Abstract
The deposition rate, composition, morphology, and structure of electroless deposits Ni–Mo–P and Ni–Co–P plated at different concentrations of \({\text{MoO}}_{4}^{2 - }\) and \({\text{Co}}^{2 + }\) from acidic tyrosine baths were conducted in this study. Electrochemical study of electroless Ni–Co–P coating was investigated. The deposition rate and the composition of Ni–Mo–P and Ni–Co–P coatings were determined using gravimetric and EDX method which depends on the corresponding ions concentration in the plating bath. The \({\text{MoO}}_{4}^{2 - }\) ion played rather an inhibitor role of electroless Ni–Mo–P coating even at low concentration in the plating bath. SEM was used to analyze the morphology of electroless alloys, whereas XRD was used to investigate the structure evolution. The Ni–P, Ni–Mo–P and Ni–Co–P deposits were characterized by distinct morphology depending on P, Mo and Co content in the coatings, whereas, the structure of each alloy depended only on P and Co contents in Ni–Co–P coating. Either \({\text{MoO}}_{4}^{2 - }\) or \({\text{Co}}^{2 + }\) ions concentration had different effects on the deposition rate, the structure and the quality of electroless Ni–Mo–P and Ni–Co–P deposits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since its discovery by Brenner and Riddle in the mid-twentieth century, electroless nickel coatings have achieved broad adoption. Electroless Ni–P deposits have a wide range of features, including great variability and corrosion resistance, as well as superior electrical and magnetic properties, and may be produced on steel alloys, polymers, glass, and ceramics. As a result, electroless Ni–P plating is widely used in many sectors such as chemical engineering, mechanical engineering, cars, electronics, and aerospace, etc. [1, 2]. Development of electroless nickel alloy deposits is considered as the most effective method to modify the chemical and physical properties of binary Ni–P. The electroless technique is predicted to have numerous advantages, including cheap cost and ease of use, good step-coverage capabilities for filling structures with high aspect ratios, low temperatures, and high selectivity. When cobalt, molybdenum, copper, or iron are added to Ni–P alloys, the characteristics of electroless Ni–P deposits can be enhanced [3,4,5]. The choice of the additional element is made on the basis of chemical and physicochemical properties to be imparted in the deposit.
Molybdenum and its alloys have a wide range of use, but their high catalytic activity makes them particularly useful in electrochemistry. They're employed as catalysts in hydrogen and oxygen evolution processes [5, 6], as well as aromatic oil hydroprocessing and benzene hydrogenation in the gas phase [7]. Electroless ternary Ni–Mo–P alloy coatings are often tougher and more corrosion resistant than binary Ni–P alloy coatings, and they can delay crystallization temperature [8,9,10]. Because of their low hydrogen over potential, electrodeposited nickel–molybdenum alloys are intriguing materials [11]. The electrodeposition of these alloys is a theoretical example of the so-called induced co-deposition process [12]. Electrodeposition from aqueous solutions cannot yield pure molybdenum, but it can be co-deposited with an iron group metal, a process known as induced electrodeposition [13].
Cobalt is thought to be the most prevalent extra element in deposits with excellent magnetic characteristics [14, 15]. The Ni–Co–P film is frequently utilized as a soft magnetic material for magnetic recording media since it has a magnetic property [16, 17], as well as diffusion barriers [18, 19], anti-corrosion materials [20, 21], electromagnetic shielding materials [22, 23], microwave absorbers [24, 25], and electro catalysis materials [26, 27].
Several processes, such as physical vapor deposition (PVD) employing radio frequency sputtering [28, 29] and wet procedures using electrochemical or electroless techniques [30, 31], can be used to deposit Ni–Co–P. The electroless deposition approach, on the other hand, is preferable to the electrolytic method because it allows for homogeneous surface coverage for plating micromagnetic patterns on a range of substrates [32]. High temperatures (60–90 °C) are generally required for autocatalytic electroless [33]. Furthermore, it may be carried out at lower temperatures (35 ± 1 °C), which reduces energy needs and allows for more precise control of solution composition and deposition parameters [34, 35]. The magnetic characteristics of electroless Ni–Co–P deposits are substantially influenced by their deposition rate and microstructure [36,37,38] which depends on the chemical species present in the plating bath and the operating conditions [36, 39,40,41]. The bulk of electroless Ni–Co–P coatings are made in two stages: sensitization and activation first, then electroless deposition. Other modified electroless Ni–Co–P deposition techniques have been used [42], with trisodium citrate (Na3C6H5O7·2H2O) as a complexant agent in the bath.
The aim of the present study is to contribute to a better understanding of the effect of \({\text{Co}}^{2 + }\) and \({\text{MoO}}_{4}^{2 - }\) ions concentration in the electroless Ni–Co–P and Ni–Mo–P elaborated on mild steel surface. The study was conducted in acidic tyrosine solution, at pH 5.5, to study the deposition rate, composition of coatings, morphology, and crystalline orientation. The cyclic voltammetry was done for Ni–Co–P to illustrate the deposit mechanism.
2 Experimental Procedure
All solutions were freshly prepared from analytical grade reagents and bi-distilled water. The plating bath composition and operation conditions of electroless Ni–Mo–P and Ni–Co–P alloy deposit are listed in Table 1. The nickel sulfate was used as the source of nickel, sodium hypophosphite as reducing agent and source of phosphorus. The cobalt sulfate was used as the source of \({\text{Co}}^{2 + }\) ions, the sodium molybdate as the source of \({\text{MoO}}_{4}^{2 - }\), the tyrosine as stabilizer and the ammonium acetate an accelerator in the plating baths. The pH was adjusted with dilute acetic acid (CH3COOH) or ammonium (NH3·H2O). The plating temperature used in this work was 85 ± 1 °C.
Prior to all testing, 6 cm2 mild steel plates were manually polished, with silicon carbide abrasive papers, ranging in grain size from 120 to 1200, then degreased in acetone, immersed in 10% dilute sulfuric acid and finally rinsed with distilled water. The weight gain measurements, after one hour of immersion, were performed to determine the deposition rate with a coating density taken equal to 8.9 g cm−3.
The morphology of the deposits was examined using scanning electron microscopy, while the content and crystalline structure were determined using EDX analysis and X-ray diffraction (XRD) using a cobalt anticathode. Indeed, the XRD technique is well suited for the determination of the structure of materials because it is non-destructive and only takes into account the surface layers due to the low penetration of X-rays used in diffraction.
Cyclic voltammetry is one of the transient electrochemical methods widely used in electrochemical process kinetics. It allows to qualitatively locate the different electrochemical processes that can occur during the deposition. It is this aspect which gives rise to numerous applications, since it is possible to observe the electrochemical phenomena taking place, oxidation or reduction of compounds, very shortly after their formation. The intensity of the current corresponding to these two processes is recorded throughout the potential scan. The curves obtained show the variation of the intensity as a function of the potential; called voltammograms. The electrochemical measurements were run with a potentiostat (VoltaLab model PGZ100) to obtain the polarization curves. Triple-electrode system was adopted with a reference electrode of saturated calomel electrode to which all potentials were referred and a counter electrode of plating.
3 Results and Discussion
3.1 Effect of Na2MoO4·2H2O Concentration
Figures 1 and 2 show the influence of different concentrations of Na2MoO4·2H2O on the deposition rate and composition of the alloys elaborated by electroless process in bath 1.
In the absence of Na2MoO4·2H2O in bath 1, the electroless process of Ni–P alloy is characterized by a high deposition rate of about 18 μm h−1 and a high P content (13 Wt.%). However, the addition of sodium molybdate, even at low concentration (5 g L−1), provoked a dramatically drop in the deposition rate from 18 to 0.5 μm h−1 accompanied with an increase of the Mo content in the coating deposit to 12.5 Wt.%. Nevertheless, when the Na2MoO4·2H2O concentration increases from 5 to 20 g L−1, in the plating bath 1, the deposition rate of electroless remains constant at 0.5 µm h−1, but the Mo content in the deposit increases from 12.5 to 33 Wt.%. Furthermore, the majority of the alloys elaborated are deposited from the first minutes of the introduction of substrate into the chemical bath 1. This low deposition rate of deposits is due to the low activity of the electroless process deposition in the surface of substrate accompanied by the appearance of bubbles of hydrogen gas which remains blocked on the surface during the rest of the deposition time without spontaneous decomposition of the bath.
The deposition rate of the electroless ternary Ni–Mo–P alloy coating is lower than that of the binary Ni–P alloy coating due to Mo co-deposition [43]. In other words, if the sodium molybdate concentration is too high, the deposition process will come to a halt, preventing the formation of the necessary coating [44]. Furthermore, when the coating reaches a sufficient thickness, the Mo–P co-deposition enhances the coating's hardness and internal stress concentration, making it more brittle and susceptible to cracking and stripping [45]. Depending on relative concentrations, mass transport of either molybdate or nickel may limit the rate of molybdenum deposition and hence alter alloy composition.
The results of the effect of Na2MoO4·2H2O concentration on the composition of the alloys deposits are summarized in Fig. 2.
The analysis of the deposition composition showed the presence of Ni, P and Mo, which justifies that the deposited coatings are Ni–Mo–P ternary alloys with low Ni and P contents. The addition of 5 g L−1 of Na2MoO4·2H2O in the plating bath caused a sharp drop in the content of Ni and P from 87 and 13% Wt.%, respectively to 2 Wt.% accompanied with an increase in Mo content to 12.5 Wt.%. However, the more increase in the concentration of \({\text{MoO}}_{4}^{2 - }\) ions in the deposition bath leads to a sharp linear increase in the Mo content to reach 33 Wt.% even if the deposition rate does not exceed 1 μm h−1, with a stabilization of Ni and P contents in the deposits. There is therefore a deposition competition between Mo, Ni and P in the procedure of the co-deposition of the ternary alloy Ni–Mo–P. Similar results were found by other authors [44, 46]. Thereby, the addition \({\text{MoO}}_{4}^{2 - }\) in the chemical deposition bath in acidic medium resulted in a strong marginalization of the hypophosphite oxidation and nickel reduction reactions by the adsorption and the occupation of Mo of the catalytic surface of the substrate. Similar results were found by Liu et al. [44] and Lu and Zangari [8] for the Ni–Mo–P alloys deposited at pH greater than 9 in the presence of trisodium citrate as complexing agent of the bath.
In general, molybdenum exhibits various isopolyanions when added the Na2MoO4·2H2O in the bath chemical as a function of pH bath [47]. In addition, \({\text{MoO}}_{4}^{2 - }\) ions have a strong tendency to polymerize in acidic medium. Polymerization leads to the formation of polyanions whose size increases with the bath acidity [48].
The addition of \({\text{MoO}}_{4}^{2 - }\) ions to the chemical bath as a source of Mo has a beneficial effect on the Mo content incorporated in the Ni–Mo–P alloys. These Mo contents increase with the rise of the added amounts of \({\text{MoO}}_{4}^{2 - }\) ions even at low quantities. On the other hand, the addition of the \({\text{MoO}}_{4}^{2 - }\) ions, has an inverse effect on the deposition rate which drops even with small quantity introduced until the total inhibition of the deposition process. The action of \({\text{MoO}}_{4}^{2 - }\) ions is generally explained by two mechanisms:
-
The displacement process deposits the \({\text{MoO}}_{4}^{2 - }\) ions supplied to the bath on the active sites of the metal surface in a substitution type manner [49]. In this scenario, the nickel content has decreased, lowering the rate of deposition.
-
The empoisoning adsorption [50], with the \({\text{MoO}}_{4}^{2 - }\) ions can prohibit the deposition of nickel by adsorption on the catalytic sites of the metal surface.
3.1.1 Morphology of Ni–Mo–P deposit
The adhesion of the alloy deposited on the substrate is an essential issue and objective for the quality of these deposits. The scotch test repeated three times on each deposit is generally used to evaluate the adhesion of the coating deposited on the substrates [51]. These tests show that the deposition of Ni–Mo–P alloys are characterized by a weak adhesion on the substrate and tend to be detached by simple application of the scotch test.
The surface morphology of the deposit, at different Mo contents, elaborated at different concentration of Na2MoO4·2H2O, in plating bath 1, is shown in Fig. 3. The examination of the surface morphology, in the absence of Na2MoO4·2H2O, showed the presence of a smooth morphology relatively homogeneous characterized by a very compact nodular aspect (Fig. 3A). The amorphous structure of Ni–P coating developed perpendicularly to the substrate surface is responsible for the dispersion of spherical nodules of various sizes. The size of grains is mostly determined by the phosphorus concentration of the deposits [52].
SEM micrographs of electroless deposition developed at different magnifications and various concentrations of Na2MoO4·2H2O in plating bath 1: A, A´: [Na2MoO4·2H2O] = 0 g L−1, Ni-13 Wt.% P; B, B´: [Na2MoO4·2H2O] = 5 g L−1, Ni-12.5 Wt.% Mo-1.5 Wt.% P; C, C’: [Na2MoO4·2H2O] = 10 g L−1, Ni-20 Wt.%Mo-1.5 Wt.% P; D, D’: [Na2MoO4·2H2O] = 20 g L−1, Ni-33 Wt.% Mo-2 Wt.% P.
However, the incorporation of Mo for Ni–Mo–P deposits causes a total change in the morphology appearance of the coating when compared to Ni–P alloy. The Ni–Mo–P alloys are very thick and marked with many cracks. These cracks are induced by the growth stress of deposits containing too much of Mo. Many electroless coatings exhibit morphologies and chemical compositions that vary with coating thickness, and the composition of Ni–Mo–P alloys is much more likely to vary with deposition time. Deposition temperature, deposition duration, and the length of the electroless process are all factors that affect the development of internal stress [53]. Similar results have been found in another work for the same high molybdenum alloys (38 to 50 Wt.% of Mo), which are characterized by cracks and small cavities that diminish the importance and practical application of Ni–Mo–P coatings [54]. Marlot et al. [11] mentioned similar results for the electrolytic Ni–Mo deposition under different conditions deposition; they noted the increase in porosity for Ni–Mo deposits with the increase of \({\text{MoO}}_{4}^{2 - }\) ions in solution. However, Chassaing et al. [55] proved a nodular aspect of these deposits.
3.1.2 Structure of Ni–Mo–P Deposit
Figure 4 shows the XRD diffractions patterns of electroless Ni–P and Ni–Mo–P deposits obtained at various sodium molybdate concentrations in bath 1 characterized by a different Mo content. The X-ray diffraction pattern of the deposit Ni–P coating (Fig. 4A) is characterized by a large single peak dominating in 2θ = 45° indicating the amorphous structure of the deposit; result in agreement with the result of EDX (Fig. 2) which reveals a content of the phosphorus in Ni–P deposit obtained at 13 Wt.%. Literature reports have shown that Ni–P deposits having greater than 7 Wt.% [38] or 8 Wt.% [39] in phosphorus are amorphous structure in as-deposited form. However, the XRD diagrams of electroless Ni–Mo–P alloy deposition prepared at different concentrations of \({\text{MoO}}_{4}^{2 - }\) ions in the plating bath 1 with a deposition time of 60 min present three very thin peaks at 2θ = 65°, 82° and 88° in addition to the one obtained at 45°.
The width mid-height of the dominant peak (2θ = 45°) indicates a crystalline nature of the Ni–Mo–P alloys, when the high peak intensity indicates a very small thickness of these deposits.
No amorphous phase was detected for the Ni–Mo–P alloy by the XRD analysis even with the increase of the molybdenum content in these deposits up to 33 Wt.% of Mo. Therefore, the increasing of Mo content does not affect the structure of the electroless Ni–Mo–P alloys. Stepanova et al. [56, 57] showed that if the Mo content does not exceed 12–13 Wt.% (18–19.6 Wt.% by weight), the obtained deposits, in ammonia and citrate baths, consist solely of a crystalline phase. Moreover, when the Mo content increases to 18 Wt.% (26 Wt.% by weight), an amorphous phase appears. Ni–Mo alloys become quite amorphous when the Mo content is greater than 22–25 Wt.% (about 31–35 Wt.% by weight). Beltowska-Lehman et al. [58] observed a similar structure for electrolytic Ni–Mo deposition even with the increase of Mo content, while Sanches [59] demonstrated an amorphous structure for electrodeposited Ni-Mo deposition from the acidic bath (at pH 4).
3.2 Effect of CoCl2·6H2O Concentration
The effect of CoCl2·6H2O concentration, on the plating rate and the deposit composition are depicted in Figs. 5 and 6, respectively. It is found that the presence of \(Co^{2 + }\) ions in the bath affects the electroless plating rate and the composition of the deposit. It can also be seen that the analysis of the composition of the deposits showed the joint presence of Ni, P and Co which justifies that the deposited coatings elaborated at different concentrations of \({\text{Co}}^{2 + }\) in the bath is a ternary Ni–Co–P alloys.
From Fig. 5, it is clear that the \({\text{Co}}^{2 + }\) ions concentration in bath 2 influences differently and in an opposite way the plating rate and the Co content for the deposited coatings according to the increase of CoCl2·6H2O concentration. When the molar ratio [\({\text{Ni}}^{2 + }\)]/[\({\text{Co}}^{2 + }\)] is greater than or equal to 0.9, at the concentration of 10 g L−1 in CoCl2·6H2O, the Ni–Co–P deposition rate remains acceptable, greater than 12 μm h−1; that is recommended industrially. However, the increase of concentration of \({\text{Co}}^{2 + }\) till 20 g L−1, the plating rate of electroless deposit decreases dramatically from 18 µm h−1 to 7 µm h−1 accompanied with an increase of Co content in the elaborated deposits to reach 14 Wt.%. With an increase in \({\text{Co}}^{2 + }\) concentration in the plating bath, Matsubara and Yamada [60] and Kim et al. [61] have both found a reduction in plating rate. Sankara Narayanan et al. [4], Wang [62] and Huang et al. [63] have concluded the increases of plating rate with the increase of [\({\text{Ni}}^{2 + }\)]/[\({\text{Co}}^{2 + }\)] ratio. However, when the CoCl2·6H2O concentration in bath 2 increases from 20 at 30 g L−1 in the plating bath, the deposition rate of electroless Ni–Co–P alloys remains constant at 7 µm h−1 accompanied with a continual increase in Co content in the deposit from 14 to 20 Wt.%. Figure 6 shows the variation of Ni, Co, and P contents in the Ni–Co–P deposit as a function of CoCl2·6H2O concentration in the plating bath.
In Fig. 6, when the concentration of CoCl2·6H2O increases to 10 g L−1, the content of phosphorus and cobalt in the Ni–Co–P coating changes. Indeed, the cobalt content increases from 0 to 5 Wt.% and reversely the phosphorus content in the deposit decreases from 13 to 8.5 Wt.% whereas the nickel content remains stable at about 87 Wt.% in Ni–Co–P alloys. However, when the concentration of CoCl2·6H2O increases from 10 at 20 g L−1, the Co content of the deposit jumps from 5 at 14 Wt.%. Because of the rise in cobalt in the Ni–Co–P alloy coating, Ni is reduced from 87 to 80 Wt.%, with a small decrease in phosphorus content. A similar effect is observed at 30 g L−1 of CoCl2·6H2O, when the Co content increases to 20 Wt.% with a decrease in Ni content till 73 Wt.% even if the rate deposition remains constant as observed in Fig. 5. This behavior is explained by the greater catalytic activity of nickel when compared to cobalt. These results led to conclude that there is a competition between the three elements Ni, P and Co deposition reactions depending on the ratio [\({\text{Ni}}^{2 + }\)]/[\({\text{Co}}^{2 + }\)] in the bath of chemical deposits of Ni–Co–P alloy. With an increase in cobalt concentration, in the plating bath, Matsubara and Yamada [60], Kim et al. [61], Wang [62], and Pang et al. [64] have all found a drop in deposition rate. This was also found in the plating of Co–P [65] and Co–B [66] alloys. These findings suggest that by changing the quantity of cobalt chloride, in a conventional bath electroless coating, the rate of deposition and composition of Ni–Co–P coating may vary and can be controlled.
3.2.1 Electrochemical Study of the Electroless Ni–Co–P Deposits
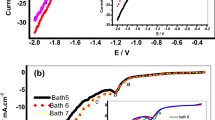
An electrochemical study by cyclic voltammetry seems interesting to apprehend the net decrease in the plating rate registered in the electroless autocatalytic Ni–Co–P alloy in acidic medium of bath 2. Figure 7 showed the superposition of cyclic voltammetry curves obtained without and with different concentrations of CoCl2·6H2O in bath 2. The operating conditions are respected in terms of temperature 85 °C and pH 5.5 for recording the voltammograms.
The superposition of voltammograms of Ni–P and Ni–Co–P deposits showed a hiding voltammograms of Ni–Co–P alloys with a part of linear shape with a very low current density when compared to Ni–P deposit voltammogram. This result indicates a very low kinetics of electrochemical reactions of bath 2 when compared to that of Ni–Co–P alloy. These results support those reported in Fig. 5 during the addition of \({\text{Co}}^{2 + }\) ions in bath 2.
In general, the voltammogram of electroless Ni–P alloys is characterized by:
-
A cathodic peak K at − 1.1 VSCE corresponds to the reaction of \({\text{Ni}}^{2 + }\), \({\text{H}}^{ + }\), and \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) ions being reduced.
-
At -0.65 VSCE, there is an anodic peak A related to the oxidation of \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) ions and hydrogen atoms adsorbed on the surface.
-
Two peaks of dissolution of the deposit Ni–P, B (at − 0.3 VSCE) and C (at 0.570 VSCE). The first peak, according to the literature, represents the dissolution of the Ni-crystalline P phase, whereas the second represents the dissolution of the amorphous phase.
For more illustration, Fig. 8 shows the general voltammograms deposition of Ni–Co–P at different concentrations of CoCl2·6H2O in the bath 2.
Globally, new peaks distinguish from peaks K, A, B and C already mentioned in the voltammogram of Ni–P deposit during the addition of CoCl2·6H2O in bath 2. The presence of zero current from the beginning of scanning rate to − 700 mVSCE reveals no reaction in this potential zone. Indeed, in the cathodic domain, a new peak is observed:
-
Under the potential − 0.7 VSCE, the cathodic current begins to increase, what entering the appearance of the first peak G; reduction reaction of \({\text{Co}}^{2 + }\).
At low concentration of \({\text{Co}}^{2 + }\) ions in bath (lower than 5 g L−1), the peak G is only observed as a change of slope of the cathodic curve because of the important contribution of the current of reduction of \({\text{Ni}}^{2 + }\), \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) and the \({\text{H}}^{ + }\) during the deposit of the alloy Ni–Co–P; which explain the reason at Co content in the deposit Ni–Co–P lower than 3 Wt.%. Similar results were found by Grujicic and Pesic [67] when studying the Ni–Co–P deposit on glassy carbon electrodes in the presence of ammonium sulfate.
In addition, in the anodic zone, two new peaks are observed:
-
The peak E, which occurs at − 0.450 VSCE, corresponds to the breakdown of the crystalline phase of the Ni–Co–P deposit.
-
At − 0.13 VSCE, the peak F related to the breakdown of the amorphous phase of the Ni–Co–P deposit.
Similar results were found by Grujicic and Bezik [67] during the study of Ni–Co–P deposit in the presence of ammonium sulfate. In the literature, the anodic domain of cyclic voltamogramms of cobalt is characterized either by the appearance of a single peak [68,69,70,71] or two peaks [72,73,74] according to the pH of the bath. With the exception of the idea that the two peaks may be due to cobalt dissolving in various ionic kinds or dissolution of cobalt under diverse phases [72]; that they represent allotropic forms of cobalt, no explanation was offered for the occurrence of two anodic peaks [73].
It can be deduced that the deposition reaction of Ni, P and Co from the equations of reduction of \({\text{Ni}}^{2 + }\), \({\text{Co}}^{2 + }\) and hypophosphite oxidation are described by the following equations:
In addition, Ni–Co–P deposits are also characterized by the integration of P. To write the Ni–Co–P deposition equation so the \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) reduction reaction must be multiplied twice (6) and 3 times the oxidation of \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) (4) in addition to reactions 3 and 4.
The overall reaction of Ni–Co–P deposits is therefore written according to the reaction:
The influence of the cobalt chloride concentration in the plating bath 2 on the evolution of the intensity of the peak (iA); hypophosphite oxidation; and the intensity of the peak (iK) correspond to the reduction of all ions existing in the bath (\({\text{Ni}}^{2 + }\), \({\text{Co}}^{2 + }\), \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) and \({\text{H}}^{ + }\)) and are given in Fig. 9.
Effect of the CoCl2·6H2O concentration on the: A: intensity of the peak (iA) corresponding to the oxidation of \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\); B: intensity of the peak (iK) corresponding to the reduction of \({\text{Ni}}^{2 + }\), \({\text{Co}}^{2 + }\),\({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) and H+
The first observation in Fig. 9, showed an identical trend of the evolution of the intensity of the two peaks iA and iK depending on the CoCl2·6H2O concentration in the plating bath. Indeed, the addition of low concentrations in \({\text{Co}}^{2 + }\) ion causes a drastic drop in the intensity of the peaks IA and iK. This low activity of oxidation of \({\text{H}}_{2} {\text{PO}}_{2 }^{ - }\) ions and the reduction of \({\text{Ni}}^{2 + }\) and \({\text{H}}_{2} {\text{PO}}_{2}^{ - }\) is due to the inclusion of Co in the catalyst surface of the deposit and then the kinetic of the autocatalytic chemical reaction of the Ni–Co–P deposit. Indeed, the P content in the.
Ni–Co–P deposit decreases depending on the concentration of \({\text{Co}}^{2 + }\). This result indicates a very low-rate deposition of electrochemical reactions in the bath during Ni–Co–P deposition which corroborate gravimetric results when \({\text{Co}}^{2 + }\) ions are added in bath 2 (Fig. 5).
3.2.2 Morphology of Ni–Co–P Deposit
The surface morphology of the deposit at different concentrations of CoCl2·6H2O in plating bath is shown in Fig. 10.
In the absence of CoCl2·6H2O, the deposit is a binary Ni–P alloy (Fig. 10A) and the surface morphology is similar to that given in Fig. 3A. This case showed the presence of a smooth morphology relatively homogeneous characterized by a very compact nodular aspect.
When the concentration of cobalt chloride is equal to 5 g L−1, the morphology of the Ni–Co–P deposit is very compact and homogeneous evolved gradually into nodular structure and circa no pits are observed (Fig. 10B). However, at 10 and 20 g L−1 of CoCl2·6H2O, the morphology of Ni–Co–P deposits changes (Fig. 10C, D). The micrography SEM shows a very marked nodular at 20 g L−1 in CoCl2·6H2O. The increase in diameter of the nodule causes the appearance of micropores, fissuring on the limit of these nodules leading to a less homogeneous surface with increasing Co content in the Ni–Co–P alloy. However, at 30 g L−1 in CoCl2·6H2O, the Ni–Co–P deposit is characterized by a macropores scattered over the entire surface although the rest of it seems very compact (Fig. 10E). Finally, the quality and morphology of Ni–Co–P alloys deteriorate as the Co content increases in the Ni–Co–P deposit obtained at pH 5.5.
3.2.3 Structure of Ni–Co–P Deposit
XRD graphs of binary electroless Ni–P and ternary Ni–Co–P alloy deposits with different cobalt concentrations at typical plating bath are shown in Fig. 11. According to the composition of these alloys, a shift in XRD diagrams is readily seen between Ni–P and Ni–Co–P deposits. The X-ray diffraction pattern of the deposit Ni–P coating (Fig. 11A) is characterized by an amorphous structure of the deposit (cf. Figure 4A). The XRD of the various Ni–Co–P deposits according to Co content in the coating is characterized by the appearance of two peaks at 2θ equal 45° and 52° corresponding to crystallization of the deposits to the plan directions (111) and (200), respectively. The intensity and the enlargement of these two peaks depend on Co and P contents. The structure of the Ni-(M)-P system is determined by the P content, according to [75, 76]. Amorphous structures were found in deposits with a P content more than 8%, while crystal structures were found in deposits with a P value less than 4%. The remainder is made up of a mix-crystal structure.
The XRD of the deposits Ni–Co–P, characterized by a content of Co inferior than 5.3 Wt.% in coating, shows that the two peaks are less visible, fused in one other to give a broad peak (111) indicating the amorphous structure of the coating (Fig. 11B). The phosphorus and cobalt contents of the Ni–Co–P deposits are higher than 7.5 Wt.% and lower than 5.3 Wt.%, respectively. The XRD of the Ni–Co–P alloys deposit, on the other hand, show that the Co content is equivalent to or greater than 14 Wt.%. Figure 11C, D shows the presence of two distinct peaks at 2θ = 45° and 2θ = 52°. With the rise in Co content in the deposits Ni–Co–P, the intensity of the plan Ni(111) falls when compared to the plan Ni(200). The intensity of the peak Ni(111) is less important when compared to the peak Ni(200), which indicates a preferential crystalline of the deposits Ni–Co–P according to Ni(200). The sharp peak, on the other hand, comes from crystal Ni diffraction, whilst the random peak comes from the amorphous Ni phase; hence, the deposit's structure is a combination of crystal and amorphous Ni. Gao et al. [22] observed the results simultaneously.
4 Conclusion
The objective of this work is to evaluate the effect of the concentration of \({\text{MoO}}_{4}^{2 - }\) and \({\text{Co}}^{2 + }\) ions on the formulation of the electroless Ni–P deposition bath for the elaboration of electroless chemical deposits of the ternary Ni–M–P alloy in an acid medium and in the presence of tyrosine as a stabilizer. The alloys proposed were Ni–Mo–P and Ni–Co–P, characterized by physical and chemical properties which open up more and more applications of these coatings. It can be concluded that the rise of Na2MoO4·2H2O and CoCl2·6H2O concentrations in baths 1 and 2, respectively gave appropriate results for each deposit. Furthermore, the microanalysis EDX confirmed that the deposits obtained were really Ni–Mo–P and Ni–Co–P alloys.
In bath 1, an almost annulment of the deposit rate that falls from 18 to 0.5 µm h−1 with the addition of the 5 g L−1 of Na2MoO4·2H2O. Furthermore, an increase in Mo content in Ni–Mo–P to 33 Wt.% was observed with an increasing of sodium molybdate concentration, although the deposition rate did not exceed 0.5 µm h−1. The Ni–Mo–P deposits produced were characterized by a degraded surface morphology with increasing Mo content and a crystal structure compared to Ni–P alloy. However, in bath 2, the addition of 30 g L−1 CoCl2·6H2O leads to a decrease in the deposition rate to become constant at 7 μm h−1 and an increase in the Co content in the Ni–Co–P alloys to 20.3 Wt.%. The study of the reaction mechanisms of Ni–Co–P alloys by cyclic voltammetry reveals a drastic drop in the intensity of the \({\text{H}}_{2} {\text{PO}}_{4}^{ - }\) oxidation peak. This result proves that the Co deposition causes a decrease in the oxidation of hypophosphite in the deposition bath, and consequently a decrease in the deposition rate of Ni–Co–P alloys, in accordance with the evolution of the deposition rate obtained by the gravimetric technique. A new structure was declared for the electroless Ni–Co–P coatings and not noted for Ni–P alloys following the appearance of the new peak Ni(200). Such a mixed structure defined a mixture structure both amorphous and crystalline of Ni–Co–P coatings.
References
Huang, Y.; Mo, W.W.; Roan, M.L.; Huang, C.Y.; Mo, W.W.; Roan, M.L.: Studies on the influence of double-layer electroless metal deposition on the electromagnetic interference shielding effectiveness of carbon fiber/ABS composites. Surf. Coat Technol. 184(2–3), 163–169 (2004)
Wojewoda-Budka, J.; Wierzbicka-Miernik, A.; Szczerba, M.; Kazimierczak, H.; Kwiecien, I.; Morgiel, J.; Stan-Glowinska, K.; Valenza, F.: The effect of Re addition on the thermal stability and structure of Ni-P electroless coatings. Mater Charact 171, 110811 (2021)
Liu, L.; Peng, J.; Du, X.; Zheng, H.; Chen, Z.; Gong, P.; Xiao, Y.; Cao, X.: Synthesis, composition, morphology, and wettability of electroless Ni-Fe-P coatings with varying microstructures. Thin Solid Films 706, 138080 (2020)
Pei, W.; Wang, X.; Liu, C.; Zhao, D.; Wu, C.; Wang, K.; Wang, Q.: Synthesis of hyperbranched Co-Ni-P nanocrystals and their splitting degree dependent HER performances. Electrochim. Acta 381, 138286 (2021)
Abdel-Karim, R.; Halim, J.; El-Raghy, S.; Nabil, M.; Waheed, A.: Surface morphology and electrochemical characterization of electrodeposited Ni-Mo nanocomposites as cathodes for hydrogen evolution. J. Alloys Comp. 530, 85–90 (2012)
Beltowska-Lehman, E.; Indyka, P.: Kinetics of Ni–Mo electrodeposition from Ni-rich citrate baths. Thin Solid Films 520(6), 2046–2051 (2012)
Kriz, J.F.; Shimada, H.; Yoshimura, Y.; Matsubayashi, N.; Nishijima, A.: Nickel-containing catalysts for hydroprocessing of aromatic oils. Fuel 74(12), 1852–1857 (1995)
Lu, G.; Zangari, G.: Study of the electroless deposition process of Ni-P-based ternary alloys. J. Electrochem. Soc. 150(11), C777 (2003)
Zhao, G.; Wang, R.; Liu, S.; Wu, D.; Zhang, Y.; Wang, T.; Zou, Y.: Study on the role of element Mo in improving thermal stability and corrosion resistance of amorphous Ni-P deposit. J. Non-Cryst. Solids 549, 120358 (2020)
Fetohi, A.E.; Hameed, R.M.A.; El-Khatib, K.M.: Ni–P and Ni–Mo–P modified aluminium alloy 6061 as bipolar plate material for proton exchange membrane fuel cells. J. Power Sources 240(58), 589–597 (2013)
Chaitree, W.; Kalu, E.E.; Liang, Z.; Yeboah, Y.D.: Effects of bath composition and thermal treatment on the performance of Co-Ni–Mo–P electrocatalyst supported on carbon for the electrooxidation of ethanol. J. Alloys Comp. 860, 158404 (2021)
Brenner, A.: Electrodeposition of Alloys, Vol. 1/2. Academic Press, New York (1963)
Kuznetsov, V.V.; Pavlov, M.R.; Kuznetsov, K.V.; Kudryavtsev, N.: Kinetics of cathodic processes of deposition of nickel–molybdenum alloys from an ammonia–citrate electrolyte. Russ. J. Electrochem. 39(12), 1338–1341 (2003)
Sharma, A.; Bhatt, P.; Brajpuriya, R.; Tripathi, S.; Chaudhari, S.M.: Correlation study between structural, magnetic and transport properties of annealed Co thin films. Vacuum 78(1), 47–51 (2005)
Kohmoto, O.; Yamamoto, T.: Hard magnetic properties of sputtered Co and Co-P films. J. Magn. Magn. Mater. 71(1), 33–38 (1987)
Flanders, P.J.: Elastic stress-induced coercive field changes in Ni-Co-P films used in a rotating disk. IEEE Trans. Magn. 9(5), 1680–1682 (1983)
Abdel Aal, A.; Shaaban, A.; Hamid, Z.; Abdel, Z.: Nanocrystalline soft ferromagnetic Ni–Co–P thin film on Al alloy by low temperature electroless deposition. Appl. Surf. Sci. 254(7), 1966–1971 (2008)
Lee, C.Y.; Lin, K.L.: Study of Co-Cr films deposited by magnetron sputtering under different nitrogen partial pressures. Thin Solid Films 239(1), 93–98 (1994)
O’Sulivan, E.J.; Schrott, A.G.; Paunovic, M.; Sambucetti, C.J.; Marino, J.R.; Bailey, P.J.; Kaja, S.; Senkow, K.W.: Electrolessly deposited diffusion barriers for microelectronics. IBM J. Res. Dev. 42(5), 607–620 (1998)
Liu, W.L.; Hsieh, S.H.; Chen, W.J.; Hsu, Y.C.: Growth behavior of electroless Ni–Co–P deposits on Fe. Appl. Surf. Sci. 255(6), 3880–3883 (2009)
Parente, M.M.V.; Mattos, O.R.; Diaz, S.L.; Neto, P.L.; Miranda, F.J.: Electrochemical characterization of Ni–P and Ni–Co–P amorphous alloy deposits obtained by electrodeposition. J. Appl. Electrochem. 31(6), 677–683 (2001)
Gao, Y.; Huang, L.; Zheng, Z.J.; Li, H.; Zhu, M.: The influence of cobalt on the corrosion resistance and electromagnetic shielding of electroless Ni–Co–P deposits on Al substrate. Appl. Surf. Sci. 253(24), 9470–9475 (2007)
Aixiang, Z.; Weilao, X.; Jian, X.: Electroless Ni–Co–P coating of cenospheres using [Ag (NH3)2]+ activator. Mater. Lett. 9(4), 524–528 (2005)
Li, Y.J.; Wang, R.; Qi, F.M.; Wang, C.M.: Preparation, characterization and microwave absorption properties of electroless Ni–Co–P-coated SiC powder. Appl. Surf. Sci. 254(15), 4708–4715 (2008)
Pan, X.F.; Mu, G.H.; Shen, H.G.; Gu, M.Y.: Preparation and microwave absorption properties of electroless Co–Ni–P coated strontium ferrite powder. Appl. Surf. Sci. 253(9), 4119–4122 (2007)
Podesta, J.J.; Piatti, R.C.V.; Arvia, A.J.; Ekdunge, P.; Juettner, K.; Kretsa, G.: The behaviour of Ni-Co-P base amorphous alloys for water electrolysis in strongly alkaline solutions prepared through electroless deposition. Int. J. Hydrogen Energy 17(1), 9–22 (1992)
Podesta, J.J.; Piatti, R.C.V.; Arvia, A.J.: The influence of iridium, ruthenium and palladium on the electrochemical behaviour of Co-P and Ni-Co-P base amorphous alloys for water electrolysis in KOH aqueous solutions. Int. J. Hydrogen Energy 20(2), 111–122 (1995)
Tunashima, S.; Maehata, Y.; Uchiyama, S.: Induced anisotropy and permeability in amorphous Fe-B and Co-Fe-B films. IEEE Trans. Magn. 17(6), 3073–3075 (1981)
Hironaka, K.; Uedaira, S.: Soft magnetic properties of Co-Fe-P and Co-Fe-Sn-P amorphous films formed by electroplating. IEEE Trans. Magn. 26(5), 2421–2423 (1990)
Riveiro, J.M.; Sanchez-Trujillo, M.C.: Magnetic anisotropy of electrodeposited Co-P amorphous alloys. IEEE Trans. Magn. 16(6), 1426–1428 (1980)
Ohashi, K., Ito, M., Maruyama, T.: Magnetic materials, processes, and devices. In: Romankiw, L.T., Herman, D.A. , Jr. (eds.) PV 90–8, The Electrochem. Soc., Proc. Series, Pennington, NJ, 247 (1990)
Yoshino, K.: Electrochemically deposited thin films. In: Paunovic, M., Ohno, I., Migoshi, Y. (eds.) PV 93–26, The Electrochem. Soc., Proc. Series, Pennington, NJ, 370 (1993)
Schlesinger, M.: Electroless deposition of nickel. Mod. Electroplat. 4, 667–684 (2000)
Zou, G.Z.; Cao, M.S.; Zhang, L.; Li, J.G.; Xu, H.; Chen, Y.J.: A nanoscale core-shell of β-SiCP–Ni prepared by electroless plating at lower temperature. Surf Coat Technol 201(1–2), 108–112 (2006)
Baskaran, I.; Kumar, R.S.; Sankara Narayanan, T.S.N.; Stephen, A.: Formation of electroless Ni–B coatings using low temperature bath and evaluation of their characteristic properties. Surf. Coat Technol. 200(24), 6888–6894 (2006)
Matsuda, H.; Jones, G.A.; Takano, O.; Grundy, P.J.: Room-temperature electroless deposition of high-coercivity Co-Ni-P films. J. Magn. Magn. Mater. 120(1–3), 338–341 (1993)
Mirzamaani, M.; Romankiw, L.; McGrath, C.; Karasinski, J.; Mahlke, J.; Anderson, N.C.: Correlation of structure and magnetic properties of thin CoP films. J. Electrochem. Soc. 135(11), 2813–2817 (1988)
Schlesinger, M.; Meng, X.; Evans, W.T.; Saunders, D.A.; Kampert, W.P.: Micromorphology and magnetic studies of electroless cobalt/phosphorus thin films. J. Electrochem. Soc. 137(6), 1706–1709 (1990)
Nicholson, E.L.; Khan, M.R.: Microstructure and magnetic properties of electroless plated Co-Ni-P and Co-P thin films for magnetic recording. J. Electrochem. Soc. 133(11), 2342–2345 (1986)
Homma, T.; Sezai, Y.; Osaka, T.: A study on growth processes of CoNiP perpendicular magnetic anisotropy films electroless-deposited at room temperature. Electrochim. Acta 42(20–22), 3041–3047 (1997)
Homma, T.; Sezai, Y.; Osaka, T.; Maeda, Y.; Donnet, D.M.: Compositional inhomogeneity in electroless-deposited CoNiP films studied by spin-echo 59Co nuclear magnetic resonance. J. Magn. Magn. Mater. 173(3), 314–320 (1997)
Liu, W.L.; Chen, W.J.; Tsai, T.K.; Hsieh, S.H.; Chang, S.Y.: Effect of nickel on the initial growth behavior of electroless Ni–Co–P alloy on silicon substrate. Appl. Surf. Sci. 253(8), 3843–3848 (2007)
Song, G.S.; Sun, S.; Wang, Z.C.; Luo, C.Z.; Pan, C.X.: Synthesis and characterization of electroless Ni–P/Ni–Mo–P duplex coating with different thickness combinations. Acta Metall. Sin 30(10), 1008–1016 (2017)
Liu, D.L.; Yang, Z.G.; Zhang, C.: Electroless Ni–Mo–P diffusion barriers with Pd-activated self-assembled monolayer on SiO2. Mater. Sci. Eng. B. 166(1), 67–75 (2010)
Agarwala, R.C.; Agarwala, V.: Electroless alloy/composite coatings: a review. Sadhana 28(3–4), 475–493 (2003)
Chou, Y.H.; Sung, Y.; Bai, C.H.; Ger, M.D.: Effects of molybdate concentration on the characteristics of Ni–Mo–P diffusion barriers grown by nonisothermal electroless deposition. J. Electrochem. Soc. 155(9), D551 (2008)
Kholmogorov, A.G.; Kononova, O.N.; Panchenko, O.N.: A review of the use of ion exchange for molybdenum recovery in Russia. Can. Metall. Q. 43(3), 297–304 (2004)
Breslin, C.; Treacy, G.; Carroll, W.: Studies on the passivation of aluminium in chromate and molybdate solutions. Corros. Sci. 36(7), 1143–1154 (1994)
Lu, G.; Zangari, G.: Corrosion resistance of ternary Ni-P based alloys in sulfuric acid solutions. Electrochim. Acta. 47(18), 2969–2979 (2002)
Yoshino, M.; Masuda, T.; Yokoshima, T.; Sasano, J.; Shacham-Diamand, Y.; Mat-suda, I.; Osaka, T.; Hagiwara, Y.; Sato, I.: Electroless diffusion barrier process using SAM on low-k dielectrics. J. Electrochem. Soc. 154(3), D122 (2007)
Zhong, S.; Yang, Z.H.; Cai, J.; He, H.J.; Wen, J.S.; Liu, C.: Electroless Cu deposition on a TiN barrier in CuSO4-HF solution. J. Electrochem. Soc. 152(7), C466 (2005)
Larhzil, H.; Cissé, M.; Touir, R.; Ebn Touhami, M.; Cherkaoui, M.: Electrochemical and SEM investigations of the influence of gluconate on the electroless deposition of Ni–Cu–P alloys. Electrochim. Acta. 53(2), 622–628 (2007)
Sudagar, J.; Lian, J.; Sha, W.: Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 571, 183–204 (2013)
Podlaha, E.J.; Landolt, D.: Induced codeposition: I. An experimental investigation of Ni-Mo alloys. J. Electrochem. Soc. 143(3), 885–892 (1996)
Chassaing, E.; Portail, N.; Levy, A.F.; Wang, G.: Characterisation of electrodeposited nanocrystalline Ni–Mo alloys. J. Appl. Electrochem. 34(11), 1085–1091 (2004)
Stepanova, L.I.; Purovskaja, O.G.; Azarko, V.N.; Sviridov, V.V.: Peculiarities of Ni-Mo alloys electrodeposition from citrate electrolytes. Proc. Acad. Sci. Belarus Ser. Chem. Sci. 1, 38–43 (1997)
Stepanova, L.I.; Purovskaja, O.G.; Sviridov, V.V.: Influence of ammonium ions on chemical and phase composition of Ni-Mo alloy films electrodeposited from citrate electrolytes. Russ. J. Appl. Chem. 73(1), 66–70 (2000)
Beltowska-Lehman, E.; Bigos, A.; Indyka, P.; Kot, M.: Electrodeposition and characterisation of nanocrystalline Ni–Mo coatings. Surf. Coat. Technol. 211, 67–71 (2012)
Sanches, L.S.; Domingues, S.H.; Marino, C.E.B.; Mascaro, L.H.: Characterisation of electrochemically deposited Ni–Mo alloy coatings. Electrochem. Commun. 6(6), 543–548 (2004)
Matsubara, H.; Yamada, A.: Control of magnetic properties of chemically deposited cobalt nickel phosphorus films by electrolysis. J. Electrochem. Soc. 141(9), 2386–2390 (1994)
Kim, D.H.; Aoki, K.; Takano, O.: Soft magnetic films by electroless Ni-Co-P plating. J. Electrochem. Soc. 142(11), 3763–3767 (1995)
Wang, S.L.: Electroless deposition of Ni–Co–B alloy films and influence of heat treatment on the structure and the magnetic performances of the film. Thin Solid Films 515(23), 8419–8423 (2007)
Huang, Y.; Shi, K.; Liao, Z.J.: Studies of electroless Ni–Co–P ternary alloy on glass fibers. Mater. Lett. 61(8–9), 1742–1746 (2007)
Pang, J.; Li, Q.; Wang, W.; Xu, X.; Zhai, J.: Preparation and characterization of electroless Ni–Co–P ternary alloy on fly ash cenospheres. Surf. Coat. Technol. 205(17–18), 4237–4242 (2011)
Bangwei, Z.; Bin, C.; Ge, Y.; Lingling, W.: Study of electroless Co-P alloys process. Electroplat. Pollut. Control. 17(6), 19–21 (1997)
Chang, Y.H.; Lin, C.C.; Hungang, M.P.; Chin, T.S.: The microstructure and magnetic properties of electroless-plated Co-B thin films. J. Electrochem. Soc. 133(5), 985–988 (1986)
Grujicic, D.; Pesic, B.: Electrochemical and AFM study of cobalt nucleation mechanisms on glassy carbon from ammonium sulfate solutions. Electrochim. Acta 49(26), 4719–4732 (2004)
Floate, S.; Hyde, M.; Compton, R.G.: Electrochemical and AFM studies of the electrodeposition of cobalt on glassy carbon: an analysis of the effect of ultrasound. J. Electroanal. Chem. 523(1–2), 49–63 (2002)
Gomez, E.; Marin, M.; Sanz, F.; Valles, E.: Nano-and micrometric approaches to cobalt electrodeposition on carbon substrates. J. Electroanal. Chem. 422(1–2), 139–147 (1997)
Myung, N., Ryu, K.H., Sumodjo, P.T.A., Nobe, K.: Fundamental aspects of electrochemical deposition and dissolution including modeling. In: Paunovic, M., Datta, M., Matlosc, M., Osaka, T., Talbot, J.B. (eds.) PV 97–27, Electrochem Soc Proc 136 (1993)
Cui, C.Q.; Jiang, S.P.; Tseung, A.C.C.: Electrodeposition of cobalt from aqueous chloride solutions. J. Electrochem. Soc. 137(11), 3418–3423 (1990)
Soto, A.B.; Arce, E.M.; Palomar-Pardave, M.; Gonzales, I.: Electrochemical nucleation of cobalt onto glassy carbon electrode from ammonium chloride solutions. Electrochim. Acta. 41(16), 2647–2655 (1996)
Palomar-Pardave, M.; Gonzales, I.; Soto, A.B.; Arce, E.M.: Influence of the coordination sphere on the mechanism of cobalt nucleation onto glassy carbon. J. Electroanal. Chem. 443(1), 125–136 (1998)
Mendoza-Huizar, L.H.; Robles, J.; Palomar-Pardave, M.: Nucleation and growth of cobalt onto different substrates: Part I. Underpotential deposition onto a gold electrode. J. Electroanal. Chem. 521(1–2), 95–106 (2002)
Daly, B.P.; Barry, F.J.: Electrochemical nickel–phosphorus alloy formation. Int. Mater. Rev. 48(5), 326–338 (2003)
Lu, K.: Nanocrystalline metals crystallized from amorphous solids: nanocrystallization, structure and properties. Mater. Sci. Eng. 16(4), 161–221 (1996)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All Authors declare that there is no any conflict of interest in this study.
Rights and permissions
About this article
Cite this article
EL Haloui, A., Driouch, M., EL Assiri, E.H. et al. Investigation of the Effect of MoO42− and Co2+ up on Electroless Ni–Mo–P and Ni–Co–P Alloys in Acidic Solution Using Tyrosine as Stabilizer: Characterization and Electrochemical Study. Arab J Sci Eng 47, 7157–7169 (2022). https://doi.org/10.1007/s13369-022-06703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-06703-x