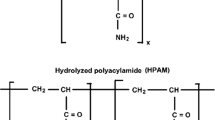
Abstract
Enhanced oil recovery (EOR) acquires global interest in the petroleum industry. Several technologies are implemented to retrieve the residual oil after conventional flooding treatment. One of these prospering technologies is the chemical EOR through polymeric nanocomposites flooding, where nanotechnology acquires incremental attention in the field of petroleum industry. Current efforts are directed toward the use of nanofluids, polymeric nanofluids, polymer nanocomposites, and polymer-g-nanoparticles to produce through chemical flooding. This review provides comprehensive highlights on the following aspects, (1) preparation and characterization of polymeric nanocomposites with their organic polymers and inorganic fillers. In addition, the mechanism of flooding and wettability alteration through nanocomposites were issued. (2) Implementation of nanofluids metal, and graphene oxide through flooding process gives promising results as it lowers the chemical adsorption onto the rock surface and enhances the rheological properties, flow diversion, specific heat improvement, emulsification improvement, and multiphase flow properties. (3) Application and advantage of biopolymers as water thickening agent and flow diversion through chemical EOR. Furthermore, the effect of zeta potential, chain length, molecular weight, and functional groups on nanocomposite stability, hydrophilicity/hydrophobicity of nanoparticles, and pressure drop and permeability limitations were reported. The application on the lab and field scale as well as the molecular dynamic (MD) simulation and theoretical modeling related to this upgrowing technology are discussed. The study outcomes encompass the optimization and utilization of synthetic polymers, biopolymers, and polymeric nanocomposites with their optimized injection and assessment conditions, in addition to, the future aspects and challenges of applications issues in enhanced oil recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Petroleum products suffer from incremental decline owing to the increased energy demand and lack of new explorations, so great efforts are directed to compensate for this critical energy demand through different technologies [1]. One of these promised and multidisciplinary technologies is enhanced oil recovery (EOR). EOR technology acquires significant attention globally owing to the decline of conventional oil resources, the persistent aspiration to enhance the producing life period of oil reservoirs, and improve crude oil extraction [2]. Based on the “US Energy Information Administration (EIA)”, the worldwide utilization of hydrocarbon products is 4.94 billion tons in 2018 with an expected increase of 38% in 2040 [3]. The residual crude oil beyond conventional primary and secondary processes reaches 7.0 billion barrels, so scientific and industrial effort is directed to EOR technology [4]. Oil-producing stages comprise the primary, secondary, and tertiary stages. The primary recovery is based on the differential pressure between the underground reservoir and the producing well yield up to 10%. Meanwhile, the second stage was conducted through either gas or water injection, producing ~ 30% [5]. Water flooding is the dominant technique owing to its low complexity and costs [4]. It is applied to compensate for the reservoir pressure and moves the fluid toward a production well. Water flooding continues until the water cut exceeds 99%, so water flooding becomes practically unacceptable on the field scale. These conventional methods (primary and secondary) recover ~ 25 to 45% of the original oil in place (OOIP) [6]. As a result, about 60–70% of oil is trapped by capillary forces in the underground porous media as discontinuous oil phase globules [7]. After that, petroleum engineers resort to tertiary techniques which are alternatively called enhanced oil recovery (EOR) [8]. Conventional EOR techniques involve chemical, gas, and thermal flooding as well as microbial treatment as summarized in Fig. 1 [9]. Recently, nanotechnology has created imprints in many industrial and technological fields, especially in the petroleum industry including the downstream and upstream [11]. Laboratory and simulation studies highlight the usage of nanotechnology in the production, exploration, stimulation jobs, formation evaluation and interpretation, reservoir seismic characterization, drilling, prediction of oil reserve, and upgrading of EOR techniques [7, 12,13,14,15]. Nanotechnology encompasses the usage of nanomaterials (1–100 nm size) [16] and nanoparticles suspensions for the enhancement of oil recovery and hydrocarbon fluids in petroleum reservoirs [7, 9, 17]. Current efforts and research directed toward the use of nanofluids (base fluid with nanoparticles), polymeric nanofluids, polymer nanocomposites, and polymer-g-nanoparticles to produce the trapped oil in the unswept regions through chemical flooding. These materials are characterized by longer stability, high thermal and ionic strength, low plugging, and retention in the porous media [18]. Reports about nanotechnology in enhanced oil recovery are reported elsewhere [19]. On the scale of EOR technology, chemical EOR techniques are considered the most efficient and sensible capital cost technique [18], in which polymer and polymer composites flooding is a widely used method for more than 40 years [3]. Improvement of oil recovery through polymer nanocomposite and nanoparticles-grafted polymer flooding relies on various aspects including rock wettability alteration, mobility control enhancement to mobilize oil, and interfacial tension (IFT) reduction [20,21,22]. Polymer nanocomposites change rock wettability to hydrophilic wetness, so reduce capillary force, control the mobility ratio, and enhance the oil recovery [1]. A comprehensive survey on the ability of polymeric composites to alter rock wettability is reported elsewhere [19, 23]. Wettability is a significant controlling parameter for the fluid distribution and flows in the porous rocks [7, 24]. Several techniques were implemented to assess reservoir wettability including contact angle, spontaneous imbibition, and Amott index [1, 8, 14, 25]. In the subsequent sections, an overview of the polymer nanocomposites, their constituents, and characterization as well as the flooding procedure and assessment of the polymeric composites will be issued in detail. Furthermore, the theoretical modeling and simulation studies relevant to the implementation of polymer composites in the enhanced oil recovery will be highlighted.
Categories of enhanced oil recovery [10]
2 Polymer Composites
2.1 Mechanism of Polymer Composite Flooding
On the field scale, polymer overflowing suffers from chain degradation under severe temperature and salinity environments [19]. Consequently, the improvement of rheological and shearing performance for polymer solutions and reduction of adsorption rate through incorporating inorganic filler through the polymeric matrices to form a polymeric composite that meets the technical and environmental requirements of EOR has been reported [19, 26]. Polymeric composite synthesized through grafting of polymeric matrices on the surface of inorganic fillers using different techniques including solvent evaporation, monomers polymerization, and dialysis [1, 27]. The chemical dispersion and grafting of these nanoparticle fillers in the polymer matrix, as displayed in Fig. 2a, lead to improvement of the rheological criteria and sweep efficiency, reduction of polymer adsorption onto the rock pores [27], owing to the creation of crosslinking bridges and enforcement of hydrophobic associations between nanoparticles and polymer matrix [28, 29]. These fillers include carbon structures (graphene oxide, graphene, carbon nanotube), clays (bentonite, and montmorillonite), metal oxides (ZnO, Fe2O3, TiO2), silica-based materials (silicon oxide, silica xerogel/aerogel), and so on [30]. The polymer matrix may be synthetic polymers, biopolymers, and hydrogels. Figure 2b is a schematic diagram of the major components used for the preparation of polymeric composites used in enhanced oil recovery. Compared with native polymers, polymeric composites are low density, durable materials [32], contain organic–inorganic hybrid, so exhibit superior thermal, and mechanical toughness, salt tolerance, as well as hydrophobic and physicochemical properties [33, 34], due to the physical H-bond creation between the fillers and polymeric chains [35,36,37]. Kumar et al. [38] reported that suspensions of polymer and nanoparticle exhibit better rheological behavior comprising high salt and thermal resistance due to enhanced bridging of nanoparticles. The stability of oil/water emulsions against creaming increases and the emulsions display solid-like performance owing to the development of a three-dimensional network by irreversible bridging of polymeric chain with nanoparticle [38]. On the scale of petroleum engineering, polymeric nanocomposite flooding is a global EOR technique established on the blending of hydrophilic polymeric nanocomposite slug with the injected fluid to intensify the injectant fluid viscosity, reduce the mobility ratio, minimize viscous fingering, decrease the formation permeability, improve the vertical and areal sweeping efficiency, and conformance (profile) control [39]. Furthermore, polymeric nanocomposite alters rock wettability to hydrophilic wetness, so recover more oil during the displacement process by pushing the oil toward the oil wellbore [22, 27, 31, 36, 40]. Polymer composite flooding is restricted for offshore processes owing to less polymer solvation rate [3, 41], also friction and wearing during the pumping action are critical issues [42, 43]. The field trials give an additional recovery factor of 5–30% OOIP through polymer composite flooding [18, 22, 26].
a Schematic illustration of nanoparticles dispersed in the polymer matrix [31]; b Schematic diagram of polymer composite and its constituents
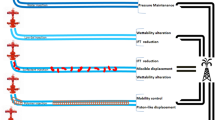
Flooding of polymeric composites in the underground reservoirs comprises long-term-injection of the dry or liquified slug with certain concentrations through the injector well followed by water injection as illustrated in Fig. 3. The slug of polymeric composite seeks to increase the water phase viscosity, so reduce the mobility ratio, and sweep the trapped oil in front of it. The polymeric composite acts as a piston-like which reduces the water relative permeability and improves oil recovery. Furthermore, the composite slug blocks channels and finger zones through the porous media leading to the recovery of the trapped oil and reduced formation water production. Several reports stated the usage of polymeric nanocomposites in EOR operations. In this regard, Maghzi et al. [44] investigated the dispersion and rheological characteristics of silica nanoparticles into the polyacrylamide matrix. The flooding was conducted in a glass micromodel. They reported that the polymer pseudoplastic behavior was enhanced by the blending of 0.1 wt% silicon oxide nanoparticles, and give rise to an additional recovery factor of 10% [1]. Shamilov et al. [15] stated the incorporation of Al and Cu nanoparticles into sodium carboxymethylcellulose and water-soluble polyacrylamide then used this nanocomposite as an oil displacement agent. Maurya et al. [27] reported experimental and simulation studies of silica nanoparticles grafted onto hydrophilic polyacrylamide chains through free-radical polymerization. Furthermore, they assess the flow properties in the porous media through STARS-CMG simulator to verify its appropriateness for polymer displacement processes. The simulation results indicate that the polymer nanocomposite can mobilize the residual oil through waterflooding processes. Yousefvand and Jafari [45] investigated the use of different concentrations of HPAM/nano-SiO2 for EOR operation. The flooding test was conducted through various salt dosages to assess the nanocomposite stability against ionic degradation. Sharma et al. [46] reported a comprehensive review of natural fiber reinforced polymer composites (NFPC’s). Raj et al. [47] prepare α-olefin sulfonate and MoS2 nanosheets hybrid and explore their foam generation ability and oil recovery efficiency through sand pack flooding. Zhang et al. [48] reported a comprehensive review of chromium-polymeric gels hybrids and their implementation in EOR. They reported that chromium gels control profile conformance, improve sweeping efficiency, and enhance oil recovery. Rezaeian et al. [49] fabricate a thin membrane film composed of polyphenyl sulfone, polyamide, and montmorillonite, for improving the oil retrieval in carbonate reservoirs through spontaneous imbibition. Gandomkar and Sharif [50] reported the use of polymeric nanocomposites as an IFT reducing and thickening agent for miscible gas flooding. Haruna et al. [51] modify the surface of silica NP with 3-aminopropyl) triethoxysilane, then polymerize it with polyacrylamide polymer and use this composite for chemically EOR applications. Furthermore, they stated that the incorporation of silica nanomaterial enhances thermal resistance and rheological criteria, in addition to the polymer aging time. Zhou et al. [2] apply betaine-based zwitterionic surfactant and polymer nanocomposites for core flooding using Berea sandstone saturated with Bakken crude oil. They reported an additional recovery factor of 9.32%. Conjugated polymers into the polymer composite for EOR applications are categorized into biopolymers, synthetic polymers, and hydrogels [19], while the most common nanoparticles include silica nanoparticles and graphene oxide. In the subsequent section, the author discusses each component with a brief highlight on the literature issued it.
Schematic representation of polymer composite injection [41]
2.2 Nanoparticles for EOR Applications
Nanoparticles “engineered materials” are nanosized fragments characterized by superior criteria including large surface area, chemical catalysis, thermal and mechanical strength, and electrical conductivity [7], and can be used in various oil field applications including cementing drilling, and EOR [52]. Nanoparticle’s incorporation into the polymer matrix gives rise to 3D networks, so enhance the solution viscosity and mitigate polymer flooding restrictions under severe reservoir circumstances leading to effective recovery of the trapped oil [7, 31, 51]. Moreover, polymer composite exerts improved sweep efficiency and lower adsorption rate onto the porous media owing to the presence of dispersed nanoparticles into the solution [27]. NPs exert different properties through flooding in porous media and fluids [1, 11]. It decreases the IFT between the O/W interface, changes rock wettability to water-wet, controls mobility ratio, and enhances the stability of emulsion in EOR operations [1, 11, 22]. Furthermore, NPs lower the chemical adsorption onto the rock surface and enhance the rheological properties, flow diversion, specific heat improvement, emulsification improvement, multiphase flow properties, shearing, and mechanical properties of drilling fluids [7, 13, 53]. Also, nanofluids “smart fluids” are a colloidal suspension of NPs in the base fluids, and exhibit higher stability through the flooding conditions [53]. Nanofluids injection into the petroleum reservoirs is a novel chemical flooding technique, which has possible application to recover the oil after conventional treatment techniques [31]. Nanofluids serve to decrease the operating pressure for the displacing fluid and the formation damage [54]. Nanofluids including SiO2, ZnO2, TiO2, CuO, ZrO2, graphene oxide, and Fe3O4 have been reported as oil displacement agents, in which SiO2 and graphene oxide nanoparticles are widely reported in enhanced oil recovery [25], owing to its ease synthesis and the preparation of the hydrophilic and hydrophobic formulation using various engineering approaches [11]. Table 1 summarized the advantage and disadvantages of the nanoparticles used in oil displacement processes. Nanofluid's performance was further improved by blending with surfactant or polymer solutions to offer greater stability and enhance the rheological criteria under shearing conditions [16]. Nanoparticles penetrate pore throats and micro-cavities of porous media without causing any permeability reduction and serve to remove the residual oil from the pore throat by the knife-edge-like mechanism [27]. Consequently, nanoparticles serve to modify the interaction between the trapped hydrocarbons and the rock pores leading to improvement of the rock wettability and heat transfer coefficients [9]. Also, these nanoparticles can act as nanosensors for gathering information during reservoir characterization [7]. A review of nanoparticles and nanoparticles-grafted polymers is listed elsewhere [13, 16,17,18, 25, 53]. In this regard, Evdokimov et al. [55] conducted flooding experiments using nano colloids solutions to investigate the phase behavior of petroleum fluids. Son et al. [56] inspected the flow performance of nanoparticles stabilized emulsion in the porous medium and movement at the binary interfaces. Furthermore, they investigated pressure differentiation and oil recovery with emulsion and water injection. Zhang et al. [17] conducted flooding tests using silica-based nanofluid and Berea sandstone through high brine solution. Esfe et al. [9] investigated the simulated and experimental activity of the nanoparticles on porous media permeability and oil recovery. The effect of capillary pressure, dose fraction of silica nanoparticles, diffusion rate, and porosity of the porous media on the oil recovery rate was investigated. The reservoir pattern is modeled as a heterogeneous 3D cone. They reported that nanoparticles flow easily through rock pore throats and cover the rock surface leading to wettability alteration. Wu et al. [25] reported the synthesis of amphiphilic Janus nanoparticles (JNPs) by Pickering emulsion. The synthesized NPs exhibits superior interfacial activity and interface stability in the EOR processes. AfzaliTabar et al. [57] synthesis different blends of graphene/MoS2 nanohybrids via the sol–gel method, and used it as IFT reducing agents and wettability modifiers through EOR applications. El-hoshoudy group stated the effect of zeolites and Fe2O3 nanofluids to reduce heavy oil viscosity as well as the IFT at the O/W contact point [58,59,60,61]. Furthermore, they investigated the flooding scenario and recovery factor using a blend formulation of alkaline/polyacrylamide/nanofluid [62].
2.2.1 Silica Oxide Nanoparticles
Silica nanoparticles possess a high surface area, so can exert an effective energy dissipation mechanism, which leads to enhanced nanocomposites criteria including favorable temperature, and salt tolerance [36, 65]. Furthermore, they exhibit well compatibility with the carbon skeleton of the polymer and are easily dispersed through the polymer matrix through H-bonding and electrostatic interactions as indicated in Fig. 4 to overcome native polymers restrictions and improve other issues in petroleum engineering [36]. On the EOR- field-scale application, SiO2 nanoparticles are the most abundant, easily produced, and applicable nanoparticles in this field, owing to their controllable chemical behavior, superior physicochemical and surface modification properties, with specific characteristics (hydrophobic to hydrophilic) [1, 18, 67, 68]. A comprehensive review of polymer composites including silica nanoparticles and their application in the field of EOR are summarized elsewhere [1, 22]. Qu et al. [69] reported inhibition of scale formation using nanoparticle formulation of silica-based zinc-phosphonate [7]. The prepared nanoparticles dispersed in surfactant solution flow easily through sandstone and calcite porous media. The surface charge and particle size distribution are the chief dominant parameters influencing the flow transportation of nanoparticles through the rock pore [69]. Onyekonwu and Ogolo [70] investigated the recovery factor through sandstone cores using three types of poly silica nanoparticles with various hydrophilicity degrees (hydrophobic, neutrally wet, and lipophilic [69]. Yousefvand and Jafari [71] reported the oil recovery factor using a 5-spot micromodel saturated with brine solution and heavy oil using nano-silica formulation. The displacement mechanism is analyzed through an image processing system. The flooding data after one pore volume designate that the incorporation of nano-silica improves the ultimate oil recovery by an additional 10% owing to the improvement of injected fluid viscosity. Wu et al. [72] reported that silica nanoparticles are effective co-injectants with surfactants in chemically improved oil recovery (CIOR). Hu et al. [66] reported the usage of hydrophilic HPAM/nanoSiO2 composite formulation as a flooding agent. Assessment of rheological properties under various temperatures, ionic strength, and aging conditions reveals that the enclosure of SiO2 nanoparticles significantly enhances the viscoelastic criteria of HPAM through the severe thermal and ionic environment. The hybrid composite displayed remarkable viscosity rises up with 0.8 wt% SiO2 loading, and thermal steadiness for 288 h. at T = 353 K. Ji et al. [36] reported the synthesis of poly(AM-co-AA)/SiO2] micro globules through inverse phase suspension polymerization. They reported that silica nanoparticles enhance the morphological criteria of the nanocomposite micro globules. Also, oil displacement efficiency and plugging ability were improved as compared to the polyacrylate polymer. Ali et al. [13] reported the use of silica nanofluids subjected to an electromagnetic field applied through a reservoir model for EOR operations. Agi et al. [18] reported the synthesis of silica nanoparticles from rice straw through wet ball milling for EOR application. The recovery factor for both the synthesized silica nanoparticles and oilfield hydrolyzed polyacrylamides under severe reservoir conditions is reported. El-hoshoudy et al. [58, 61, 73] stated the fabrication of SiO2 nanoparticles surfaces, then used it for reducing oil viscosity and IFT during EOR operations.
Interaction mechanism between SiO2 and polymer [66]
2.2.2 Graphene and Graphene Oxide (GO)
Carbon nanoparticles including fullerenes, carbon nanotubes, graphene sheets, and platelets, are acquiring incremental attention in the development of next-generation to satisfy the required energy demands [74]. Graphene particles exhibit superior optical, thermodynamic conductivity, electronic, and mechanical criteria [75], owing to their distinct sp2- carbon structure which is packed into 2D-honeycomb frameworks [76]. Graphene is the toughest and robust crystallized structure, as it exhibits a tensile strength of 125 GPa and elastic moduli of 1.1 TPA [1]. Graphene oxide (GO) is a pseudo-2D-crystallized geometry with uniform single-atom thickness and plentiful hydrophilic oxygen functionalized moieties (comprising the -OH, -COOH, and epoxy groups) which is available for various chemical modifications, and grafting of polymer macromolecules [76,77,78]. Graphene oxide is a reinforced structure that enhances the thermal, and mechanical behavior of the polymer at a very low concentration [75]. Furthermore, GO is a hydrophilic surface-active agent that possesses mixed sp2- sp3 hybridized carbon atoms, so can modify the rock wettability to water-wetness through electrostatic attraction and reduce IFT at the O/W interface [1]. These superior properties extend its usage in the field of polymeric nanocomposites and their application in EOR. Also, the functional groups on GO sheets allow H-bond formation with polymer chains as indicated in Fig. 5, so it acts as reinforcing nanofillers for the polymer matrix especially polyacrylamide (PAM) hydrogel, to enhance the thermal and mechanical criteria of the composite under harsh reservoir conditions [79, 81]. Polyacrylamides are deemed the most appropriate composite hydrogels with GO sheets for enhanced oil recovery owing to the ease formation of H-bonding between its -NH2 and the GO surface functional groups [82, 83]. Depending on this concept, Liu et al. [84] investigated the mechanical stability and tensile strength through preparing crosslinked PAM hydrogels with GO sheets as a physical crosslinker, and N,N-methylenebisacrylamide as a chemical crosslinker. They found that physically crosslinked hydrogels exhibited higher mechanical strength and elasticity as compared with the other one, due to the presence of GO sheets which offer tough crosslinking degrees, and give rise to 3D- network structure in the polymeric matrix [77]. Zhang et al. [79] grafted PAM macromolecules on GO sheets to form a uniform formulation of dispersed GO sheets into the polymer matrix [80, 85]. Tang et al. [76] verified the grafting of modified GO on the polyacrylamide surface to prepare PAM-g-GO composite via spectroscopic methods for oil displacement in sandstone reservoirs. Alam et al. [80] reported the facile, low-cost synthesis of conductive graphene sheets through water processing, then the formation of graphene/PAM composite via in-situ polymerization between acrylamide monomers and graphene sheets. The H-bond originated between the two constituents that lead to highly functionalized mechanical properties and IFT reduction.
Evingür and Pekcan [77] reported on the synthesis of GO-PAM composites through the incorporation of graphene oxide nanofillers into polyacrylamide hydrogels. They experimentally investigated the composite's mechanical behavior before and after swelling in water through a compressive performance and modeled it by the rubber elasticity theory. Jafarbeigi et al. [14] reported the synthesis and application of (graphene oxide (N-(1-naphthyl) ethylenediamine)) using Hummer’s method as a wettability alternating and flooding agent. They investigated the effect of contact angle, IFT, and displacement scenarios on the core scale. El-hoshoudy et al. stated the combination of ACTF as a modified graphene oxide derivative into polyacrylate polymer to form a highly strengthen composite for flooding operations under severe reservoir conditions [86].
2.3 Biopolymers
Biopolymers or naturally occurring polymers are considered as available, eco-friendly, non-toxic, and superior chemical stable EOR candidates [26, 87,88,89,90,91,92,93,94]. In the nearest future, biopolymers are anticipated to replace HPAM in EOR operations, owing to their high viscosity at low concentration and their higher resistance to shear degradation, thermal and ionic strength [21]. These superior properties resort to their distinct double to triple-helical structure render the biopolymers, as exceptional flooding agents, in harsh reservoir conditions [26]. On the other hand, the application of biopolymers in EOR applications suffers from high economic cost and microbial degradation by the reservoir microflora [95]. To overcome microbial degradation, petroleum engineers resort to injecting biocides in the fluid formulation [21]. On the research scale, chemists are resorted to injecting biopolymer composites through the incorporation of nanomaterials fillers into the biopolymer matrix or by grafting biopolymers onto the surface of the nanoparticles. Biopolymers contain highly active hydroxyl groups available for different chemical modifications including polymerization, grafting, esterification, and so on. Consequently, many inorganic/biopolymer composites can be synthesized and modified for polymer flooding in EOR operations [96]. El-hoshoudy et al. [96, 97] design the application and economic profit of the biopolymer flooding scenario in EOR operations. Biopolymers and natural gums are considered effective additives for EOR [98], as they control fluid mobility through viscosifying the injectant fluid and withstand thermal and ionic degradation [26]. In this section, the author highlights the most used biopolymers in enhanced oil recovery. At the end of the section, Table 2 summarizes the chemical structure and advantages of the used biopolymers.
2.3.1 Cyclodextrin (CD)
CD are cyclic truncated oligosaccharides structures of glucose units linked by β1 → 4 bonds [102]. Its toroidal structure with a hydrophobic cavity and a hydrophilic outer surface makes it an excellent host material for molecular recognition [103]. The macrocyclic CD ring displays a central cavity where various guest molecules can be accommodated, resulting in the formation of host–guest complexes. CDs are environmentally friendly candidates prepared from renewable biodegradable and biocompatible sources. They exhibit wettability alteration effects which are beneficial for oil remediation from the underground reservoirs. Figure 6 summarizes the most common types of cyclodextrins [104].
Geometric structures of cyclodextrins [104]
Zou et al. [105] synthesize anionic and cationic polymers combined with B-cyclodextrin and evaluate their performance in EOR operations. Qin et al. [106] use cationic β-cyclodextrin inclusion complexes for enhancing oil recovery. De Lara et al. [8] reported the implementation of cyclodextrins as a prospective EOR candidate for petroleum application recovery and wettability alteration. Wei, 2015 [107] reported a comprehensive evaluation of β-cyclodextrin molecules in heavy oil recovery applications. Furthermore, they assess the rheological properties and sandpack flooding tests at reservoir conditions. Da Cruz et al. [104] reported the use of cyclodextrins (CDs) as wettability modifiers for EOR. Romero-Zerón and Espinosa [108] evaluated the self-association of XG with anionic and cationic surfactants through non-covalent β-cyclodextrin host–guest interactions.
2.3.2 Xanthan Gum
It is a global biopolymer applied in petroleum reservoirs on a commercial scale. It is rigid, negatively charged double-helical polysaccharide that can withstand shearing action, water hardness, and ionic degradation resulting from divalent ions and electrolytes in the petroleum reservoirs [21]. Ghoumrassi-Barr and Aliouche [109] evaluated the potential applicability of xanthan as a flooding agent in the Devonian oilfield. The flooding data indicate that the solution viscosity increased by ~ 400% in the formation water after 30 days of storage and ~ 60% of the initial viscosity was retained at 68 °C due to the rigid structure of xanthan polysaccharide chains. On other petroleum applications, Elkholy et al. [94] reported the usage of xanthan and guar gums as scale inhibitors in oil reservoirs. El-hoshoudy group investigated the flooding scenario using xanthan and xanthan-grafted silica composite [110]. They stated that the incorporation of silica nanoparticles improves the rheological performance of the xanthan solution, also changes rock wettability to water-wet to enhance the recovery factor. Other reports stated the usage of polyacrylamide-g-xanthan composite for chemical EOR [111].
2.3.3 Starch
Owing to its hydrophilic characteristics, starch can be used as an effective additive in EOR operations [4]. El-Hamshary et al. [112] stated the grafting of N-vinyl imidazole on carboxymethyl starch for heavy metal removal from aqueous solutions and improved oil recovery (IOR) operation. El-hoshoudy group discussed the synthesis of different starch composites through the grafting of silica nanoparticles and vinyl-containing monomers on the starch surface [95, 113, 114]. The rheological properties and flooding scenarios were assessed at simulated reservoir conditions. They concluded that the incorporation of silica and vinyl monomers acquire superior properties to starch composites as a flooding candidate.
2.3.4 Welan Gum (WLG)
WLG is an anionic three-fold double-helical polysaccharide formed by microbial fermentation of the sugar. In the petroleum industry, it is used as a common EOR viscosifying agent, cementing additive, and water shut-off agent [26]. Gao et al. [115] assess the rheological properties of WLG solutions at reservoir conditions. They reported that WLG is insensitive to harsh conditions upon long thermal aging and exhibits higher viscosity and viscoelasticity rather than xanthan gum. Moreover, it is a pseudoplastic fluid with shearing-thinning performance. This behavior resort to the network build-up by the neighboring double-helical structure [26].
2.3.5 Guar Gum (GG)
GG is a naturally occurring hydrophilic, non-ionized biopolymer. It has a rod-like polymeric geometry of mannose backbone with galactose side chains (molecular ratio 2:1) and contains plentiful surface hydroxyl groups, so exerts excessive H-bonding and unique gelling properties in the aqueous phase [26], and allow manufacturing of guar gum-based nanocomposite for industrial applications [93]. On an industrial scale, guar gum is reported in many industrial activities including the textile industry, mining, and petrochemical products. In the field of EOR, it is used as a flooding agent, emulsifier, gelling and stabilizing agent in conventional reservoirs [93, 116]. Wang et al. [75] stated the synthesis of sulfonated hydroxypropyl guar gum with superior rheological and hydrophilic properties in saline solutions for use in fracturing fluid. Bera et al. [22] reported the synthesis of guar gum-based silica nanocomposite with reasonable rheological properties for flooding purposes in sandstone reservoirs. They conducted flooding runs through sandstone cores soaked with waxy crude oil and saline solution of 2000 ppm salinity and reported that this composite can modify the wettability of the rock to water-wet via changing the contact angle toward the water-wet state. El-hoshoudy et al. investigated the implementation of guar-g- palmitate composite as a flooding agent and wettability modifier in sandstone reservoirs [117]. The synthesized composite obeys the Hershel-Bulkley model with shear-thinning performance, and higher adsorption rate on the sandstone rocks, so exhibit remarkable, and well-suited application in the EOR process.
2.3.6 Schizophyllan (SPG)
SPG is a hydrophilic, non-ionic, triple-helical biopolymer with a high average M.wt of 2–6*106 Da and excellent thickening efficiency. It is composed of linearly associated β-(1–3)-D-glucose units with single β (1,6) D-glucose residue. SPG resists thermal and salinity effects under reservoir conditions due to its intermolecular interactions and stiff conformation through H-bond. It exhibits a low adsorption rate on the rock surface since it has a neutralized backbone structure [118]. Moreover, it exhibits pseudoplastic (non-Newtonian) fluids with distinct shear-thinning behavior which are favored for polymer flooding [26].
2.3.7 Cellulose
Cellulose is abundant, linear, and hierarchical polysaccharide that consists of D-glucopyranose moieties, linked through β(1,4) bonds [119]. Cellulose can resist higher temperatures and high mechanical shearing owing to its 3D-network nanofibrillar structure [120], so can be considered as a nanomaterial candidate [121] for improved oil recovery. Cellulosic nanomaterials include cellulose nanocrystal and nanofibrils cellulose, in which cellulose nanocrystal is rod-like nanometer particles, while nanofibrils cellulose are effective EOR candidates owing to their outstanding rheological properties [99, 100]. Two common cellulose derivatives are implemented in the field of EOR including carboxymethyl cellulose, and hydroxyethylcellulose.
2.3.7.1 Carboxymethyl Cellulose (CMC)
CMC is multiple carboxylic groups of glucopyranose moieties that have higher coordination ability with metal ions, [79, 122, 123]. Bishop and Parlman, 1988 [124] reported that carboxymethyl cellulose functions as an effective polymer formulation in underground oil reservoirs. Yadav et al. [125] synthesized a graphene oxide/ alginate/CMC composite through the solution/mixing evaporation technique. Zhang et al., 2014 [79] stated about the incorporation of GO into the CMC/ PAM hydrogel pursued by ionic crosslinking of Al+3 ions with superior mechanical properties.
2.3.7.2 Hydroxyethylcellulose (HEC)
It is a thickening and gelling cellulose derivative, used in well-completion jobs and EOR operations [126]. It can be grafted by several inorganic fillers and nanoparticles to form polymeric composites that can withstand harsh reservoir conditions. Several reports stated the application of HEC in the field of chemical flooding and enhanced oil recovery as stated in literature [126, 127].
2.3.8 Lignin
Lignin is a natural complex polymer with quite low molecular weight and contains plentiful aromatic rings, so it is a valuable biosource for industrial applications [128]. The advantage of using lignin in water flooding processes relies on two aspects; (1) lignin exhibits pseudoplastic behaviors with shear-thinning properties [26], so can be implemented in waterflooding processes as a polymer agent. (2) Furthermore, lignin can be used in EOR formulations as a cheap source of surfactant, where lignin polyether sulfonate surfactants were effective in lowering the IFT between brine and crude oil [129]. In this regard, Sulaiman et al. [130] reported the use of lignin from black liquor waste as a surfactant or sacrificial agent in enhanced oil recovery.
2.3.9 Scleroglucan
Scleroglucan is a rigid, triple-helical biopolymer, consisting of β (1,3) D-glucose linked with β (1,6) D-glucose moieties [101]. It exhibits superior rheological criteria including temperature and shearing action withstanding as well as pH and salinity resistance [26]. Scleroglucan can be applied in reservoir conditions with temperature up to 100–120 °C, and within a broad range of pH (1–13), and exhibit pseudoplastic behavior even in the presence of various salts, such as NaCl, KCl, CaCl2, MgCl2, and MnCl2 [101]. Over 30 years ago, field-scale injection using scleroglucan solution has been conducted in the North Sea Reservoir, where about 140 polymers were tested under severe reservoir conditions and scleroglucan exhibited the best EOR performance [26].
2.4 Synthetic Polymers
Polymer flooding in petroleum reservoirs comprises biopolymers and synthetic polymers [131]. Regarding the EOR synthetic polymers, partially hydrolyzed polyacrylamide (HPAM) has attracted incremental attention in petroleum applications including drilling fluid formulations, EOR, wastewater treatment, water shut-off, and plugging agents [31, 66, 132,133,134]. The worldwide application of HPAM in oil field applications resort to its availability, ease of handling, and cheap manufacturing cost [1, 26]. HPAM is synthesized by copolymerization of vinyl-containing polymers such as acrylamide and acrylic acid [21]. Furthermore, it can be copolymerized or grafted with nanoparticles to form polymeric composites. HPAM performs superior rheological properties under harsh reservoir conditions, as it exhibits shear-thinning behavior so favored for flooding processes as it mitigates the pumping action and electrical work overload during the operation. Furthermore, it exhibits reasonable microbial resistance and controls the mobility ratio through increasing displacing fluid viscosity, so enhance the sweeping efficiency [19, 66, 135]. HPAM performance in the flooding process is controlled by several factors including.
-
(a)
Degree of polymer degradation under high temperature and high ionic strength [136].
-
(b)
Blockage of pore throats due to polymer retention [137].
-
(c)
Rate of polymer adsorption on the rock surface [51].
-
(d)
Formation pressure and temperature, pH, degree of salinity, and hardness [31].
On the other hand, HPAM suffers from polymer disintegration and viscosity drop under high salinity environments > 30,000 ppm, due to hydrolysis of -NH2 groups of acrylates into COOH-groups. To overcome these shortages, a few strategies have been proposed. One of them is the incorporation of nanoparticles through a polymer framework to form a polymer composite [66]. Nanoparticles incorporation into HPAM reinforces the intermolecular hydrophobic associations that strengthen the network structure of the polymeric composite and enhance the rheological performance of the composite solution [66]. In this regard, Bhardwaj et al. [138] stated that PAM/SiO2 nanocomposites exhibited enhanced thermo resistance performance. Ponnapati et al. [139] synthesized ethylene oxide/SiO2-based nanocomposite. They included that SiO2 polymer nanocomposite improves oil recovery without pore blockage. Ye et al. [65] demonstrated the synthesis of hydrophilic, thermostable, and easily flowable polymer/SiO2 nanocomposites. Maurya and Mandal [140] reported the use of polyacrylamide (PAM) and silica nanoparticle suspension for high temperature and high saline reservoirs. The results reveal that silica/PAM suspension has a higher viscosity and viscosity retention owing to the formation of a complex macromolecular structure. Maghzi et al. [44] indicated that the viscosity of PAM increased by incorporating SiO2 nanoparticles, with an additional 10% oil recovery during a polymer displacement. Maurya et al. [27] reported the synthesis of soluble nanocomposite for application in the high-temperature reservoir using a free-radical polymerization. The polymer chains were grafted on the surface of the silica nanoparticle. El-hoshoudy et al. stated the synthesis of synthetic polymer composites through grafting or copolymerization of various silica derivatives with polyacrylamides [28, 141,142,143,144]. The rheological criteria at simulated severe conditions and the optimum polymerization conditions were reported [145,146,147]. The studies concluded that the grafting or copolymerization of silica nanoparticles into the polyacrylates matrix enhances their rheological, mechanical, and viscoelastic properties. Furthermore, the recovery factor was enhanced, and rock wettability changed from oil-wetness to water-wetness, so it improved sweeping and displacement efficiency.
2.5 Hydrogels
Hydrogels are 3D-crosslinked networks of synthetic polymers that can absorb large aquatic fluid quantities, so attracted outstanding attention in the field of enhanced oil recovery [77, 80]. In petroleum industries, gellants and hydrogels were used on a wide scale for the treatment of water shut-off during production operations [148]. Hydrogels suffer from deprived mechanical and thermal behavior, so they are incorporated with nanomaterials fillers including clay, graphene sheets, silica nanoparticles, and carbon nanotubes to form nanocomposites with enhanced mechanical toughness, dense structure, improved rheological and mechanical performance [77, 79, 80], where Young’s moduli depend on the concentrations of nanofiller crosslinker [77]. Hatzignatiou et al. [149] use gel polymers for water control and enhanced oil recovery in fractured chalk reservoirs. Hasankhani et al. [148] reported hydrolyzed polyacrylamide (HPAM) crosslinked with polyethyleneimine (PEI) hydrogel as a flooding agent. El-hoshoudy et al. reported the experimental and theoretical application of polyacrylate hydrogel for water shut-off and permeability reduction through highly permeable sandstone reservoirs [150]. The experimental study indicates the enhancement of recovery factor up to 85.35 Sor%, while the simulation study reported a recovery factor of 150,000 bbl/d on a field scale with a polymer dosage of 0.6 Pv.
3 Experimental Section
In this section, the author gives short hints on the generalized mechanism and strategy of polymeric sample preparation and characterization, in addition to displacement operations based on the literature survey and our experience in this field as reported in our previous relevant publications [28, 60, 78, 95, 96, 110, 116, 146, 150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167]. The oil composition, rock types, and properties, and experimental conditions are detailed in our previous book [10], pages 49–72.
3.1 Sample Preparation and Handling
First, the oil sample was deoxygenated and left under an N2 blanket until usage. The polymer composite solution is prepared by blending the composite flakes or powder with distilled or brine solution until complete dissolution at neutral PH. The mixture was left for 48 h to obtain a homogeneous formulation. Before the flooding runs, the composite solutions were diluted to the various slug concentrations [168].
3.2 Flooding and Displacement Mechanism
The mechanism of polymeric nanocomposite flooding to enhance the oil recovery resort to the capability of nanoparticles to retard chain degradation and intensify the polymer viscosity under reservoir conditions. Core or sandpack flooding on the lab scale is conducted on a pilot scale to simulate the process of chemical injection through the injector well in the reservoir as displayed in Fig. 7 [1, 51, 168]. The pilot model may be a linear 1D- or ¼ five-spot model. Several reports about core flooding are reported by Lashari and Ganat, 2020 [1]. Steps of sand pack preparation and composite flooding can be summarized as follow;
-
(a)
The sand sieved, acid-washed, and dried as detailed in our previous literature [10].
-
(b)
The fittings, lines, and valves of the stainless-steel model (linear or ¼ five-spot) were checked for leakage under pressurized air of 500 psi.
-
(c)
The stainless-steel model is associated with a positive displacement pump for fluids displacing, two inlet and outlet pressure gauges for monitoring the pressure fluctuations during the flooding process,
-
(d)
The stainless-steel model was evacuated, then packed slowly with the sand under mechanical shaking to ascertain optimum packing without any voids.
-
(e)
The model is saturated with brine solution, then record the model physical properties including bulk volume, porosity, and model dimensions, brine and oil permeability, Darcy velocity for water and oil, initial water and oil saturation, and residual oil saturation (Sor). All the fluids were displaced at a constant rate of 1.0 cc/min [47].
-
(f)
The formation water displaced for 10 Pv at a constant flow rate, where the pressure drop (ΔPw)before polymer was monitored, then the polymeric composite was flooded to displace formation water and pressure difference (ΔPp) was recorded.
-
(g)
Flush the formation water again to displace the non-absorbed polymer, where the pressure difference (ΔPw) after the polymer was recorded.
-
(h)
From the recorded pressure difference values, the resistance factor (Rf), and a residual resistance factor (Rrf) are calculated by the following expressions [150].
$$ Rf = \frac{(\Delta P)p}{{(\Delta P)w({\text{Beforepolymer}})}} $$(1)$$ Rrf = \frac{{(\Delta P)w({\text{Afterpolymer)}}}}{{(\Delta P)w({\text{Beforepolymer)}}}} $$(2) -
(i)
Inject the crude oil under the required conditions (pressure and temperature) until the ejected fluid is 99% oil, then the model is left for 21 days for oil aging.
-
(j)
Flush the brine solution to displace the oil until oil production ceased off.
-
(k)
Inject the polymeric composite slug with different concentrations to recover residual trapped oil.
-
(l)
The oil recovery factor is calculated relative to the original oil in place (OOIP) or residual oil saturation (Sor) as a function of injected pore volume, as displayed in Fig. 8.
Schematic representation of core flooding [168]
Oil recovery factor relative to injected pore volume (PV) [41]
3.3 Polymeric Nanocomposite Preparation
Polymeric composites synthesized through grafting of inorganic fillers such as graphene oxide, carbon nanotubes, and silica nanoparticles into polymer chain through reversible addition-fragmentation chain transfer processes (RAFT), free-radical emulsion polymerization (FRP), atom transfer radical polymerization (ATRP), and nitroxide mediated polymerization (NMP). Emulsion polymerization is a common and widely used method for macromolecule build-up [76]. Composites prepared through subjecting modified nanoparticles including (silica particles and GO sheets) to free-radical emulsion polymerization with the aid of water-soluble initiator and surfactants [76]. SiO2 nanoparticles are synthesized according to the Stober technique [36, 169]. GO sheets are adapted using various chemical modifications to achieve functionalized GO sheets as reported by Tang et al. [76]. After composites preparation, it is layered to slices or disks of 4 mm thickness and 10 mm diameter [77].
3.4 Polymeric Composite Characterization
Characterization of polymeric composites is an important issue through composite implementation in EOR processes. Rheological and viscoelastic characteristics were conducted using a rotational rheometer equipped with concentric cylinder geometry [170] according to ASTM D7945-16 and ASTM D2196-18 with an uncertainty of 2.0% [171]. The test was done at a temperature range of 25–150 °C depending on the reservoir operating conditions [21]. The rheological criteria are estimated under dynamic and steady shearing to assess dynamic and steady viscoelastic criteria relevant to shear rate, salinity, and temperature at an angular frequency (1–100 rad s−1) [150, 172]. Shear stress is tested at a shearing rate of 0.1–1000 s−1 [21], but most are assigned at 7.34 s−1 since it is the accepted shearing applied to the fluids in the underground reservoirs [145]. The swelling capability is estimated in distilled water or brine solutions for 1–30 days [36]. Compression measurements were conducted by an Instron 3345 testing machine with a 10 N force transducer, and a probe size of 10 cm [77]. Long-term thermal steadiness was conducted by storing the samples in sealed glass bottles covered by screw caps in the heater at 80–100 °C for 12–30 days and monitoring the viscosity loss rate [66]. Aging of polymeric composites assessed by Raman spectroscopy through keeping thin sheets in an oven at aging temperatures for 30 days. Surface and interfacial tension of polymer composite solutions were estimated using a Sigma 703 ring tensiometer at various temperature ranges with an uncertainty of 0.5 mN/m [21]. Molecular weight is assessed by gel permeation chromatography. Infrared bands of evacuated polymeric composite samples were assigned by FTIR spectrometer in the scanning optical range of 4000–400 cm−1 using KBr pellets [66]. The surface of the polymer composite was screened by an atomic force microscope (AFM) [36]. AFM images were conducted on (AFM-NanosurfFlex-AxiomFlexAFM 5 scan head specifications with C3000 controller) operating in dynamic force mode at 25 ºC. Thermal gravimetric analysis (TGA) was conducted under N2 atmosphere at 5 ml/min as a heating rate, where 5.0 mg of the composite sample was heated in an aluminum pan from 25 TO 800 °C [76]. The internal structure was screened by HRTEM analysis on a high-resolution transmission electron microscope. The samples were dispersed in ethanol, then plummeted on copper grids for imaging. 1H-NMR spectrum was conducted by using TMS as internal standard and D2O/DMSO as a solvent. The particle size distribution was determined by (Zetasizer 6.32) supplied with He–Ne laser at scattering angles of 90° and 25 °C. The particle zeta (z) potential was determined by (Zetasizer ver. 6.32, Serial Number: MAL1071664 Nano Series, Nano-ZS, Malvern Instruments, UK)-dynamic light scattering equipped with a cuvette rotation/translation unit (CRTU) and a He–Ne laser at 25 °C [28, 38]. All experiments were performed at scattering angles 90° and 25 °C. The acquisition time for each run was 30 s [28].
3.4.1 Effect of Zeta Potential Value on the Stability of the Aqueous Solution
Zeta potential is the measure of the surface charge on nanoparticles in solution, which controls the stability of the solution. The potential values may be either positive or negative. Normally values greater than 30 mV indicate good stability, while those with values lower than 30 mV eventually aggregate due to Van Der Waal associations [38, 173]. Polymer chains reduce the repulsive interactions between oil droplets but contribute toward steric hindrance to impede droplet coalescence. Silica nanoparticles in the range 5–15 nm aid in the formation of robust oil-aqueous interfaces, by adsorbing onto available ‘‘interfacial sites” for adsorption; and strengthening electrostatic as well as steric barrier influences. With increasing SiO2 concentration, the stabilizing constituents are more closely arranged at oil droplet surfaces; and retard droplet flocculation and recoalescence [173]. Normally, van der Waals's force increases with the interaction distance while electrostatic force strongly depends on zeta potential and is sensitive to salinity, pH, and ionic type. When the attraction between NP and rock is larger than the repulsion, NP adsorption and retention would occur, and the adsorption/desorption rates are closely correlated with the magnitude of energy barrier [174]. Insertion of silica nanoparticles in the nanocomposite structure exhibit negative zeta potential values of which indicate the ability of nanocomposites to induce wettability alteration on positively charged sandstone reservoirs at pH = 2–6 during polymer flooding processes [28].
3.4.2 Effect of Hydrophilicity/Hydrophobicity of Nanoparticles
Zhong et al. [174] reported that nanoparticles can act as emulsion stabilizers. In general, NPs with higher hydrophilicity are good choices for O/W emulsions, otherwise are selected to stabilize W/O emulsions. However, strong hydrophobic or hydrophilic NPs can hardly go to the oil–water interface unless with huge external energy input by ultrasonication or homogenization. They stated that surface activated partially hydrophobic NPs with both hydrophobic and hydrophilic parts are better preferred considering their higher tendency to go and to stay at the oil/water interface with a low or medium energy input like shaking or stirring. By temperature increasing, the thermal motion of hydrophobic interactions is intensified, which weakens the degree of hydration of hydrophobic (tail) groups and reduces the hydrodynamic volume of stabilized oil droplets [173]. Nanoparticles show synergetic interactions with dissolved crude oil components at micro-interfaces in solution. This behavior resort to their hydrophilic or lipophilic properties [175]. Nanoparticles are energetically adsorbed at the fluid/fluid interface and replace water and/or oil molecules of the original interface, so the interface exists between the hydrophilic part of the complexes and water molecules on one side and between the hydrophobic part of the complexes and oil molecules on the other side of the interface. Thus the tension across the interface is reduced by the presence of nanoparticle complexes, as the interactions now are considered to be much stronger than the interaction earlier between the highly dissimilar oil and water molecules [176].
3.4.3 Effect of Chain Length, Molecular Weight, and Functional Groups on Nanocomposite Stability
With the increase of backbone chain length, the d-spacing of π-electron delocalization increase through nanocomposite structure, as a result, the degree of and rigidity crystallinity decreases, which in turn promotes the molecular packing within the layer structure [177, 178]. This behavior resorts to, with the increase of backbone chain-length, the interactions of intercalated modifier with polymer could increase leading to greater disruption of orderly orientation of polymer chain and hence greater reduction in the polymer crystallinity [179]. Moreover, increasing of backbone chain-length increases the elastic modulus of nanocomposite solution. As a result, it will possess viscoelastic properties which are favorable for flooding operations [110]. Functional groups on nanocomposite surface determine its performance during the flooding process. Electron donating groups like (NH2-, OH-) which exist on polyacrylamide surface can give rise to H-bond formation through aqueous acidic phase, and form 3D- network structure which in turn increase displacing fluid viscosity and improve sweeping efficiency [157]. Moreover, these protonated groups are stronger in disrupting the crystallinity of nanocomposite [179]. In the case of biopolymers, their surface has a plentiful hydroxyl group, which can be polymerized or form H-bonding leading to viscosity enhancement. Reinforced nanocomposites with functionalized nanoparticles enhance their thermal and mechanical properties [180] during the flooding operations. It is known that the radius of gyration (Rg) of polymeric random coil increases with molecular weight (MW). The change of MW affects thermochromic properties of the resultant nanocomposites [177]. The low MW nanocomposites have relatively small Rg, implying low viscosity and small size in solution, so not favored for EOR applications. On the other hand, high molecular weight nanocomposites exhibit high viscosity values which improve sweeping efficiency and thus recover more oil. At this point, one should note that nanocomposites with excessive molecular weight will cause plugging and injectivity disorders.
3.4.4 Effect of Droplet Size Measurement and DLS Studies
The polymer solution samples were tested for droplet size stability by dynamic light scattering (DLS) to investigate the synergistic effect of polymer and nanoparticles. The solution stability relies on the size of the dispersed droplets, as a result, the sample with a smaller droplet size is more kinetically stable than the sample with a larger droplet size. Kumar et al. [38] stated that cohesive force between the emulsion/polymer solution drops decreases by the accumulation of nanoparticles on the drop surface, resulting in lower stability. By increasing nanoparticle concentration, the number of droplets with smaller size increases, representing better stability owing to enhanced Brownian motion [38]. The effective diameter of nanocomposites and pore throat diameter of cores is a crucial factor to predict the effectiveness of flooding through the enhanced oil recovery process. The stability of nanocomposite overtime is required for its effective performance in the reservoir [176]. Nanoemulsions as well as nanocomposite solution function by entering into pore throat pores, where trapped crude oil exists [173]. The nanocomposite solution, and polymer/nanoparticle solution serve to alter the wetting properties of rock. Furthermore, they modify the IFT between water/oil surface as well as rock/oil surface, which in turn improves flooding effectiveness. El-hoshoudy and Desouky [10] stated that nanocomposite solutions exhibit higher values of resistance factor (RF) and a residual resistance factor (RRF), so exert improved sweep efficiency and penetrating or spreading to the more rock porous region, which would greatly improve the oil recovery inside the rock pores. In the oilfield practical flooding process, definite relationships between the medium resistant factors and oil flooding efficiency have been established. That is, the higher value of RF and RRF for nanocomposite solution produced the higher efficiency of oil recovery in the rock reservoir from middle to low porosity. Consequently, it is expected that the low efficiency of water injection would be improved with nanocomposite injection. Although the risk of decreased oil permeability with polymer injection is minimal, because the polymer injected into the reservoir rocks alters preferentially the permeability of the small pores, whereas the oil drops are found in the large pores, but the possibility of formation damage due to polymer adsorption/retention in the pores should not be overlooked and can be measured by a decrease in permeability. The adsorbed polymer layer thickness (e) was determined using the following equation.
In most scenarios, the adsorbed layer thickness (e) of the nanocomposite, are thin layers and will not cause pores plugging.
3.4.5 Pressure Drop and Permeability Limitations
The presence of polymer as well as nanoparticles in nanocomposite systems improves the steric, viscous effects in displacing fluid, and forces the trapped oil in low-permeability regions [173]. Nanocomposite possesses a 3D-network structure exhibiting viscoelastic behavior which displaces oil from low-permeable zones and plugs high-permeable zones [173]. The extraction of tertiary oil is achieved by overcoming the capillary pressure in pore spaces, which aids in the mobilization of crude oil droplets [173]. Nanocomposites are expected to transport smoothly in the porous media if there is no severe particle aggregation or precipitation, but the physicochemical interactions between NPs, formation fluids, and reservoir rocks are complex and may lead to significant NP retention especially at incompatible conditions [174]. NP retention might lead to severe pore plugging and irreversible permeability damage [174]. The transportation and retention behaviors of nanocomposites are driven by electrostatic, van der Waals forces, and non-DLVO forces (born repulsion, capillary, steric repulsive, entropic, hydration/hydrophobic, hydrodynamic forces) forces between nanocomposites, formation fluids, and the rock [174]. Permeability reduction was determined by measuring the pressure drop during the core flood experiments which were used to calculate the resistance factor (RF) and a residual resistance factor (RRF). The higher values of RF and RRF factors for nanocomposite solutions resort to; (1) high gel effect in aqueous solution, so increase mobility retardation, resulting in higher pressure difference during flooding and increase resistance factors, (2) formation of hydrophobic microdomain and enhancement of the sweeping efficiency through a proper inorganic–organic synergistic or nanosize effect. In the case of a physical mixture of polymer or biopolymer/ nanoparticles, the capture of nanoparticles on rock surface may form the stagnant boundary layer, giving rise to a 3D-network structure inside the porous space of rock which greatly increases the permeation resistance force. The larger the resistance factor was, the larger the permeation resistance of polymer solution in the porous medium was. Nanocomposite solutions exhibit high values of RF and RRF, so they exert improved sweep efficiency and penetrate or spread to the more rock porous region, which would greatly improve the oil recovery inside the rock pores. However, extreme increase in RF and RRF values may lead to injectivity disorders specially in the low-permeable zones, which lead to porous media blocking and failure of the flooding process [10].
4 Assessment of the Rheological Properties
The addition of nanoparticle fillers enhances the rheological properties of polymeric nanocomposites, changes the rock wettability, reduces surface, and interfacial tension at O/W contact leading to a high recovery factor [1]. Khalilinezhad et al. [20] examined the rheological properties of hydrophilic silica nanoparticles blended with the polymer solution for improving the oil recovery throughout polymer flooding. They concluded that silica nanoparticles enhance the viscoelastic properties of the blend, so the oil recovery factor reaches 60% OOIP. The detailed effect of the rheological characters on the polymer composites' performance during the flooding process is discussed elsewhere [20]. Rheological properties can be modeled mathematically as discussed in the literature [110, 117, 143, 150, 181]. Assessment of rheological properties for polymer composite in EOR operation conducted relative to shearing action, salinity effect, and thermal effect. The following section highlights these parameters through polymeric composite flooding in EOR operation.
4.1 Shearing Action
Polymeric composites are subjected to high shearing action during the flooding operations and flow through rock pore throats [182]. The shearing force affects adversely the composite compactness leading to chain destruction and loss of viscosity [183]. Injectant fluids are subjected to a shearing rate of 7.34 s−1 in the underground reservoirs [172, 184]. Resistance to shear stress is estimated at various shear rates as exhibited in Fig. 9. The composite beads dried then dispersed in deionized water at different concentrations and subjected to different shear rates [78]. For a favorable chemical flooding agent, it must obey non-Newtonian fluid performance (shear-thinning effect) to reduce the pumping action, electrical operating cost, and maintain viscosity after the shearing end [161, 172, 185, 186]. Polymeric composites exhibit pseudoplastic behavior owing to the decline of molecular entanglement at the incremental shearing rate [187]. Shear stress is related to the shear rate through the Hershel-Bulkley model [188,189,190,191] as shown in Eq. 3.
Shearing rate and shearing stress relevant to composite viscosity [161]
Non-Newtonian fluids exhibit a lower n value less than unity (n ≤ 1)[172, 185], due to the decreasing of viscosity by the shearing increase. The presence of inorganic fillers (SiO2, GO, carbon nanotubes) through composite matrix mitigates the viscosity loss by shearing action due to; (1) the development of intra/intermolecular associations and 3D-network, which strengthen the composite stiffness during the shearing force [163, 172]. (2) Dispersion of inorganic fillers into the composite matrix enhances shear resistance criteria. The Allometric1 power-law model fits the viscosity-shear rate profile as indicated in Eq. 4.
yield stress occurrence confirms the development of crosslinked 3D-structure between the dispersed inorganic fillers and composite matrix [191, 192].
4.2 Salinity Effect
The higher reservoir salinity adversely affects the gelling effect of the polymer [193, 194]. The effect of formation water salinity on the polymeric nanocomposite viscosity was assessed at simulated reservoir ionic strength at various shear rates. The concentration of mineral composition in most petroleum reservoirs ranges from (4–12 *104 ppm) depending on the producing layer depth and location. In the flooding process, polymers suffer from chain degradation at high salinity and hardness encountered by reservoir formation brines, where the carbon chain tends to conform and coagulate from the solution [19]. On the other hand, polymeric nanocomposites can withstand the degradative effect of the reservoir ions due to the formation of intermolecular linkage between inorganic fillers and composite matrix which intensifies the crosslinking of molecular chain and increase the composite hydrodynamic volumes [195, 196]. Furthermore, at high ionic strength, divalent ions are absorbed by polymeric composites, so the gelling effect is enhanced, and solution viscosity increased [197, 198]. The assessment of the salinity effect relevant to the composite viscosity is expressed as displayed in Fig. 10. The viscosity gradually declines with salinity increase until reaching an intangible limit.
Salinity-viscosity profile [95]
Saline solution with different ionic compositions greatly affects the polymer chain degradation and polymer viscosity. Most polyacrylamides and biopolymers are grafted with COO− and SO3− groups to increase their hydrodynamic volumes and enhance their solution viscosities during the flooding process. Different ions (Na+, Mg2+, Ca2+, Cl−) have different effects on the polymer chains and their grafted functional groups. For COO− and SO3− groups, Mg2+ basically cannot enter the hydration layer around the groups, while Na+ and Ca2+ can enter the hydration layer of both groups and tend to be distributed between two oxygen atoms. Moreover, Mg2+ and Ca2+ ions have the main influence on the carbon chains through polymer backbone structure leading to reduction of hydrodynamic volume and viscosity decreasing [150]. COO− and SO3− groups exhibit higher affinity to Na+ ions compared to Mg2+ and Ca2+ ions. Mg2+ ions can interact with the carbon chain groups on the polymer architecture. It can be concluded that Mg2+ and Ca2+ ions have the chief effect on the carbon chain groups. On the other hand, COO− and SO3− are greatly affected by Cl−, where chloride ions cannot enter the first hydration shell of polymers. Finally, we can conclude that the cation is the chief influence issue to the viscosities of the polymer since it can penetrate the first hydration shell of the polymers [199].
4.3 Temperature Effect
Longitudinal thermal withstanding for synthetic polymer in HPHT reservoirs is a crucial factor of profile conformance in chemically EOR [200]. Synthetic polymer flooding in HPHT reservoirs suffers from chain degradation and polymer precipitation due to the hydrolysis of the pendant-acrylamide group into carboxylate ions [19, 201]. The temperature of most petroleum reservoirs ranges from 50 to 170 °C. The thermal effect was evaluated through monitoring the viscosity change at different shear rates and simulated reservoir temperature as displayed in Fig. 11. In the case of polymeric composites, the higher temperature values enhance the stress modulus, and gel strength of the composite so can withstand thermal degradation [163, 172, 193, 202,203,204]. Also, the inorganic fillers as a crosslinking agent mitigate the association of divalent ions to the polymer chains, so enhance the thickening efficiency at reservoir conditions [205,206,207].
Temperature-viscosity profile [150]
4.4 Viscoelastic Properties
Viscoelasticity evaluated through measurement of elastic or shearing modulus (G′) and viscous or dynamic modulus (G″) in the range of 1–100 rad s−1 of the angular frequency (ω) at a constant strain of 5% within the linear viscoelastic regime [208] through the application of a sinusoid oscillating shear strain [161, 191, 209,210,211,212] as shown in Fig. 12. Dynamic properties represent the material response to an external force field [213]. G′ modulus represents the reversible stored deformation energy (material elastic properties), while G″ modulus measures the irreversible dissipated energy during one cycle (viscous properties of the material) [161] [214]. For chemical flooding agents, to perform gel-like behavior, the G′ modulus must predominate the G″ modulus [210]. The polymeric composites exhibit superior viscoelastic criteria owing to the incorporation of inorganic fillers which strengthen the molecular chains, give rise to the crosslinked network structure, and delay the composite agglomeration [201, 209], so improve viscoelastic characteristics [210].
G′ and G″ moduli versus angular frequency (ω) [161]
The shear stress G′, G″, G* moduli, and tan δ as a function of the applied torque represented mathematically as formulated in Eqs. 5–9, respectively [215, 216];
The loss factor (tan δ) related to G′ and G″ moduli and measure the ratio of the stored to dissipated energy [215]. For polymeric composites, tan δ represents the gel viscoelasticity [191, 217]. For a flooding agent, tan δ should be less than unity to assure the predominance of elastic nature over viscous nature [217].
5 Simulation and Theoretical Studies
5.1 Molecular Dynamic (MD) Simulation
MD simulation was performed to scrutinize the optimized interaction and mixing energies between polymeric composites and the modeled oil [116, 218, 219]. MD explores the molecular interactions involved in the wettability alteration through adsorption on reservoir rocks. Furthermore, it can be conducted to screen the mechanical properties and tensile of the polymer composites in the flooding process [181]. Software used for MD simulation includes Schrodinger, Ds Discovery studio, and DS-Biovia Materials studio which is considered the most common one [220]. The composites build up depending on their constituting monomers and polymerization degree, then subjected to geometry optimization, random rotation, and translation under the applied forcefield. After that the amorphous packed cell was constructed, then the MD and Monte Carlo calculations were conducted through Blends and Forcite modules [221], to estimate some physical parameters including the solubility parameter, cohesive energy density (CED), mixing energy (\(E_{{}}^{mix}\)), and Flory–Huggins chi parameter (χ bs) [222]. These parameters screen the binary mixtures compliance, and the system thermodynamics [181, 223,224,225,226,227].
Although the usage of MD and MC simulation in the field of EOR suffers from rare publications, few reports stated this issue. In this regard, De Lara et al., 2015 [8] investigated the ability of cyclodextrin composite to alter the wettability of a quartz surface through molecular dynamics simulations. El-hoshoudy group [181] conducted an MD simulation to explore the interaction energy of the DES/oil system. They reported that DES exhibit high cohesive energy with the oil system, so can solubilize the oil and improve displacement efficiency during the flooding process. In other work, they reported wettability alteration through adsorption of guar gum and palmitate guar gum on SiO2-quartz crystal through Monte Carlo simulation [152]. The simulation output reveals that palmitate guar gum exhibit higher adsorption and electrostatic interaction than guar gum on the surface of SiO2-quartz crystal, leading to stable film coverage and more wettability alteration, so enhance the oil recovery factor. In the same field, they conducted MD simulation to explore the mechanical criteria of silica/polyacrylate composite including bulk modulus, Poisson's ratio, Young’s, and shear moduli [143]. The inoculation of the silane-coupling agent enhances bonding and torsion energies as well as the mechanical and rheological performance of the composite [228]. Consequently, this composite will perform well in the underground severe reservoir conditions.
Molecular simulation technology implies computing methods to simulate the molecular and atomic models’ behavior through calculating the physical properties which cannot be estimated experimentally. Molecular simulation acquires incremental attention in the study of fluid equilibrium, reservoir characteristics, rock heterogeneity, and rock properties through analyzing the motion of the atoms and coordinates in terms of statistical parameters, dynamics, and thermodynamics equilibrium [229]. Furthermore, molecular simulation investigates the sweeping mechanism, most favorable oil-displacing agents, and oil displacement at the microscopic scale [229]. Future advances of molecular dynamic simulation are critical for the characterization of oil and gas reservoirs through screening of the production mechanisms and flow-related phenomena. The prospects of molecular dynamic (MD) simulation in the field of the oil and gas industry can be directed in the following trends.
-
1.
Conducting computational and MD simulations to predict the physical properties and interaction energies through oil/water/rock, then employing these properties in the optimization of polymeric nanocomposites flooding.
-
2.
In the field of EOR engineering, molecular simulation investigates the interaction mechanism between surfactants and crude oil, the design of oil displacement agent molecules, and the analysis of oil and gas structure [229].
-
2.
Directing MD simulation to study the mechanistic of polymer flooding process including collision and friction forces, viscoelastic and dynamic criteria, which in turn help in screening microscopic oil displacement mechanism of polymer flooding.
-
3.
Screening performance analysis and conformation profile of polymer flooding.
-
4.
Investigation of nano-scale dynamics in the process of surfactant flooding.
-
5.
Understanding the interfacial phenomena during N2 and CO2 flooding.
-
6.
Investigating molecular and solubility behavior of asphaltenic crude oil during waterflooding processes.
-
7.
Solving the foam, viscosity, and density issues during gas injection processes.
-
8.
Evaluating the synergistic effects during polymer/surfactant combined flooding.
-
9.
Applying MD simulation in new displacement technologies including nanoparticles flooding, CO2 WAG flooding, low salinity water flooding, and microemulsion flooding.
-
10.
Molecular simulation to design generally acceptable models and procedures to represent complex reservoir fluids and heterogeneous porous media, develop fluid and rock property prediction toolboxes, study cemented formations and production paths in reservoir rocks.
-
11.
Study of fracturing fluids and the mechanism of profile control and water plugging agent.
5.2 Numerical Simulation and Field-Scale Modeling
Although the flooding runs performed on a wide scale in the laboratory through the sand pack and core models, the numerical studies have been validated and confirmed in the oil recovery process as summarized elsewhere [9]. The process of petroleum engineering gives a crucial interest to the numerical, and statistical modeling of the flooding agents especially polymeric nanocomposites to determine the optimum injection parameters and optimized scenarios for reservoir characterization and evaluation before the field operation [230], owing to the high cost of laboratory tests, and heterogeneous physical properties of polymer composites and nanofluids [11]. The modeling of polymer nanocomposites through EOR operations including adsorption and retention of composite, IFT reduction, oil improving relative to composite concentration, molecular weight and viscosity, injection rate, fracture and matrix permeability, wettability alteration, slug size, reservoir simulation, and flow behavior through porous media are reported in the literature [7, 230]. The modeling and simulation study relies on the following aspects, (1) surface charge of the nanocomposites which control the process of wettability alteration, (2) electrostatic charge on the rock surface (sandstone and carbonate reservoirs) which adjust the adsorption process during the flooding strategy, (3) the capillary pressure and relative permeability through the porous media, and (4) history matching with actual historical reservoir data before simulation commencement [7, 231,232,233,234,235]. Several commercial simulators are reported in the simulation studies conductance to model reservoir characterization and polymer flooding. These simulators include Schlumberger Eclipse, Computer modeling group (CMG), UTCHEM (University of Texas Chemical Compositional Simulator), and Petrel. Although UTCHEM conducts lab-scale runs, then validates it to the field-scale polymer injection [1], but STARS-CMG and GEM-CMG simulators are the predominant software in EOR thermal and chemical flooding [27, 236]. STARS is implemented in petroleum companies as an optimum reservoir simulator for modeling thermal, compositional, geomechanics, chemical, and thermal flooding issues [237]. STARS-CMG can model polymer and composites adsorption through carbonate and sandstone reservoirs by using the Langmuir isotherm equation on the field scale as well as on the lab scale (consolidated packed models and core models) [27, 40]. In the simulation scenario, some laboratory measurements are introduced as inputs into the simulator including rheological data of the polymer composite (viscosity, composite concentration, and shear rate) as well as, oil density, rate of adsorption on the pore surface, and other fluid physical properties [238]. Also, some field data are reported as key parameters including porosity, relative water/oil permeabilities, relative oil/gas permeabilities, reservoir pressure, rock compressibility, and temperature, bubble point pressure, initial oil, and water saturation, depth of water /oil contact, maximum BHP for the injector and the producer, oil-producing rate, and composite injection rate [157, 239]. The model is built in 3D dimensions where several grid blocks and directions are defined. The oil and water saturation, as well as pressure profiles, are illustrated as shown in Fig. 13. The tertiary flooding using polymer composite commences in the simulation model when oil cut approach 1% and the water cut approach 99% after secondary recovery stage through saline flooding [27].
Khalilinezhad et al. [240] conduct a UTCHEM simulator to simulate the effect of clay and hydrophilic silica nanoparticles hybrid on the flow performance of the injectant fluid through the porous media [1, 20]. Furthermore, the simulation model was confirmed by laboratory core flooding runs to ensure proper implementation on the field scale [19]. Druetta and Picchioni [241] simulate the behavior of nanoparticles-assisted polymer flooding in the chemical EOR process. The simulation output concluded that this nanoparticle blend can recover 40/45% OOIP. El-hoshoudy et al. [157] conducted a simulation study on a 3D-reservoir model using deep eutectic solvents (DES) through a GEM-CMG simulator. The simulation study continued for two years and concluded that DES are effective EOR agents. In other work, El-hoshoudy group employed a UTCHEM simulator for simulating the hydrogel capability for water shut-off and oil recovery through sandstone reservoirs. The simulation continued for 800 days and concluded that the oil recovery achieves 150*104 barrels/day at polymer dose = 0.6 pore volume [150]. Firozjaii and Saghafi [40] employ a CMG-STARS simulator to simulate polymer adsorption on the rock surface [242]. They reported that polymer adsorption is not related linearly to salinity and polymer mole fraction. Esfe et al. [11] reported a simulation study of nanofluids using various slug concentrations. The nanofluid behavior through porous media and flooding efficacy were simulated relevant to the diffusion rate and capillary pressure [243].
6 Future Recommendation
Polymeric nanocomposites for enhanced oil recovery face several challenges related to the cost and mechanism of preparation from commercial and cheap sources since the flooding process requires a high slug amount. This section summarizes the future aspirations in which R&D research should be directed toward it in the nearest future.
-
1.
Development of polymer composite from cheap natural sources, and commercial inorganic fillers.
-
2.
Photoimaging of the reservoir through the development of ferrofluids and electromagnetic sensors and nano Roberts for collecting the underground reservoir data.
-
3.
Scaling-up of the field trials to adjust and validate the real injection and flooding scenarios, besides overcoming the polymeric nanocomposite shortage through the underground harsh reservoir conditions.
-
4.
Development of more optimized numerical models and simulators especially for heterogeneous reservoirs.
-
5.
Conducting computational and MD simulations to predict the physical properties and interaction energies through oil/water/rock, then employing these properties in the optimization of polymeric nanocomposites flooding.
7 Conclusion
Chemical enhanced oil recovery through nanocomposite flooding as well as nanofluids, biopolymers, and synthetic polymers acquire incremental imprints on the industrial and environmental scale. Polymeric composite synthesized through grafting of polymeric matrices on the surface of inorganic particles using different polymerization techniques. The polymer matrix may be synthetic polymers, biopolymers, and hydrogels. The main purpose of using these materials is to increase incremental oil recovery after conventional treatment methods through altering wettability, reducing IFT at W/O interface, and improvement of sweeping efficiency. The present article reviews and highlighted each of these materials with their advantage and disadvantages through the flooding process. The author tries to illustrate the popular experimental techniques including flooding and displacement mechanism as well as the simulation techniques used in this field of research. Furthermore, the rheological properties comprising shearing action, salinity and thermal effect, and viscoelastic properties, as well as physical parameters affecting the solution stability including zeta potential, hydrophilicity/hydrophobicity of nanoparticles, chain length, molecular weight, and functional groups, droplet size measurement and DLS studies were reported. Finally, the optimum numerical models and simulators As well as computational and MD simulations used for predicting the physical properties and interaction energies through heterogeneous reservoirs were reported with the emphasis on the future prospects in this field.
Abbreviations
- EOR:
-
Enhanced oil recovery
- MD:
-
Molecular dynamics
- OOIP:
-
Original oil in place
- HPAM:
-
Hydrolyzed polyacrylamides
- IFT:
-
Interfacial tension
- NPs:
-
Nanoparticles
- CIOR:
-
Chemically improved oil recovery
- GO:
-
Graphene oxide
- O/W interface:
-
Oil/water interface
- CD:
-
Cyclodextrin
- IOR:
-
Improved oil recovery
- WLG:
-
Welan gum
- GG:
-
Guar gum
- SPG:
-
Schizophyllan
- CMC:
-
Carboxymethyl cellulose
- HEC:
-
Hydroxyethylcellulose
- RAFT:
-
Reversible addition-fragmentation chain transfer processes
- FRP:
-
Free-radical emulsion polymerization
- ATRP:
-
Atom transfer radical polymerization
- NMP:
-
Nitroxide mediated polymerization
- HRTEM:
-
High-resolution transmission electron microscope
- G′:
-
Elastic modulus
- G″:
-
Viscous modulus
- ω:
-
Angular frequency
- poly (AM-co-AA)/SiO2 :
-
Poly (acrylamide-co-acrylic acid)/silica
- TMS:
-
Tetramethyl silane
- D2O:
-
Deuterium oxide
- DMSO:
-
Dimethyl sulfoxide
- ACTF:
-
Amorphous carbon thin film
- CED:
-
Cohesive energy density
- \(E_{{}}^{mix}\) :
-
Mixing energy
- χbs :
-
Flory–Huggins chi parameter
- MC simulation:
-
Monte Carlo simulation
- PV:
-
Pore volume
- Sor :
-
Residual oil saturation
- DES:
-
Deep eutectic solvent
- CMG:
-
Computer modeling group
- UTCHEM:
-
University of Texas Chemical Compositional Simulator
- \(\Gamma\) :
-
Shear stress, Pa
- γ:
-
Shear rate, s−1
- τ 0 :
-
The yield stress, Pa
- K:
-
The coefficient of flow consistency, Pa. s–n
- n:
-
The flow behavior index, a dimensionless parameter
- HPHT:
-
High-pressure high temperature
- (G′):
-
Elastic modulus
- (G″):
-
Viscous modulus
- (G*):
-
Complex moduli
- δ:
-
The phase angle
- tan δ:
-
Loss factor
- µ:
-
Viscosity, cp
- 3D- reservoir:
-
Three-dimensional reservoir
References
Lashari, N., Ganat, T. Emerging applications of nanomaterials in chemical enhanced oil recovery: Progress and perspective. Chin. J. Chem. Eng. (2020)
Zhou, Y.; Wu, X.; Zhong, X.; Reagen, S.; Zhang, S.; Sun, W., et al.: Polymer nanoparticles based nano-fluid for enhanced oil recovery at harsh formation conditions. Fuel 267, 117251 (2020)
Fan, Y-.W., Liu, K-.X., Zhang, L-.L., Zhao, H., Sun, B-.C., Chu, G-.W., et al. Rapid and continuous polymer dissolution by rotating packed bed for enhanced oil recovery. Chem. Eng. Process. Process Intensif., 107952 (2020)
López, O.V., Castillo, L.A., Ninago, M.D, Ciolino A.E., Villar, M.A. Modified starches used as additives in enhanced oil recovery (EOR). Industrial Applications of Renewable Biomass Products. Springer; (2017), p 227–48
Kögler, F.; Mahler, E.; Dopffel, N.; Schulze-Makuch, D.; Borovina, A.; Visser, F., et al.: The microbial enhanced oil recovery (MEOR) potential of halanaerobiales under dynamic conditions in different porous media. JPet SciEng 196, 107578 (2021)
Dudek, M.; Vik, E.A.; Aanesen, S.V.; Øye, G.: Colloid chemistry and experimental techniques for understanding fundamental behaviour of produced water in oil and gas production. Adv. Colloid Interface Sci. 276, 102105 (2020)
Bera, A.; Belhaj, H.: Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery-a comprehensive review. J. Natl. Gas Sci. Eng. 34, 1284–1309 (2016)
de Lara, L.S.; Voltatoni, T.; Rodrigues, M.C.; Miranda, C.R.; Brochsztain, S.: Potential applications of cyclodextrins in enhanced oil recovery. Colloids Surf. A 469, 42–50 (2015)
Esfe, M.H.; Hosseinizadeh, E.; Mosaferi, M.: Investigation on nanofluid flooding effect on enhancement oil recovery process in a random pore distribution incomplete cone. Int. Commun. Heat Mass Transf. 117, 104629 (2020)
El-hoshoudy, A.N.; Desouky, S.M.: Hydrophobic polymers for enhanced oil recovery: preparation, characterization, rheological properties. Lambert Academic Publishing (2016)
Esfe, M.H.; Esfandeh, S.; Hosseinizadeh, E.: Nanofluid flooding for enhanced oil recovery in a heterogeneous two-dimensional anticline geometry. Int. Commun. Heat Mass Transf. 118, 104810 (2020)
Michael, F.M.; Krishnan, M.R.; Li, W.; Alsharaeh, E.H.: A review on polymer-nanofiller composites in developing coated sand proppants for hydraulic fracturing. J. Natl. Gas Sci. Eng. (2020). https://doi.org/10.1016/j.jngse.2020.103553
Ali, H.; Soleimani, H.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M., et al.: Enhanced oil recovery by using electromagnetic-assisted nanofluids: a review. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.113095
Jafarbeigi, E.; Kamari, E.; Salimi, F.; Mohammadidoust, A.: Experimental study of the effects of a novel nanoparticle on enhanced oil recovery in carbonate porous media. JPet SciEng 195, 107602 (2020)
Shamilov, V.; Babayev, E.; Kalbaliyeva, E.; Shamilov, F.: Polymer nanocomposites for enhanced oil recovery. Mater. Today Proc. 4, S70–S74 (2017)
Sharma, T.; Iglauer, S.; Sangwai, J.S.: Silica nanofluids in an oilfield polymer polyacrylamide: interfacial properties, wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 55(48), 12387–12397 (2016)
Zhang, H.; Nikolov, A.; Wasan, D.: Enhanced oil recovery (EOR) using nanoparticle dispersions: underlying mechanism and imbibition experiments. Energy Fuels 28(5), 3002–3009 (2014)
Agi, A.; Junin, R.; Jaafar, M.Z.; Mohsin, R.; Arsad, A.; Gbadamosi, A., et al.: Synthesis and application of rice husk silica nanoparticles for chemical enhanced oil recovery. J. Market. Res. 9(6), 13054–13066 (2020)
Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Augustine, A.: Hybrid suspension of polymer and nanoparticles for enhanced oil recovery. Polym. Bull. 76(12), 6193–6230 (2019)
Khalilinezhad, S.S.; Mohammadi, A.H.; Hashemi, A.; Ghasemi, M.: Rheological characteristics and flow dynamics of polymer nanohybrids in enhancing oil recovery from low permeable carbonate oil reservoirs. JPet SciEng (2020). https://doi.org/10.1016/j.petrol.2020.107959
Sveistrup, M.; van Mastrigt, F.; Norrman, J.; Picchioni, F.; Paso, K.: Viability of biopolymers for enhanced oil recovery. J. Dispersion Sci. Technol. 37(8), 1160–1169 (2016)
Bera, A., Shah, S., Shah, M., Agarwal, J., Vij, R.K. Mechanistic study on silica nanoparticles-assisted guar gum polymer flooding for enhanced oil recovery in sandstone reservoirs. Colloids Surf. Physicochem. Eng. Asp. 124833 (2020)
Zargartalebi, M.; Kharrat, R.; Barati, N.; Zargartalebi, A.: Slightly hydrophobic silica nanoparticles for enhanced oil recovery: interfacial and rheological behaviour. Int. J. Oil Gas Coal Technol. 6(4), 408–421 (2013)
Mugele, F.; Bera, B.; Cavalli, A.; Siretanu, I.; Maestro, A.; Duits, M., et al.: Ion adsorption-induced wetting transition in oil-water-mineral systems. Sci. Rep. 5, 10519 (2015)
Wu, H.; Gao, K.; Lu, Y.; Meng, Z.; Gou, C.; Li, Z., et al.: Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 586, 124162 (2020)
Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y.: A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 61, 1–11 (2018)
Maurya, N.K.; Kushwaha, P.; Mandal, A.: Studies on interfacial and rheological properties of water soluble polymer grafted nanoparticle for application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 70, 319–330 (2017)
El-Hoshoudy, A.; Desouky, S.; Betiha, M.; Alsabagh, A.: Use of 1-vinyl imidazole based surfmers for preparation of polyacrylamide–SiO2 nanocomposite through aza-Michael addition copolymerization reaction for rock wettability alteration. Fuel 170, 161–175 (2016)
Aliabadian, E.; Sadeghi, S.; Moghaddam, A.R.; Maini, B.; Chen, Z.; Sundararaj, U.: Application of graphene oxide nanosheets and HPAM aqueous dispersion for improving heavy oil recovery: effect of localized functionalization. Fuel 265, 116918 (2020)
Kaya, G.G., Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: a review. J. Ind. Eng. Chem. (2020)
Corredor, L.M.; Husein, M.M.; Maini, B.B.: A review of polymer nanohybrids for oil recovery. Adv. Colloid Interface Sci. 272, 102018 (2019)
Ayre, D.: Technology advancing polymers and polymer composites towards sustainability: a review. Curr. Opin. Green Sustain. Chem. 13, 108–112 (2018)
Karami, P.; Khorshidi, B.; McGregor, M.; Peichel, J.T.; Soares, J.B.; Sadrzadeh, M.: Thermally stable thin film composite polymeric membranes for water treatment: a review. J. Clean. Prod. 250, 119447 (2020)
Chi, H.; Wang, S.; Li, T.; Li, Z.: Recent progress in using hybrid silicon polymer composites for wastewater treatment. Chemosphere (2020). https://doi.org/10.1016/j.chemosphere.2020.128380
Zhu, D.; Wei, L.; Wang, B.; Feng, Y.: Aqueous hybrids of silica nanoparticles and hydrophobically associating hydrolyzed polyacrylamide used for EOR in high-temperature and high-salinity reservoirs. Energies 7(6), 3858–3871 (2014)
Ji, J.; Zeng, C.; Ke, Y.; Pei, Y.: Preparation of poly (acrylamide-co-acrylic acid)/silica nanocomposite microspheres and their performance as a plugging material for deep profile control. J. Appl. Polym. Sci. 134(46), 45502 (2017)
Xin, H.; Ao, D.; Wang, X.; Zhu, Y.; Zhang, J.; Tan, Y.: Synthesis, characterization, and properties of copolymers of acrylamide with sodium 2-acrylamido-2-methylpropane sulfonate with nano silica structure. Colloid Polym. Sci. 293(5), 1307–1316 (2015)
Kumar, N.; Gaur, T.; Mandal, A.: Characterization of SPN Pickering emulsions for application in enhanced oil recovery. J. Ind. Eng. Chem. 54, 304–315 (2017)
Tian, M.; Xu, Y.J.: The preparation and characterization of AM/2-EHA/VTEOS copolymer as profile control agent. Adv. Mater. Res. 602, 732–738 (2013)
Firozjaii, A.M.; Saghafi, H.R.: Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 6, 115–122 (2019)
Druetta, P.; Raffa, P.; Picchioni, F.: Chemical enhanced oil recovery and the role of chemical product design. Appl. Energy 252, 113480 (2019)
Friedrich, K.: Polymer composites for tribological applications. Adv. Ind. Eng. Polym. Res. 1(1), 3–39 (2018)
Shu, B.; Wu, S.; Dong, L.; Norambuena-Contreras, J.; Li, Y.; Li, C., et al.: Self-healing capability of asphalt mixture containing polymeric composite fibers under acid and saline-alkali water solutions. J. Clean. Prod. 268, 122387 (2020)
Maghzi, A.; Kharrat, R.; Mohebbi, A.; Ghazanfari, M.H.: The impact of silica nanoparticles on the performance of polymer solution in presence of salts in polymer flooding for heavy oil recovery. Fuel 123, 123–132 (2014)
Yousefvand, H.A.; Jafari, A.: Stability and flooding analysis of nanosilica/NaCl/HPAM/SDS solution for enhanced heavy oil recovery. JPet SciEng 162, 283–291 (2018)
Sharma, S.; Sudhakara, P.; Misra, S.; Singh, J.: A comprehensive review of current developments on the waste-reinforced polymer-matrix composites for automotive, sports goods and construction applications: materials, processes and properties. Mater. Today Proc. (2020). https://doi.org/10.1016/j.matpr.2020.06.523
Raj, I.; Liang, T.; Qu, M.; Xiao, L.; Hou, J.; Xian, C.: An experimental investigation of MoS2 nanosheets stabilized foams for enhanced oil recovery application. Colloids Surf. A Physicochem. Eng. Asp. 606, 125420 (2020)
Zhang, H.; Yang, H.; Sarsenbekuly, B.; Zhang, M.; Jiang, H.; Kang, W., et al.: The advances of organic chromium based polymer gels and their application in improved oil recovery. Adv. Colloid Interface Sci. (2020). https://doi.org/10.1016/j.cis.2020.102214
Rezaeian, M.S.; Mousavi, S.M.; Saljoughi, E.; Amiri, H.A.A.: Evaluation of thin film composite membrane in production of ionically modified water applied for enhanced oil recovery. Desalination 474, 114194 (2020)
Gandomkar, A.; Sharif, M.: Nano composites performance as direct thickeners for gas based enhanced oil recovery, a new approach. JPet SciEng (2020). https://doi.org/10.1016/j.petrol.2020.107491
Haruna, M.A.; Gardy, J.; Yao, G.; Hu, Z.; Hondow, N.; Wen, D.: Nanoparticle modified polyacrylamide for enhanced oil recovery at harsh conditions. Fuel 268, 117186 (2020)
Aurand, K. Enhanced oil recovery using silica nanoparticles: an experimental evaluation of oil production, recovery mechanisms and nanofluid stability (2017)
Foroozesh, J., Kumar, S. Nanoparticles behaviors in porous media: application to enhanced oil recovery. J. Mol. Liq. 113876 (2020)
Chaturvedi, K.R., Sharma, T. Single-step silica nanofluid for improved carbon dioxide flow and reduced formation damage in porous media for carbon utilization. Energy 117276 (2020)
Evdokimov, I.N., Eliseev, N.Y., Losev, A.P., Novikov, M.A. Emerging Petroleum-Oriented Nanotechnologies for Reservoir Engineering (Russian). SPE Russian oil and gas technical conference and exhibition. Society of Petroleum Engineers (2006)
Son, H.; Kim, H.; Lee, G.; Kim, J.; Sung, W.: Enhanced oil recovery using nanoparticle-stabilized oil/water emulsions. Korean J. Chem. Eng. 31(2), 338–342 (2014)
AfzaliTabar, M.; Rashidi, A.; Alaei, M.; Koolivand, H.; Pourhashem, S.; Askari, S.: Hybrid of quantum dots for interfacial tension reduction and reservoir alteration wettability for enhanced oil recovery (EOR). J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.112984
El-hoshoudy, A.N.; Gomaa, S.; Taha, M.: Investigating the effect of different nanoparticles on the interfacial tension reduction. Pet. Petrochem. Eng. J. 2(7), 175 (2018)
El-hoshoudy, A.N.; Gomaa, S.; Taha, M.: Improving oil recovery using zeolite nanoparticles flooding. Pet. Petrochem. Eng. J. 3(1), 1–14 (2019)
El-hoshoudy, A.; Gomaa, S.; Taha, M.: Improving oil recovery using fe2o3 nanoparticles flooding. Pet. Coal (2019). https://doi.org/10.23880/PPEJ-16000186
El-hoshoudy, A.N.; Gomaa, S.; Taha, M.: Investigating the effect of different nanofluids types on crude oil viscosity. Pet. Petrochem. Eng. J. 2(7), 177 (2018)
El-hoshoudy, A.N.; Gomaa, S.; Hassan, A.; Attia, A.M.: Effects of alkaline/polymer/nanofluids on oil recovery at harsh reservoir conditions. Pet. Coal 61(6), 1556–1567 (2019)
Sun, L.; Guan, J.; Xu, Q.; Yang, X.; Wang, J.; Hu, X.: Synthesis and applications of molecularly imprinted polymers modified TiO2 nanomaterials: a review. Polymers 10(11), 1248 (2018)
Tran, T.H.; Nguyen, V.T.: Copper oxide nanomaterials prepared by solution methods, some properties, and potential applications: a brief review. Int. Sch. Res. Not. (2014). https://doi.org/10.1155/2014/856592
Ye, Z.; Qin, X.; Lai, N.; Peng, Q.; Li, X.; Li, C.: Synthesis and performance of an acrylamide copolymer containing nano-SiO2 as enhanced oil recovery chemical. J. Chem. (2013). https://doi.org/10.1155/2013/437309
Hu, Z.; Haruna, M.; Gao, H.; Nourafkan, E.; Wen, D.: Rheological properties of partially hydrolyzed polyacrylamide seeded by nanoparticles. Ind. Eng. Chem. Res. 56(12), 3456–3463 (2017)
Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J.: Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 22(16), 1805–1825 (2010)
Agista, M.N.; Guo, K.; Yu, Z.: A state-of-the-art review of nanoparticles application in petroleum with a focus on enhanced oil recovery. Appl. Sci. 8(6), 871 (2018)
Qu, Y.; Tian, Y.; Zou, B.; Zhang, J.; Zheng, Y.; Wang, L., et al.: A novel mesoporous lignin/silica hybrid from rice husk produced by a sol–gel method. Bioresour. Technol. 101(21), 8402–8405 (2010)
Onyekonwu, M.O., Ogolo, N.A. Investigating the use of nanoparticles in enhancing oil recovery. Nigeria Annual international conference and exhibition. Society of Petroleum Engineers (2010)
Yousefvand, H.; Jafari, A.: Enhanced oil recovery using polymer/nanosilica. Proc. Mater. Sci. 11, 565–570 (2015)
Wu, Y.; Chen, W.; Dai, C.; Huang, Y.; Li, H.; Zhao, M., et al.: Reducing surfactant adsorption on rock by silica nanoparticles for enhanced oil recovery. JPet SciEng 153, 283–287 (2017)
El-hoshoudy, A.N.: Synthesis and fabrication of silica based superhydrophobic surfaces. Pet. Petrochem. Eng. J. (2019). https://doi.org/10.23880/PPEJ-16000181
Alazemi, M.; Dutta, I.; Wang, F.; Blunk, R.; Angelopoulos, A.: Adsorption kinetics and nanostructure development during layer by layer assembly of graphene nanoplatelets and amorphous carbon nanospheres. Carbon 48(14), 4063–4073 (2010)
Wang, K.; Pang, J.; Li, L.; Zhou, S.; Li, Y.; Zhang, T.: Synthesis of hydrophobic carbon nanotubes/reduced graphene oxide composite films by flash light irradiation. Front. Chem. Sci. Eng. 12(3), 376–382 (2018)
Tang, M.; Xu, X.; Wu, T.; Zhang, S.; Li, X.; Li, Y.: Polyacrylamide grafting of modified graphene oxides by in situ free radical polymerization. Mater. Res. Bull. 60, 576–583 (2014)
Evingür, G.A.; Pekcan, Ö.: Mechanical properties of graphene oxide–polyacrylamide composites before and after swelling in water. Polym. Bull. 75(4), 1431–1439 (2018)
El-hoshoudy, A.; Hosny, R.; Fathy, M.; Abdelraheem, O.; Gomaa, S.; Desouky, S.: Enhanced oil recovery using polyacrylates/ACTF crosslinked composite: preparation, characterization and coreflood investigation. JPet SciEng 181, 106236 (2019)
Zhang, H.; Zhai, D.; He, Y.: Graphene oxide/polyacrylamide/carboxymethyl cellulose sodium nanocomposite hydrogel with enhanced mechanical strength: preparation, characterization and the swelling behavior. RSC Adv. 4(84), 44600–44609 (2014)
Alam, A.; Kuan, H.-C.; Zhao, Z.; Xu, J.; Ma, J.: Novel polyacrylamide hydrogels by highly conductive, water-processable graphene. Compos. A Appl. Sci. Manuf. 93, 1–9 (2017)
Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M.: Mechanical, thermal and swelling properties of poly (acrylic acid)–graphene oxide composite hydrogels. Soft Matter 8(6), 1831–1836 (2012)
Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M.: Classification, processing and application of hydrogels: a review. Mater. Sci. Eng., C 57, 414–433 (2015)
Wang, H.; Yuan, X.; Zeng, G.; Wu, Y.; Liu, Y.; Jiang, Q., et al.: Three dimensional graphene based materials: synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv. Colloid Interface Sci. 221, 41–59 (2015)
Liu, R.; Liang, S.; Tang, X.-Z.; Yan, D.; Li, X.; Yu, Z.-Z.: Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 22(28), 14160–14167 (2012)
Alam, A.; Zhang, Y.; Kuan, H.-C.; Lee, S.-H.; Ma, J.: Polymer composite hydrogels containing carbon nanomaterials—morphology and mechanical and functional performance. Prog. Polym. Sci. 77, 1–18 (2018)
El-hoshoudy, A.N.; Hosny, R.; Fathy, M.; Abdelraheem, O.H.; Gomaa, S.; Desouky, S.M.: Enhanced oil recovery using polyacrylates/ACTF crosslinked composite: preparation, characterization and coreflood investigation. J. Pet. Sci. Eng. 181, 106236 (2019)
Chen, Q.; Ye, Z.; Tang, L.; Wu, T.; Jiang, Q.; Lai, N.: Synthesis and solution properties of a novel hyperbranched polymer based on chitosan for enhanced oil recovery. Polymers 12(9), 2130 (2020)
Wever, D.; Picchioni, F.; Broekhuis, A.: Polymers for enhanced oil recovery: a paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 36(11), 1558–1628 (2011)
Zuber, M., Zia, K.M., Barikani, M. Chitin and chitosan based blends, composites and nanocomposites. Advances in natural polymers. Springer; (2013), p. 55–119.
Thomas, M.S.; Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A.: Starch, chitin and chitosan based composites and nanocomposites. Springer (2019)
Visakh, P.; Mathew, A.P.; Thomas, S.: Natural polymers: their blends, composites and nanocomposites: state of art, new challenges and opportunities. In: Thomas, S.; Visakh, P.M.; Mathew, A.P. (Eds.) Advances in natural polymers, pp. 1–20. Springer (2013)
Anitha, A.; Sowmya, S.; Kumar, P.S.; Deepthi, S.; Chennazhi, K.; Ehrlich, H., et al.: Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 39(9), 1644–1667 (2014)
Sharma, G.; Sharma, S.; Kumar, A.; Ala’a, H.; Naushad, M.; Ghfar, A.A., et al.: Guar gum and its composites as potential materials for diverse applications: a review. Carbohydr. Polym. 199, 534–545 (2018)
Elkholy, A.E.; Heakal, F.E.-T.; Rashad, A.M.; Zakaria, K.: Monte carlo simulation for guar and xanthan gums as green scale inhibitors. J. Pet. Sci. Eng. 166, 263–273 (2018)
El-Hoshoudy, A.: Synthesis of acryloylated starch-g-poly acrylates crosslinked polymer functionalized by emulsified vinyltrimethylsilane derivative as a novel EOR agent for severe polymer flooding strategy. Int. J. Biol. Macromol. 123, 124–132 (2019)
El-hoshoudy, A., Zaki, E., Elsaeed, S. Biopolymers composites as oil improving candidates-article review. Pet. Coal, 61(6) (2019)
Noha Khedr, O.A.; Badr, M.; Hamza, I.; Awad, M.; Ibrahim, N.E.; El-hoshoudy, A.N.: Design and economical evaluation of biopolymer injection-design project. Pet. coal 61(6), 1528–1545 (2019)
Gao, C.: Application of a novel biopolymer to enhance oil recovery. J. Pet. Explor. Prod. Technol. 6(4), 749–753 (2016)
Ching, Y.C.; Ali, M.E.; Abdullah, L.C.; Choo, K.W.; Kuan, Y.C.; Julaihi, S.J., et al.: Rheological properties of cellulose nanocrystal-embedded polymer composites: a review. Cellulose 23(2), 1011–1030 (2016)
Molnes, S.N.; Torrijos, I.P.; Strand, S.; Paso, K.G.; Syverud, K.: Sandstone injectivity and salt stability of cellulose nanocrystals (CNC) dispersions—Premises for use of CNC in enhanced oil recovery. Ind. Crops Prod. 93, 152–160 (2016)
Castillo, N.A.; Valdez, A.L.; Fariña, J.I.: Microbial production of scleroglucan and downstream processing. Front. Microbiol. 6, 1106 (2015)
Wei, B.; Romero-Zerón, L.; Rodrigue, D.: Improved viscoelasticity of xanthan gum through self-association with surfactant: β-cyclodextrin inclusion complexes for applications in enhanced oil recovery. Polym. Eng. Sci. 55(3), 523–532 (2015)
Yu, W.; Liu, L.; Li, F.; Tan, Z.: β-Cyclodextrin-based poly (ionic liquids) membranes enable the efficient separation of the amino acids mixture. J. Ind. Eng. Chem. 103, 322–328 (2021)
Da Cruz, A.F.T.; Sanches, R.D.; Miranda, C.R.; Brochsztain, S.: Evaluation of cyclodextrins as environmentally friendly wettability modifiers for enhanced oil recovery. Colloids Interfaces 2(1), 10 (2018)
Zou, C.; Zhao, P.; Ge, J.; Lei, Y.; Luo, P.: β-Cyclodextrin modified anionic and cationic acrylamide polymers for enhancing oil recovery. Carbohydr. Polym. 87(1), 607–613 (2012)
Qin, Y.; Zou, C.; Yan, X.; Zhou, L.; Luo, P.: High performance acid composition based on cationic β-cyclodextrin inclusion complexes for enhancing oil recovery. Chem. Eng. Res. Des. 94, 301–306 (2015)
Wei, B.: β-Cyclodextrin associated polymeric systems: rheology, flow behavior in porous media and enhanced heavy oil recovery performance. Carbohydr. Polym. 134, 398–405 (2015)
Romero-Zerón, L.; Espinosa, C.: Advantageous supramolecular system through self-association of xanthan gum/cationic surfactant via β-cyclodextrin host-guest complexations for enhanced oil recovery applications. JPet SciEng 185, 106644 (2020)
Ghoumrassi-Barr, S.; Aliouche, D.: A rheological study of xanthan polymer for enhanced oil recovery. J. Macromol. Sci. Part B 55(8), 793–809 (2016)
Soliman, A.A.; El-Hoshoudy, A.N.; Attia, A.M.: Assessment of xanthan gum and xanthan-g-silica derivatives as chemical flooding agents and rock wettability modifiers. Oil Gas Sci. Technol. Revue d’IFP Energies nouvelles 75, 12 (2020)
El-Hoshoudy, A.N.; Desouky, S.M.; Attia, A.M.; Gomaa, S.: Synthesis and evaluation of xanthan-G-Poly (Acrylamide) CoPolymer for enhanced oil recovery applications. Pet. Petrochem. Eng. J. (2018). https://doi.org/10.23880/PPEJ-16000154
El-Hamshary, H.; Fouda, M.M.; Moydeen, M.; Al-Deyab, S.S.: Removal of heavy metal using poly (N-vinylimidazole)-grafted-carboxymethylated starch. Int. J. Biol. Macromol. 66, 289–294 (2014)
El-Hoshoudy, A.N.; Desouky, S.M.: Synthesis and evaluation of acryloylated starch-g-poly (Acrylamide/ Vinylmethacrylate/1-Vinyl-2-pyrrolidone) crosslinked terpolymer functionalized by dimethylphenylvinylsilane derivative as a novel polymer-flooding agent. Int. J. Biol. Macromol. 116, 434–442 (2018)
El-Hoshoudy, A.N.; Desouky, S.M.; Attia, A.M.: Synthesis of starch functionalized sulfonic acid co-imidazolium/silica composite for improving oil recovery through chemical flooding technologies. Int. J. Biol. Macromol. (2018). https://doi.org/10.1016/j.ijbiomac.2018.07.009
Gao, F.; Xue, Y.; Gao, Y.; Zhang, Z.; Teng, T.; Liang, X.: Fully coupled thermo-hydro-mechanical model for extraction of coal seam gas with slotted boreholes. J. Natl Gas Sci. Eng. 31, 226–235 (2016)
Abou-alfitooh, S.A.; El-Hosiny, F.; Ramzi, M.; Mansour, E.; Elnaggar, O.M.; El-hoshoudy, A.: Chemical modification of guar by different synthetic vinyl monomers for enhancing oil recovery under severe sandstone reservoir conditions. Egypt. J. Pet. (2021). https://doi.org/10.1016/j.ejpe.2021.07.001
El-hoshoudy, A.N.; Zaki, E.G.; Elsaeed, S.M.: Experimental and Monte Carlo simulation of palmitate-guar gum derivative as a novel flooding agent in the underground reservoir. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.112502
Leonhardt, B., Ernst, B., Reimann, S., Steigerwald, A., Lehr, F. Field testing the polysaccharide schizophyllan: results of the first year. SPE Improved Oil Recovery Symposium. Society of Petroleum Engineers (2014)
Douglass, E.F.; Avci, H.; Boy, R.; Rojas, O.J.; Kotek, R.: A review of cellulose and cellulose blends for preparation of bio-derived and conventional membranes, nanostructured thin films, and composites. Polym. Rev. 58(1), 102–163 (2018)
Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J.: Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 40(7), 3941–3994 (2011)
Gupta, V.; Carrott, P.; Singh, R.; Chaudhary, M.; Kushwaha, S.: Cellulose: a review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 216, 1066–1076 (2016)
Asl, S.A.; Mousavi, M.; Labbafi, M.: Synthesis and characterization of carboxymethyl cellulose from sugarcane bagasse. J. Food Process. Technol. 8(8), 687 (2017)
Sunardi, S. Preparation of carboxymethyl cellulose produced from purun tikus (Eleocharis dulcis). AIP Conference Proceedings 1868, 020008 (2017)
Bishop, M.D., Parlman, R.M. Stable suspensions of carboxymethyl cellulose and their preparation. Google Patents (1988)
Yadav, M.; Rhee, K.Y.; Park, S.: Synthesis and characterization of graphene oxide/carboxymethylcellulose/alginate composite blend films. Carbohyd. Polym. 110, 18–25 (2014)
Abbas, S., Sanders. A.W., Donovan, J.C. Applicability of hydroxyethylcellulose polymers for chemical EOR. SPE Enhanced Oil Recovery Conference. Society of Petroleum Engineers (2013)
Bai, Y.; Shang, X.; Wang, Z.; Zhao, X.: Experimental study on hydrophobically associating hydroxyethyl cellulose flooding system for enhanced oil recovery. Energy Fuels 32(6), 6713–6725 (2018)
Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A., et al.: Valorization of lignin in polymer and composite systems for advanced engineering applications–a review. Int. J. Biol. Macromol. 131, 828–849 (2019)
Ganie, K.; Manan, M.A.; Ibrahim, A.; Idris, A.K.: An experimental approach to formulate lignin-based surfactant for enhanced oil recovery. Int. J. Chem. Eng. (2019). https://doi.org/10.1155/2019/4120859
Sulaiman, W.; Abbas, A.; Jaafar, M.; Idris, A.: Lignin black liquor in chemical enhanced oil recovery: a review for sacrificial agent. J. Pet. Environ. Biotechnol. 7(5), 5–8 (2016)
Rock, A.; Hincapie, R.E.; Tahir, M.; Langanke, N.; Ganzer, L.: On the role of polymer viscoelasticity in enhanced oil recovery: extensive laboratory data and review. Polymers 12(10), 2276 (2020)
Sharma, T.; Velmurugan, N.; Patel, P.; Chon, B.; Sangwai, J.: Use of oil-in-water pickering emulsion stabilized by nanoparticles in combination with polymer flood for enhanced oil recovery. Pet. Sci. Technol. 33(17–18), 1595–1604 (2015)
Pereira, K.A.; Aguiar, K.L.; Oliveira, P.F.; Vicente, B.M.; Pedroni, L.G.; Mansur, C.R.: Synthesis of hydrogel nanocomposites based on partially hydrolyzed polyacrylamide, polyethyleneimine, and modified clay. ACS Omega 5(10), 4759–4769 (2020)
Ghriga, M.A.; Gareche, M.; Khodja, M.; Andreu, N.; Lebouachera, S.E.I.; Khoukh, A., et al.: Structure–property relationships of the thermal gelation of partially hydrolyzed polyacrylamide/polyethylenimine mixtures in a semidilute regime. Polym. Bull. 77(3), 1465–1488 (2020)
Silva, I.P.; Aguiar, A.A.; Rezende, V.P.; Monsores, A.L.; Lucas, E.F.: A polymer flooding mechanism for mature oil fields: laboratory measurements and field results interpretation. JPet SciEng 161, 468–475 (2018)
Jouenne, S.: Polymer flooding in high temperature, high salinity conditions: selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 195, 107545 (2020)
Wang, F.; Yang, H.; Jiang, H.; Kang, X.; Hou, X.; Wang, T., et al.: Formation mechanism and location distribution of blockage during polymer flooding. J. Pet. Sci. Eng. (2020). https://doi.org/10.1016/j.petrol.2020.107503
Bhardwaj, P.; Singh, S.; Singh, V.; Aggarwal, S.; Mandal, U.: Nanosize polyacrylamide/SiO2 composites by inverse microemulsion polymerization. Int. J. Polymer. Mater. 57(4), 404–416 (2008)
Ponnapati, R.; Karazincir, O.; Dao, E.; Ng, R.; Mohanty, K.; Krishnamoorti, R.: Polymer-functionalized nanoparticles for improving waterflood sweep efficiency: characterization and transport properties. Ind. Eng. Chem. Res. 50(23), 13030–13036 (2011)
Maurya, N.K.; Mandal, A.: Studies on behavior of suspension of silica nanoparticle in aqueous polyacrylamide solution for application in enhanced oil recovery. Pet. Sci. Technol. 34(5), 429–436 (2016)
El-hoshoudy, A.N.; Gomaa, S.; Selim, O.A.: Application of acrylamide polymer grafted with sio2 nanoparticles in enhanced oil recovery- design project. Pet. Coal 61(6), 1505–1520 (2019)
El Hoshoudy, A.; Desouky, S.; Al-sabagh, A.; El-kady, M.; Betiha, M.; Mahmoud, S.: Synthesis and characterization of polyacrylamide crosslinked copolymer for enhanced oil recovery and rock wettability alteration. Int. J. Oil Gas Coal Eng. 3(4), 47–59 (2015)
El-hoshoudy, A.; Mansour, E.; Desouky, S.: Experimental, computational and simulation oversight of silica-co-poly acrylates composite prepared by surfactant-stabilized emulsion for polymer flooding in unconsolidated sandstone reservoirs. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.113082
El-hoshoudy, A.N.: Quaternary ammonium based surfmer-co-acrylamide polymers for altering carbonate rock wettability during water flooding. J. Mol. Liq. (2018). https://doi.org/10.1016/j.molliq.2017.11.119
El-Hoshoudy, A.N.; Desouky, S.E.; Al-Sabagh, A.M.; Betiha, M.A.; My, E.K.; Mahmoud, S.: Evaluation of solution and rheological properties for hydrophobically associated polyacrylamide copolymer as a promised enhanced oil recovery candidate. Egypt. J. Pet. (2017). https://doi.org/10.1016/j.ejpe.2016.10.012
El-Hoshoudy, A.; Desouky, S.; Elkady, M.; Alsabagh, A.; Betiha, M.; Mahmoud, S.: Investigation of optimum polymerization conditions for synthesis of cross-linked polyacrylamide-amphoteric surfmer nanocomposites for polymer flooding in sandstone reservoirs. Int. J. Polym. Sci. (2015). https://doi.org/10.1155/2015/318708
El-hoshoudy, A.; Desouky, S.; Elkady, M.; Alsabagh, A.; Betiha, M.; Mahmoud, S.: Hydrophobically associated polymers for wettability alteration and enhanced oil recovery–article review. Egypt. J. Pet. (2017). https://doi.org/10.1016/j.ejpe.2016.10.008
Hasankhani, G.M.; Madani, M.; Esmaeilzadeh, F.; Mowla, D.: Experimental investigation of asphaltene-augmented gel polymer performance for water shut-off and enhancing oil recovery in fractured oil reservoirs. J. Mol. Liq. 275, 654–666 (2019)
Hatzignatiou, D.G.; Giske, N.H.; Stavland, A.: Polymers and polymer-based gelants for improved oil recovery and water control in naturally fractured chalk formations. Chem. Eng. Sci. 187, 302–317 (2018)
El-Hoshoudy, A.N.; Mohammedy, M.M.; Ramzi, M.; Desouky, S.M.; Attia, A.M.: Experimental, modeling and simulation investigations of a novel surfmer-co-poly acrylates crosslinked hydrogels for water shut-off and improved oil recovery. J. Mol. Liq. 277, 142–156 (2019)
El-hoshoudy, A.; Mansour, E.; Desouky, S.: Experimental, computational and simulation oversight of silica-co-poly acrylates composite prepared by surfactant-stabilized emulsion for polymer flooding in unconsolidated sandstone reservoirs. J. Mol. Liq. 308, 113082 (2020)
El-hoshoudy, A.; Zaki, E.; Elsaeed, S.: Experimental and Monte Carlo simulation of palmitate-guar gum derivative as a novel flooding agent in the underground reservoir. J. Mol. Liq. 302, 112502 (2020)
Khedr, N., Abdelaal, O., Badr, M., Hamza, I., Awad, M., Ibrahim, N.E., et al. Design and economical evaluation of biopolymer injection-design project. Pet. Coal, 61(6) (2019)
El-hoshoudy A, Gomaa S, Selim OA. Application of acrylamide polymer grafted with sio 2 nanoparticles in enhanced oil recovery-design project. Pet. Coal, 61(6) (2019)
El-hoshoudy, A., Gomaa, S., Hassan, A., Attia, A. Effects of alkaline/polymer/nanofluids on oil recovery at harsh reservoir conditions. Pet. Coal, 61(6) (2019)
El-hoshoudy, A. Application of proteins in enhanced oil recovery-a review. Pet. Coal, 61(6) (2019)
El-hoshoudy, A.; Soliman, F.; Mansour, E.; Zaki, T.; Desouky, S.: Experimental and theoretical investigation of quaternary ammonium-based deep eutectic solvent for secondary water flooding. J Mol Liq 294, 111621 (2019)
El-Hoshoudy, A.; Desouky, S.; Gomaa, S.: Application of acrylates in enhanced oil recovery. J. New Dev. Chem. 2(3), 1 (2019)
El-Hoshoudy, A.N.; Desouky, S.: CO2 miscible flooding for enhanced oil recovery. In: Agarwal, R.K. (Ed.) Carbon capture utilization and sequestration. InTech (2018)
El-Hoshoudy, A.; Desouky, S.; Attia, A.: Synthesis of starch functionalized sulfonic acid co-imidazolium/silica composite for improving oil recovery through chemical flooding technologies. Int. J. Biol. Macromol. 118, 1614–1626 (2018)
El-Hoshoudy, A.; Desouky, S.: Synthesis and evaluation of acryloylated starch-g-poly (Acrylamide/Vinylmethacrylate/1-Vinyl-2-pyrrolidone) crosslinked terpolymer functionalized by dimethylphenylvinylsilane derivative as a novel polymer-flooding agent. Int J Biol. Macromol. 116, 434–442 (2018)
El-hoshoudy, A.N.M.B.: Emulsion polymerization mechanism. In: Cankaya, N. (Ed.) Recent Research in Polymerization. InTech (2018)
El-Hoshoudy, A.: Quaternary ammonium based surfmer-co-acrylamide polymers for altering carbonate rock wettability during water flooding. J. Mol. Liq. 250, 35–43 (2018)
El-Hoshoudy, A.N.; Desouky, S.E.; Al-Sabagh, A.M.; Betiha, M.A.; My, E.K.; Mahmoud, S.: Evaluation of solution and rheological properties for hydrophobically associated polyacrylamide copolymer as a promised enhanced oil recovery candidate. Egypt. J. Pet. 26(3), 779–785 (2017)
El-Hoshoudy, A.; Desouky, S.; Elkady, M.; Al-Sabagh, A.; Betiha, M.; Mahmoud, S.: Hydrophobically associated polymers for wettability alteration and enhanced oil recovery–article review. Egypt. J. Pet. 26(3), 757–762 (2017)
El-hoshoudy, A.N.; Desouky, S.M.; Betiha, M.H.; Alsabagh, A.M.: Hydrophobic Polymers Flooding. In: Najjar, R. (Ed.) Application and Characterization of Surfactants, pp. 75–95. InTechopen (2017)
El Hoshoudy, A.; Desouky, S.; Al-sabagh, A.; El-kady, M.; Betiha, M.; Mahmoud, S.: Synthesis and characterization of polyacrylamide crosslinked copolymer for enhanced oil recovery and rock wettability alteration. Int. J. Oil Gas Coal Eng. 3(4), 43–55 (2015)
Salehi, M.B.; Vasheghani-Farahani, E.; Sefti, M.V.; Moghadam, A.M.; Naderi, H.: Rheological and transport properties of sulfonated polyacrylamide hydrogels for water shutoff in porous media. Polym. Adv. Technol. 25(4), 396–405 (2014)
Stober, W.; Fink, A.; Bohn, E.: Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26(1), 62–69 (1968)
Aho, J. Rheological characterization of polymer melts in shear and extension: measurement reliability and data for practical processing, (2011)
Vraneš, M.; Panić, J.; Tot, A.; Popsavin, M.; Jocić, A.; Gadžurić, S.: Physicochemical characterization of choline based ionic liquids with chelating anions. J. Chem. Thermodyn. 131, 80–87 (2019)
El-hoshoudy, A.; Desouky, S.; Betiha, M.; Alsabagh, A.: Use of 1-vinyl imidazole based surfmers for preparation of polyacrylamide–SiO 2 nanocomposite through aza-Michael addition copolymerization reaction for rock wettability alteration. Fuel 170, 161–175 (2016)
Pal, N.; Mandal, A.: Enhanced oil recovery performance of gemini surfactant-stabilized nanoemulsions functionalized with partially hydrolyzed polymer/silica nanoparticles. Chem. Eng. Sci. 226, 115887 (2020)
Zhong, X.; Chen, J.; An, R.; Li, K.; Chen, M.: A state-of-the-art review of nanoparticle applications with a focus on heavy oil viscosity reduction. J. Mol. Liq. (2021). https://doi.org/10.1016/j.molliq.2021.117845
Pal, N.; Mandal, A.: Oil recovery mechanisms of Pickering nanoemulsions stabilized by surfactant-polymer-nanoparticle assemblies: a versatile surface energies’ approach. Fuel 276, 118138 (2020)
Pillai, P.; Saw, R.K.; Singh, R.; Padmanabhan, E.; Mandal, A.: Effect of synthesized lysine-grafted silica nanoparticle on surfactant stabilized O/W emulsion stability: application in enhanced oil recovery. JPet SciEng 177, 861–871 (2019)
Kamphan, A.; Khanantong, C.; Traiphol, N.; Traiphol, R.: Structural-thermochromic relationship of polydiacetylene (PDA)/polyvinylpyrrolidone (PVP) nanocomposites: effects of PDA side chain length and PVP molecular weight. J. Ind. Eng. Chem. 46, 130–138 (2017)
Song, N.; Yang, J.; Ding, P.; Tang, S.; Shi, L.: Effect of polymer modifier chain length on thermal conductive property of polyamide 6/graphene nanocomposites. Compos. A Appl. Sci. Manuf. 73, 232–241 (2015)
Sikdar, D.; Katti, D.R.; Katti, K.S.; Mohanty, B.: Influence of backbone chain length and functional groups of organic modifiers on crystallinity and nanomechanical properties of intercalated clay-polycaprolactam nanocomposites. Int. J. Nanotechnol. 6(5–6), 468–492 (2009)
Zabihi, Z.; Araghi, H.: Effect of functional groups on thermal conductivity of graphene/paraffin nanocomposite. Phys. Lett. A 380(45), 3828–3831 (2016)
El-hoshoudy, A.N.; Soliman, F.S.; Mansour, E.M.; Zaki, T.; Desouky, S.M.: Experimental and theoretical investigation of quaternary ammonium-based deep eutectic solvent for secondary water flooding. J. Mol. Liq. 294, 111621 (2019)
Lai, N.; Wu, T.; Ye, Z.; Zhou, N.; Xu, Q.; Zeng, F.: Preparation and properties of hyperbranched polymer containing functionalized Nano-SiO 2 for low-moderate permeability reservoirs. Russ. J. Appl. Chem. 89(10), 1681–1693 (2016)
Wei, Q.; Wang, Y.; Zhang, Y.; Chen, X.: Aggregation behavior of nano-silica in polyvinyl alcohol/polyacrylamide hydrogels based on dissipative particle dynamics. Polymers 9(11), 611 (2017)
Maia, A.M.; Borsali, R.; Balaban, R.C.: Comparison between a polyacrylamide and a hydrophobically modified polyacrylamide flood in a sandstone core. Mater. Sci. Eng. C 29(2), 505–509 (2009)
Zhang, D.L.; Liu, S.; Puerto, M.; Miller, C.A.; Hirasaki, G.J.: Wettability alteration and spontaneous imbibition in oil-wet carbonate formations. J. Petrol. Sci. Eng. 52(1), 213–226 (2006)
Zhang, D.L.; Liu, S.; Puerto, M.; Miller, C.A.; Hirasaki, G.J.: Wettability alteration and spontaneous imbibition in oil-wet carbonate formations. J. Petrol. Sci. Eng. 52(1–4), 213–226 (2006)
Xie, F.; Halley, P.J.; Avérous, L.: Rheology to understand and optimize processibility, structures and properties of starch polymeric materials. Prog. Polym. Sci. 37(4), 595–623 (2012)
Chen, Z. Polyacrylamide and its derivatives for oil recovery, (2016)
Ye, Z.; Feng, M.; Gou, S.; Liu, M.; Huang, Z.; Liu, T.: Hydrophobically associating acrylamide-based copolymer for chemically enhanced oil recovery. J. Appl. Polym. Sci. 130(4), 2901–2911 (2013)
Chhabra, R.P.; Richardson, J.F.: Non-Newtonian Flow: Fundamentals and Engineering Applications. Elsevier (1999)
Angar, N.-E.; Aliouche, D.: Rheological behavior and reversible swelling of pH sensitive poly (acrylamide-co-itaconic acid) hydrogels. Polym. Sci. Ser. A 58(4), 541–549 (2016)
Barnes, H.A.; Hutton, J.F.; Walters, K.: An Introduction to Rheology. Elsevier (1989)
Singh, R.; Mahto, V.: Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. Pet. Sci. 14(4), 765–779 (2017)
Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Ran, Y., et al.: Ultrastable hydrogel for enhanced oil recovery based on double-groups cross-linking. Energy Fuels 29(11), 7196–7203 (2015)
Yang-Chuan, K.; Guang-Yao, W.; Yi, W.: Preparation, morphology and properties of nanocomposites of polyacrylamide copolymers with monodisperse silica. Eur. Polymer J. 44(8), 2448–2457 (2008)
Fu, J.; Qiao, R.; Zhu, L.; Zhu, W.; Hao, S.: Application of a novel cationic starch in enhanced oil recovery and its adsorption properties. Korean J. Chem. Eng. (2013). https://doi.org/10.1007/s11814-012-0147-4
Tereshchenko, A.G.: Deliquescence: hygroscopicity of water-soluble crystalline solids. J. Pharm. Sci. 104(11), 3639–3652 (2015)
Vicini, S.; Castellano, M.; Faria Soares Lima, M.C.; Licinio, P.; Goulart Silva, G.: Polyacrylamide hydrogels for stone restoration: effect of salt solutions on swelling/deswelling degree and dynamic correlation length. J. Appl. Polym. Sci. (2017). https://doi.org/10.1002/app.44726
Du, Y.; Zhu, Y.; Ji, Y.; Xu, H.; Zhang, H.; Yuan, S.: Effect of salt-resistant monomers on viscosity of modified polymers based on the hydrolyzed poly-acrylamide (HPAM): a molecular dynamics study. J. Mol. Liq. 325, 115161 (2021)
Wang, L.; Long, Y.; Ding, H.; Geng, J.; Bai, B.: Mechanically robust re-crosslinkable polymeric hydrogels for water management of void space conduits containing reservoirs. Chem. Eng. J. 317, 952–960 (2017)
Aalaie, J.; Alvand, E.; Hemmati, M.; Sajjadian, V.: Preparation and probing of the steady shear flow and viscoelastic properties of weakly crosslinked hydrogels based on sulfonated polyacrylamide for oil recovery applications. Polym. Sci. Ser. A 57(5), 680–687 (2015)
Mousa, M.H.; Dong, Y.; Davies, I.J.: Recent advances in bionanocomposites: preparation, properties, and applications. Int. J. Polym. Mater. Polym. Biomater. 65(5), 225–254 (2016)
Tian, H.; Wang, K.; Liu, D.; Yan, J.; Xiang, A.; Rajulu, A.V.: Enhanced mechanical and thermal properties of poly (vinyl alcohol)/corn starch blends by nanoclay intercalation. Int. J. Biol. Macromol. 101, 314–320 (2017)
Zhao, G.; Dai, C.; You, Q.; Zhao, M.; Zhao, J.: Study on formation of gels formed by polymer and zirconium acetate. J. Sol-Gel. Sci. Technol. 65(3), 392–398 (2013)
Liu, R.; Pu, W.; Sheng, J.J.; Du, D.: Star-like hydrophobically associative polyacrylamide for enhanced oil recovery: comprehensive properties in harsh reservoir conditions. J. Taiwan Inst. Chem. Eng. 80, 639–649 (2017)
Boal, A.K.; Ilhan, F.; DeRouchey, J.E.; Thurn-Albrecht, T.; Russell, T.P.; Rotello, V.M.: Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 404(6779), 746 (2000)
Deans, R.; Ilhan, F.; Rotello, V.M.: Recognition-mediated unfolding of a self-assembled polymeric globule. Macromolecules 32(15), 4956–4960 (1999)
Qiu, L.; Shen, Y.; Wang, T.; Wang, C.: Rheological and fracturing characteristics of a novel sulfonated hydroxypropyl guar gum. Int. J. Biol. Macromol. 117, 974–982 (2018)
Adewunmi, A.A.; Ismail, S.; Sultan, A.S.: Investigation into the viscoelastic response at various gelation performance, thermal stability and swelling kinetics of fly ash reinforced polymer gels for water control in mature oilfields. Asia-Pac. J. Chem. Eng. 12(1), 13–24 (2017)
Teodorescu, M.; Morariu, S.; Bercea, M.; Săcărescu, L.: Viscoelastic and structural properties of poly (vinyl alcohol)/poly (vinylpyrrolidone) hydrogels. RSC Adv. 6(46), 39718–39727 (2016)
Pu, J.; Zhou, J.; Chen, Y.; Bai, B.: Development of thermotransformable controlled hydrogel for enhancing oil recovery. Energy Fuels 31(12), 13600–13609 (2017)
Tongwa, P.; Bai, B.: Degradable nanocomposite preformed particle gel for chemical enhanced oil recovery applications. J. Petrol. Sci. Eng. 124, 35–45 (2014)
Zhang, F.; Shen, Y.; Ren, T.; Wang, L.; Su, Y.: Synthesis of 2-alkenyl-3-butoxypropyl guar gum with enhanced rheological properties. Int. J. Biol. Macromol. 97, 317–322 (2017)
Wang, C.; Qiu, L.; Wang, T.: Self-assembly and rheological behaviors of intermacromolecular complexes consisting of oppositely charged fluorinated guar gums. Carbohyd. Polym. 184, 333–341 (2018)
Aalaie, J.; Rahmatpour, A.: Preparation and swelling behavior of partially hydrolyzed polyacrylamide nanocomposite hydrogels in electrolyte solutions. J. Macromol. Sci. Part B 47(1), 98–108 (2007)
Koohi, A.D.; Seftie, M.V.; Ghalam, A.; Moghadam, A.M.; Sabet, S.Z.: Rheological characteristics of sulphonated polyacrylamide/chromium triacetate hydrogels designed for water shut-off. Iran Polym. J. 19(10), 757–770 (2010)
Song, J.; Fan, W.; Long, X.; Zhou, L.; Wang, C.; Li, G.: Rheological behaviors of fluorinated hydrophobically associating cationic guar gum fracturing gel. J. Petrol. Sci. Eng. 146, 999–1005 (2016)
Naik, P.K.; Mohan, M.; Banerjee, T.; Paul, S.; Goud, V.V.: Molecular Dynamic simulations for the extraction of quinoline from heptane in the presence of a low-cost phosphonium-based deep eutectic solvent. J. Phys. Chem. B 122(14), 4006–4015 (2018)
El-hoshoudy, A.; Ghanem, A.; Desouky, S.: Imidazolium-based ionic liquids for asphaltene dispersion; experimental and computational studies. J. Mol. Liq. 324, 114698 (2021)
Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y., et al.: Reverse screening methods to search for the protein targets of chemopreventive compounds. Front. Chem. 6, 138 (2018)
Yuan, J.; Zhong, L.; Vakili, M.; Segun, G.A.: New modeling method to simulate asphaltenes at oil sands process in water management. J. Mol. Graph. Modell 91, 1–9 (2019)
Goodarzi, F. A closer look at water/oil emulsions in energy and environment: modeling strategy. Memorial University of Newfoundland, (2019)
Goodarzi, F.; Zendehboudi, S.: Effects of salt and surfactant on interfacial characteristics of water/oil systems: molecular dynamic simulations and dissipative particle dynamics. Ind Eng. Chem. Res. (2019). https://doi.org/10.1021/acs.iecr.9b00504
Flory, P.: Principles of polymer chemistry Ithaca. Cornell University, NY (1953)
Liang, X.; Wu, J.; Yang, X.; Tu, Z.; Wang, Y.: Investigation of oil-in-water emulsion stability with relevant interfacial characteristics simulated by dissipative particle dynamics. Colloids Surf. Physicochem. Eng. Asp. 546, 107–114 (2018)
Mohan, M.; Naik, P.K.; Banerjee, T.; Goud, V.V.; Paul, S.: Solubility of glucose in tetrabutylammonium bromide based deep eutectic solvents: experimental and molecular dynamic simulations. Fluid Phase Equilib. 448, 168–177 (2017)
Wahab, O.O.; Olasunkanmi, L.O.; Govender, K.K.; Govender, P.P.: Prediction of aqueous solubility by treatment of COSMO-RS data with empirical solubility equations: the roles of global orbital cut-off and COSMO solvent radius. Theor. Chem. Acc. 138(6), 80 (2019)
Hall, S.; Hamerton, I.; Howlin, B.; Mitchell, A.: Validating software and force fields for predicting the mechanical and physical properties of poly (bisbenzoxazine) s. Mol. Simul. 34(10–15), 1259–1266 (2008)
Xu, J., Yuan, Y., Xie, Q., Wei, X. Research on the application of molecular simulation technology in enhanced oil-gas recovery engineering. E3S Web of Conferences. 233. EDP Sciences; (2021)
Bai, M.; Zhang, Z.; Cui, X.; Song, K.: Studies of injection parameters for chemical flooding in carbonate reservoirs. Renew. Sustain. Energy Rev. 75, 1464–1471 (2017)
Ahmadi, P.; Asaadian, H.; Khadivi, A.; Kord, S.: A new approach for determination of carbonate rock electrostatic double layer variation towards wettability alteration. J. Mol. Liq. 275, 682–698 (2019)
Dehghan Monfared, A.; Ghazanfari, M.H.: Wettability alteration of oil-wet carbonate porous media using silica nanoparticles: electrokinetic characterization. Ind. Eng. Chem. Res. 58(40), 18601–18612 (2019)
Keykhosravi, A.; Simjoo, M.: Insights into stability of silica nanofluids in brine solution coupled with rock wettability alteration: an enhanced oil recovery study in oil-wet carbonates. Colloids Surf. A Physicochem. Eng. Asp. 583, 124008 (2019)
Mohammed, M.; Babadagli, T.: Wettability alteration: a comprehensive review of materials/methods and testing the selected ones on heavy-oil containing oil-wet systems. Adv. Colloid Interface Sci. 220, 54–77 (2015)
Ali, J.A.; Kolo, K.; Manshad, A.K.; Mohammadi, A.H.: Recent advances in application of nanotechnology in chemical enhanced oil recovery: effects of nanoparticles on wettability alteration, interfacial tension reduction, and flooding. Egypt. J. Pet. 27(4), 1371–1383 (2018)
CMG W. Advanced processes and thermal reservoir simulator, (2008)
Tagavifar, M.; Fortenberry, R.; de Rouffignac, E.; Sepehrnoori, K.; Pope, G.A.: Heavy-oil recovery by combined hot water and alkali/cosolvent/polymer flooding. SPE J. 21(01), 74–86 (2016)
Bao, K.; Lie, K.-A.; Møyner, O.; Liu, M.: Fully implicit simulation of polymer flooding with MRST. Comput. Geosci. 21(5–6), 1219–1244 (2017)
Panja, P.; Conner, T.; Deo, M.: Factors controlling production in hydraulically fractured low permeability oil reservoirs. Int. J. Oil Gas Coal Technol. 13(1), 1–18 (2016)
Khalilinezhad, S.S.; Cheraghian, G.; Roayaei, E.; Tabatabaee, H.; Karambeigi, M.S.: Improving heavy oil recovery in the polymer flooding process by utilizing hydrophilic silica nanoparticles. Energy Sour. Part A Recovery Util. Environ. Eff. (2017). https://doi.org/10.1080/15567036.2017.1302521
Druetta, P.; Picchioni, F.: Polymer and nanoparticles flooding as a new method for enhanced oil recovery. JPet. Sci. Eng. 177, 479–495 (2019)
Mohsenatabar Firozjaii, A.; Akbari, M.; Zargar, G.: Sensitivity analysis and optimization on effective parameters during chemical enhanced oil recovery (CEOR) using experimental design and numerical simulation. Energy Sour. Part A Recovery Util. Environ. Effects 41(15), 1847–1861 (2019)
Esfe, M.H.; Esfandeh, S.: Nanofluid flooding in a randomized heterogeneous porous media and investigating the effect of capillary pressure and diffusion on oil recovery factor. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.113646
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-hoshoudy, A.N. Experimental and Theoretical Investigation for Synthetic Polymers, Biopolymers and Polymeric Nanocomposites Application in Enhanced Oil Recovery Operations. Arab J Sci Eng 47, 10887–10915 (2022). https://doi.org/10.1007/s13369-021-06482-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-06482-x