Fe3O4 (0.5 g) was added and stirred for another 1 h. The mixture was then added to a syringe and dropped into 250 mL NaOH (3 M) to form PEDGE cross-linked magnetic chitosan (PEDGE-MCh) microspheres. The obtained PEDGE-MCh microspheres were filtered and washed with distilled water until neutral pH was reached. The PEDGE-MCh microspheres were dried overnight at 40 °C. The PEDGE-MCh microspheres were characterized using Shimadzu XRD-700 Series X-Ray Diffractometer (Kyoto, Japan), Shimadzu FT-IR 8400 (Kyoto, Japan), and SEM-EDS (Jeol. Jsm-6510 LA, Tokyo, Japan), as described in details previously [22].
2.4 Batch Adsorption
The PEDGE-MCh microspheres (0.1 g) were placed in an Erlenmeyer flask containing 25 mL of cadmium solution (25 mg/L). The mixture was shaken at a constant speed of 150 rpm for various contact times (5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 min). After an adsorption process, the adsorbent was separated from the mixture. The cadmium concentration in the solution was determined by a UV–Vis spectrophotometer. The adsorption was also conducted under different pH (2–8) and initial concentrations of cadmium. The solution pH was adjusted with NaOH and HCl before the adsorption process. The initial concentration of Cd2+ was varied from 25 to 85 mg/L.
2.5 Adsorption Regeneration
0.1 gram of the used PEDGE-MCh microspheres was immersed in 250 mL HNO3 solution (1.0 mM), shaken and separated from the solution. The used PEDGE-MCh microspheres were then rinsed until neutral pH was reached and dried overnight at 40 °C. The regenerated PEDGE-MCh microspheres were used again for the adsorption process. The same procedure was conducted for several cycles.
3 Results and Discussion
3.1 FT-IR
FT-IR analysis was performed to study the functional groups of PEDGE-MCh microspheres. The results of FT-IR analysis are shown in Fig. 1. A broad absorption band at wave number 3427 cm−1 in chitosan corresponds to overlapping –OH and –NH2 vibrations. Furthermore, the absorption band at wave number 1633 cm−1 is assigned to amide II vibration. The absorption band at 1078 cm−1 corresponds to ether (C–O–C) vibration of chitosan. After modification with PEDGE and Fe3O4 nanoparticles, the absorption band intensity of –OH vibration increased due to cross-linking that occurred between –NH2 functional groups from chitosan and epoxide functional groups from PEDGE formed new –OH functional groups. Absorption band intensity of ether vibration also increased due to the presence of same functional group from PEDGE. These results confirmed the formation of cross-linking in chitosan polymer chains by PEDGE. A new absorption band was observed at wave number 582 cm−1 corresponding to Fe–O stretching vibration, suggesting the successful embedment. In addition, the shifting of certain absorption bands of chitosan to lower wavenumbers was observed due to physical interaction among components in the material.
3.2 XRD
XRD patterns of the local iron sand, isolated Fe3O4, chitosan, and PEDGE-MCh are presented in Fig. 2. The local iron sand was exhibited to contain Fe3O4 magnetite through the appearance of multiple crystalline peaks at 2θ= 30.06, 35.38, 43.04 and 56.84° (Fig. 2a). XRD peak observed at 2θ=31.84° corresponds to the crystalline lattice of Fe2O3 maghemite. Following the isolation process, the presence of Fe3O4 nanoparticles is indicated by its diffractogram patterns at 2θ=35.52 and 58.18° (Fig. 2b). The presence of Fe2O3 maghemite is still observable at 2θ=32.58°. The identification of XRD patterns of Fe3O4 and Fe2O3 was according to the Joint Committee on Powder Diffraction Standards (JCPDS) number 19-0629 and 39-1346, respectively. To determine the change of its crystalline property, we employed a calculation of crystalline index based on the entire diffractogram using the previously reported method [23, 24]. We found that the untreated iron sand had higher crystallinity index (52.14%) in comparison with that of the isolated Fe3O4 nanoparticles (23.67%). The reduced crystallinity could be seen from lower intensities of diffractogram peaks of Fe3O4 nanoparticles than that of iron sand, which is in line to a previously published study [25].
X-ray diffraction patterns of iron sand (a), iron oxide (b), chitosan microspheres (c), and PEDGE-MCh microspheres (d). Value of d represents the distance from one plane of atoms to another (Å). The diffractograms had been processed with subtracted baseline and smoothed signal using OriginPro 2019b (Northampton, MA, USA)
Figure 2c represents the XRD pattern of chitosan, showing a strong typical peak at 2θ=19.62° (Fig. 2c). Other crystalline peaks at 2θ=37.8, 39.5, 43.98, and 57.4° also correspond to the crystalline lattice of chitosan but with lower intensities. Upon its modification with PEDGE and Fe3O4 nanoparticles (Fig. 2d), most of the crystalline peaks experienced intensities reduction. Several peaks that are observable include those appear at 2θ=29.94, 31.84, and 35.22, and 56.84° which confirm the successful immobilization of Fe3O4 nanoparticles. Crystallinity indices of chitosan and PEDGE-MCh were obtained 78.08 and 25.52%, respectively. A dramatic reduction in crystallinity was due to the cross-linking and intercalation of Fe3O4 nanoparticles among chitosan polymer chains. Cross-linking prevents the mobility of chitosan polymer chains to form crystallite phase at the inversion process of chitosan from liquid to a solid phase [26]. This amorphous material is favorable for the adsorption process due to the high accessibility of adsorbate to reach active sites of the adsorbent [27].
3.3 SEM–EDS
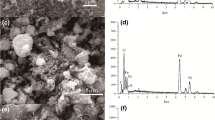
SEM analysis was conducted to study the morphology of materials. SEM image of Fe3O4 confirms the presence of polydispersed nanoparticles of various sizes (Fig. 3a). Some particles formed aggregates contributing to larger particle sizes. SEM images of PEDGE-MCh microsphere with different magnifications are presented in Fig. 3b, c. Figure 3b shows the image of PEDGE-MCh microsphere at 100× magnification. The size of PEDGE-MCh microsphere was about 900 μm. Fe3O4 nanoparticles used as a filler of chitosan matrix were not observed on the microsphere’s surface due to the covering by chitosan polymer (Fig. 3c). The surface of the PEDGE-MCh microsphere was rough, probably due to the existence of Fe3O4 nanoparticles inside the chitosan and drying process. Similar SEM appearances of materials after the addition of fillers have been reported previously [3, 22, 28].
EDS spectrum of the PEDGE-MCh microsphere is presented in Fig. 3d. Elemental composition of PEDGE-MCh microsphere (Fig. 3e) suggested the dominance of C and O which are typical for organic material. Among the metal elements detected (Si, Ti, and Fe), the highest weight percentage was shown by Fe (14.12%). It confirmed the successful embedment of magnetic Fe particles within the PEDGE-MCh microsphere. Further analysis with elemental mapping revealed that the Fe particles had been distributed covering the entire surface of the microsphere (Fig. 3f).
3.4 Adsorption Studies
3.4.1 Contact Time
Several factors influence the adsorption capacity of an adsorbent, such as contact time, solution pH, and concentration of adsorbate. In this work, in order to study the influence of contact time, the adsorption experiments were conducted for several contact times (5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 min). Figure 4 shows the adsorption capacity of Cd2+ by PEDGE-MCh microspheres with several contact times. At initial phase, the increase in adsorption capacity was contact time dependent. The adsorption capacity under 10 minutes did not increase significantly, assumed to be associated with the low swelling of chitosan polymer. The chitosan swelling allows more diffusion of adsorbate to the adsorbent surface [29,30,31]. Later, the adsorption increased in linear correlation due to increased adsorbate diffusion concomitant to the swelling, and the optimum contact time was recorded at around 40 minutes. A decrease in adsorption capacity afterward was due to the detachment of iron oxide particles. It might be attributed to the physical force of the liquid rotation and over-swelling of the chitosan that detached the physically attached iron oxide particles.
3.4.2 pH
In order to study the influence of pH on the adsorption of Cd2+ onto PEDGE-MCh, the adsorption experiments were conducted with initial pH variations (pH 2–8). The results are presented in Fig. 5a, where the highest adsorption capacity was obtained at pH 5. At low pH, Cd2+ is more likely to be repelled by amino groups protonation-induced electrostatic repulsion. Moreover, at low pH range, Cd+2 competes with H+ ions to bind onto the PEDGE-MCh microspheres. At pH 5, the surface was more negatively charged, becoming electrostatically favorable for Cd2+ ions. This phenomenon is induced by the deprotonation of the chitosan surface, as suggested previously [21]. The role of electrostatic interaction of adsorbate-adsorbent was further confirmed by the point of zero charge pH (pHpzc) of PEDGE-MCh microspheres calculated from pH drift method, as used by other reported studies [22, 24]. The pHpzc of PEDGE-MCh adsorbent was obtained pH 5.29 (Fig. 5b). When the pH level of the solution was lower than the pHpzc, the material would form positive charge. Since the adsorbate (Cd2+) has a positive charge, its interaction with the adsorbent would be preferable at higher pH level. Nonetheless, we observed a declining pattern of Cd2+ removal efficiency when the pH was increased to be more than pH 5. This occurrence could be associated with the reduced mobility of chitosan polymeric chains at alkaline pH range [29,30,31], in which the polymer was unable to sufficiently swell to allow more access for Cd2+ to reach the binding sites. This finding is in line with a previous report that has thoroughly studied the effect of pH against the chitosan swelling [24].
3.4.3 Adsorption Isotherm
Interaction between the adsorbate and the adsorbent is dependent to the initial concentration. Hence, isotherm modeling could be used to predict the adsorption outcome by plotting the adsorption capacity (qe) and final concentration at equilibrium (Ce). Herein, we employed the two-parameters isotherm model, Freundlich, and three-parameters isotherm model, Redlich–Peterson. Langmuir isotherm model was not used as the model requires the adsorption to reach equilibrium. Freundlich isotherm model equation is based on the assumption that the adsorbate could form a multilayer adsorption, where the surface energy of each binding site is heterogenous [32]. The Freundlich isotherm model shows a good correlation by R2 = 0.9292 and root-mean-square errors (RMSE) = 0.661 (Table 1). However, Redlich–Peterson isotherm model has a better fit with the experimental data (R2 = 0.9996; RMSE = 0.064). Therefore, the Redlich-Peterson isotherm model best predicts the adsorption outcome of Cd2+ adsorption onto the PEDGE-MCh microsphere. The Redlich–Peterson itself is constructed by the features applied in Langmuir and Freundlich isotherm model. The isotherm model has superiority in a wide range concentration of pollutant with versatility against homogenous or heterogenous system [33, 34]. The isotherm exponent g that is close to one suggests the adsorption is closely represented by the assumption of Freundlich model where the adsorbate-adsorbent interaction occurs on heterogenous surface in multilayer fashion (Fig. 6).
3.4.4 Adsorption Mechanism
To understand the role of functional group in the adsorption mechanism of Cd2+ onto PEDGE-MCh microspheres, FT-IR analysis was conducted on the adsorbent before and after the adsorption, where the results have been presented (Fig. 7). In this part of the study, the batch adsorption was carried out at pH 7 (neutral) with 45-min contact time. Alterations of the spectral profile before and after the adsorption were observed at around 1381 and 1087 cm−1 which correspond to the stretching vibrations of O–C and C–N, respectively. This finding strengthens the claim that both O– and N–containing functional groups are playing significant role in Cd2+ adsorption [35, 36]. In previous report, EDTA-modified chitosan could form a complex interaction with Cd2+ [22], observed from a new emergence of spectral peak at around 1382 cm−1. Interestingly, in this present study, such phenomenon did not occur, suggesting the adsorption was dependent to electrostatic interaction. The change of spectral profile is observable at a range of 3600–3500 cm−1, where after the adsorption, the intensity and width of the absorption band are found increased. Those phenomena could be associated with the introduction of new hydroxyl deriving from the adsorbed Cd(OH)+ species. A spectral peak corresponding to the Fe–O stretching vibration (578 cm−1) was observed lower after the adsorption, suggesting the detachment of Fe3O4 nanoparticle from the chitosan matrix. It confirms our previous finding, where after the 45-min contact time, the UV–Vis absorbance increased leading to the error of analyzing the real value of Cd2+ removal. To enhance the ability of chitosan matrix in immobilizing the Fe3O4 nanoparticle, several modifications are suggested including the grafting with amino acids [24, 37].
3.4.5 Regeneration
In this work, adsorbent regeneration was also studied to support the circular economy. The results of regeneration until five cycles have been presented (Fig. 8). At the first cycle, the adsorption capacity of PEDGE-MCh fell up to 15.14% from the initial adsorption capacity, which was higher than that of chitosan (8.06%). It is ascribed to the detachment of iron oxide particles from the PEDGE-chitosan matrix. As the adsorbents underwent five cycles of regeneration, the depletion percentage of the adsorption capacity was found higher in chitosan (96.3% from initial adsorption capacity) than that in PEDGE-MCh. It is owing to less iron oxide particles detached from the matrix. Poorer Cd2+ adsorption onto chitosan after the 5th cycle could be attributed to the loss of active sites as reported previously [26]. As a distinction, our previous work used the adsorbent in a shape of film with activated carbon acting as the filler, which is completely different to this present study using bead adsorbents with magnetic fillers. Taken altogether, it proves that regeneration is still a challenging factor for PEDGE-cross-linked chitosan adsorbents prepared in our works.
4 Conclusions
PEDGE-MCh microspheres with a high adsorption capacity of Cd2+ ions had been prepared and characterized using FT-IR, XRD, and SEM-EDS. The adsorption was influenced by operating parameters such as contact time and pH, associated with the physical and chemical properties of chitosan. The adsorption of Cd2+ ions on PEDGE-MCh microspheresis had the best fitness with Redlich–Peterson isotherm model. PEDGE-MCh microspheres could be used several times, although maintaining the removal efficiency is still a challenging factor.
Data availability
None.
Code availability
None.
References
Pires, C.T.G.V.M.T.; Vilela, J.A.P.; Airoldi, C.: The effect of chitin alkaline deacetylation at different condition on particle properties. Procedia Chem. 9, 220–225 (2014). https://doi.org/10.1016/j.proche.2014.05.026
Younes, I.; Rinaudo, M.: Chitin and chitosan preparation from marine sources. Structure properties and applications. Mar. Drugs 13, 1133–1174 (2015). https://doi.org/10.3390/md13031133
Safitri, E.; Humaira, H.; Murniana, M.; Nazaruddin, N.; Iqhrammullah, M.; Md Sani, N.D.; Esmaeili, C.; Susilawati, S.; Mahathir, M.; Latansa Nazaruddin, S.: Optical pH sensor based on immobilization anthocyanin from Dioscorea alata L. onto polyelectrolyte complex pectin-chitosan membrane for a determination method of salivary pH. Polymers (Basel) 13, 1276 (2021). https://doi.org/10.3390/polym13081276
Kyzas, G.; Bikiaris, D.: Recent modifications of chitosan for adsorption applications: a critical and systematic review. Mar. Drugs 13, 312–337 (2015). https://doi.org/10.3390/md13010312
Jayanudin, J.; Rochmadi, R.; Fahrurrozi, M.; Wirawan, S.: Microencapsulation technology of ginger oleoresin with chitosan as wall material: a review. J. Appl. Pharm. Sci. 6(12), 209–223 (2016). https://doi.org/10.7324/JAPS.2016.601232
Song, Z.; Li, G.; Guan, F.; Liu, W.: Application of chitin/chitosan and their derivatives in the papermaking industry. Polymers (Basel) 10, 389 (2018). https://doi.org/10.3390/polym10040389
Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P.: Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohydr. Polym. 113, 115–130 (2014). https://doi.org/10.1016/j.carbpol.2014.07.007
Fan, C.; Li, K.; He, Y.; Wang, Y.; Qian, X.; Jia, J.: Evaluation of magnetic chitosan beads for adsorption of heavy metal ions. Sci. Total Environ. 627, 1396–1403 (2018). https://doi.org/10.1016/j.scitotenv.2018.02.033
Iqhrammullah, M.; Mustafa, I.: The application of Chitosan modified polyurethane foam adsorbent. RASĀYAN J. Chem. 12, 494–501 (2019). https://doi.org/10.31788/RJC.2019.1225080
Sobahi, T.R.A.; Abdelaal, M.Y.; Makki, M.S.I.: Chemical modification of Chitosan for metal ion removal. Arab. J. Chem. 7, 741–746 (2014). https://doi.org/10.1016/j.arabjc.2010.12.011
Islam, S.; Bhuiyan, M.A.R.; Islam, M.N.: Chitin and Chitosan: structure, properties and applications in biomedical engineering. J. Polym. Environ. 25, 854–866 (2017). https://doi.org/10.1007/s10924-016-0865-5
Gutha, Y.; Zhang, Y.; Zhang, W.; Jiao, X.: Magnetic-epichlorohydrin crosslinked chitosan schiff’s base (m-ECCSB) as a novel adsorbent for the removal of Cu(II) ions from aqueous environment. Int. J. Biol. Macromol. 97, 85–98 (2017). https://doi.org/10.1016/j.ijbiomac.2017.01.004
Kildeeva, N.R.; Perminov, P.A.; Vladimirov, L.V.; Novikov, V.V.; Mikhailov, S.N.: About mechanism of chitosan cross-linking with glutaraldehyde. Russ. J. Bioorgan. Chem. 35, 360–369 (2009). https://doi.org/10.1134/S106816200903011X
Mirzaei, B.E.; Ramazani, S.A.A.; Shafiee, M.; Danaei, M.: Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. 62, 605–611 (2013). https://doi.org/10.1080/00914037.2013.769165
Zhang, X.; Jin, X.; Xu, C.; Shen, X.: Preparation and characterization of glutaraldehyde crosslinked chitosan nanofiltration membrane. J. Appl. Polym. Sci. 128, 3665–3671 (2013). https://doi.org/10.1002/app.38580
Jozwiak, T.; Filipkowska, U.; Rodziewicz, J.; Nowosad, E.: Effect of cross-linking with glutaraldehyde on adsorption capacity of chitosan beads. Prog. Chem. Appl. Chitin Deriv. 28, 35–48 (2013)
Kyzas, G.Z.; Deliyanni, E.A.: Mercury removal with modified magnetic Chitosan adsorbents. Molecules 18, 6193–6214 (2013)
Zhao, W.; Huang, X.; Wang, Y.; Sun, S.; Zhao, C.: A recyclable and regenerable magnetic chitosan absorbent for dye uptake. Carbohydr. Polym. 150, 201–208 (2016). https://doi.org/10.1016/j.carbpol.2016.05.037
Liu, Z.; Wang, H.; Liu, C.; Jiang, Y.; Yu, G.; Mu, X.; Wang, X.: Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem. Commun. 48, 7350 (2012). https://doi.org/10.1039/c2cc17795a
Zhang, X.; Sun, C.; Zhang, L.; Liu, H.; Cao, B.; Liu, L.; Gong, W.: Adsorption studies of cadmium onto magnetic Fe3O4@FePO4 and its preconcentration with detection by electrothermal atomic absorption spectrometry. Talanta 181, 352–358 (2018). https://doi.org/10.1016/j.talanta.2018.01.023
Alizadeh, B.; Delnavaz, M.; Shakeri, A.: Removal of Cd(ӀӀ) and phenol using novel cross-linked magnetic EDTA/chitosan/TiO2 nanocomposite. Carbohydr. Polym. 181, 675–683 (2018). https://doi.org/10.1016/j.carbpol.2017.11.095
Iqhrammullah, M.; Audina, U.; Husin, H.; Fathana, H.: Adsorptive removal of Cd (II) using oil palm empty fruit bunch-based charcoal/chitosan-EDTA film composite. Sustain. Chem. Pharm. 21, 100449 (2021). https://doi.org/10.1016/j.scp.2021.100449
Rahmi, R.; Lubis, S.; Az-Zahra, N.; Puspita, K.; Iqhrammullah, M.: Synergetic photocatalytic and adsorptive removals of metanil yellow using TiO2/grass-derived cellulose/chitosan (TiO2/GC/CH) film composite. Int. J. Eng. 34, 1827–1836 (2021). https://doi.org/10.5829/ije.2021.34.08b.03
Nina, M.; Fathana, H.; Iqhrammullah, M.: Preparation and characterization of new magnetic chitosan-glycine-PEGDE (Fe3O4/Ch-G-P) beads for aqueous Cd(II) removal. J. Water Process Eng. 45, 102493 (2022). https://doi.org/10.1016/j.jwpe.2021.102493
Zein, I.; Jalil, Z.: Identification of magnetite material (Fe3O4) based on natural materials as catalyst for industrial raw material application. J. Phys. Conf. Ser. 1232, 012054 (2019). https://doi.org/10.1088/1742-6596/1232/1/012054
Nurfatimah, R.: Preparation of polyethylene glycol diglycidyl ether (PEDGE) crosslinked chitosan/activated carbon composite film for Cd2+ removal. Carbohydr. Polym. 199, 499–505 (2018). https://doi.org/10.1016/J.CARBPOL.2018.07.051
Hameed, A.M.: Synthesis of Si/Cu amorphous adsorbent for efficient removal of methylene blue dye from aqueous media. J. Inorg. Organomet. Polym. Mater. 30, 2881–2889 (2020). https://doi.org/10.1007/s10904-019-01436-1
Iqhrammullah, M.; Saleha, S.; Maulina, F.P.; Idroes, R.: Polyurethane film prepared from ball-milled algal polyol particle and activated carbon filler for NH3–N removal. Heliyon 6, e04590 (2020). https://doi.org/10.1016/j.heliyon.2020.e04590
Nagarpita, M.V.; Roy, P.; Shruthi, S.B.; Sailaja, R.R.N.: Synthesis and swelling characteristics of chitosan and CMC grafted sodium acrylate-co-acrylamide using modified nanoclay and examining its efficacy for removal of dyes. Int. J. Biol. Macromol. 102, 1226–1240 (2017). https://doi.org/10.1016/j.ijbiomac.2017.04.099
Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z.: Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 234, 115890 (2020). https://doi.org/10.1016/j.carbpol.2020.115890
Ibrahim, A.G.; Sayed, A.Z.; Abd El-Wahab, H.; Sayah, M.M.: Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye. Int. J. Biol. Macromol. 159, 422–432 (2020). https://doi.org/10.1016/j.ijbiomac.2020.05.039
Freundlich, H.M.F.: Over the adsorption in solution. J. Phys. Chem. 57, 385–471 (1906)
Foo, K.Y.; Hameed, B.H.: Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156, 2–10 (2010). https://doi.org/10.1016/j.cej.2009.09.013
Al-Ghouti, M.A.; Da’ana, D.A.: Guidelines for the use and interpretation of adsorption isotherm models: a review. J. Hazard. Mater. 393, 122383 (2020). https://doi.org/10.1016/j.jhazmat.2020.122383
Iqhrammullah, M.; Suyanto, H.; Pardede, M.; Karnadi, I.; Kurniawan, K.H.; Chiari, W.; Abdulmadjid, S.N.: Cellulose acetate-polyurethane film adsorbent with analyte enrichment for in-situ detection and analysis of aqueous Pb using Laser-Induced Breakdown Spectroscopy (LIBS). Environ. Nanotechnol. Monit. Manag. 16, 100516 (2021). https://doi.org/10.1016/j.enmm.2021.100516
Yin, W.; Zhao, C.; Xu, J.; Zhang, J.; Guo, Z.; Shao, Y.: Removal of Cd(II) and Ni(II) from aqueous solutions using activated carbon developed from powder-hydrolyzed-feathers and Trapa natans husks. Colloids Surf. A Physicochem. Eng. Asp. 560, 426–433 (2019). https://doi.org/10.1016/J.COLSURFA.2018.10.031
Fathana, H.; Iqhramullah, M.; Rahmi, R.; Adlim, A.; Lubis, S.: Tofu wastewater-derived amino acids identification using LC-MS/MS and their uses in the modification of chitosan/TiO2 film composite. Chem. Data Collect. 35, 100754 (2021). https://doi.org/10.1016/j.cdc.2021.100754
Acknowledgements
Authors appreciate the collaboration between Universitas Syiah Kuala, Aceh, Indonesia and The University of Agriculture Peshawar, Pakistan during the research and the making of this article.
Funding
This research is funded by Universitas Syiah Kuala through Penelitian Lektor Kepala scheme.
Author information
Authors and Affiliations
Contributions
RR, LL and FF contributed to conceptualization; RR and JJ contributed to methodology; MI contributed to software; MI, MF and LL contributed to validation; MI and RR contributed to formal analysis; RS and MI contributed to investigation; RR contributed to resources; JJ and MI contributed to data curation; RR contributed to writing—original draft preparation; MI, MF, and MI contributed to writing—review and editing; JJ contributed to visualization; RR, LL, and FF contributed to supervision; RR contributed to project administration; RR contributed to funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors delcare that they have no conflict of interest.
Consent for Publication
All authors have read and agreed to the submitted version of the manuscript.
Rights and permissions
About this article
Cite this article
Rahmi, R., Lelifajri, L., Iqbal, M. et al. Preparation, Characterization and Adsorption Study of PEDGE-Cross-linked Magnetic Chitosan (PEDGE-MCh) Microspheres for Cd2+ Removal. Arab J Sci Eng 48, 159–167 (2023). https://doi.org/10.1007/s13369-022-06786-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-06786-6