Abstract
In this study, the bioaccumulation of some metals by Phragmites australis plants exposed to poultry slaughterhouse wastewater was investigated. For this aim, Mo, As, Cd, Se, Cu, Zn, Cr, Ba, Ti, Fe, Al, and Mn metals were determined in slaughterhouse wastewaters, P. australis plants, and sediment samples. The uptake of metals by P. australis was investigated. Also, BCF and TF values were calculated and compared with reference plant values. The maximum metal accumulation in P. australis plants exposed to poultry slaughterhouse wastewaters was 5900% for Al and 33.5% for Mn when compared with reference plant values. According to the BCF values, P. australis was bioaccumulative for Mo, Se, and Al. As a result, it was determined that P. australis plant can be used in the remediation of metals found in poultry slaughterhouse wastewaters and would be a good accumulator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to industry and rapid urbanization, water use has also led to an increase in the amount of wastewater. The wastewaters that have heavy metal sourced from metal processing plants, fertilizer plants, battery, battery production, paper industry and slaughterhouses are either directly discharged into the receiving environment or treated. Wastewaters containing heavy metals and untreated or not properly treated have toxic effects on living organisms in receiving environments and cause environmental pollution [1].
The poultry slaughterhouse wastewater has medium strength because the wastewater has a high concentration of organics, inorganics (i.e., heavy metals), suspended solids, fats, disease-causing microorganisms and nutrients [2,3,4,5]. Wastewaters containing heavy metals cause contamination of drinking water when they enter the groundwater. When heavy metals enter the food chain, they have a carcinogenic effect on living organisms and adversely affect their living health. In order to eliminate these negative effects, heavy metal in wastewater needs to be removed by different treatment methods. Adsorption, membrane purification technology, biological treatment, and physicochemical processes are used in the removal of heavy metals. However, they are not economical and not easy to operate. Therefore, more economical and easier-to-use phytoremediation technologies are preferred to remove heavy metals from wastewaters.
Phytoremediation technology can be describable as the accumulation of pollutants such as heavy metals, nutrients, pesticides causing environmental pollution by plants. Rooted, floating and submerged plants can be used in phytoremediation technology. Example of plants used in phytoremediation technology is P. australis. P. australis plant commonly found in Turkey is a rooted plant. It is usually found in water basins, swamps, rivers, lakes and streams along the coastline. P. australis is used in phytoremediation technology for wastewater treatment in environmental engineering. Therefore, information is needed on the metal bioaccumulation properties of P. australis plant to improve environmental environments and reduce the environmental impact of metals.
In this study, the accumulation of some metals in P. australis plants exposed to PSW was investigated. The studies in the literature are generally laboratory-scale studies. The reason for a large number of studies in the literature under controlled conditions in the laboratory is that there are many environmental matrices in the real-scale wastewater environment. The difference of this study from the other studies is the investigation of the remediation potential of P. australis in the polluted areas taking the discharged wastewaters of a real-scale poultry slaughterhouse. In the study, P. australis, water, and sediment were sampled by a poultry slaughterhouse wastewater system to determine whether P. australis can bioaccumulate certain metals in the water. This is an interesting study to measure metals from a point that many researchers ignore. In addition, stating that some metals accumulate in different organs by P. australis increases the authenticity of the study. Therefore, the translocation factor (TF) and bioconcentration factor (BCF) values were calculated. In this respect, the study is an original and novelty study.
2 Materials and Methods
2.1 Study Area
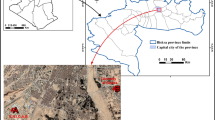
The area including a poultry slaughterhouse and a stream as the receiving environment (Elazığ, Turkey) was chosen (Fig. 1). The stream (Hinsor river) has about 6–7 km length. The stream flows to a dam lake named Cip which is used for irrigation purposes. Fruit and vegetable cultivation is carried out in the irrigation areas. Therefore, it is possible that the Cip Dam Lake will be exposed to metal pollution due to poultry slaughterhouse wastewater. The P. australis plant selected in this study is commonly found at the point where the slaughterhouse wastewater is discharged. The amount of wastewater from the slaughterhouse is approximately 1600 m3/day.
2.2 Sampling and Analysis
P. australis plants were selected in the study. P. australis plants were collected from the point where poultry slaughterhouse wastewater was discharged. The samples were transported to the laboratory. The organs of the plants were separated. Plant roots were washed with water to separate sediment particles. The dried plants were ground by a grinder. Wastewater samples were collected from slaughterhouse wastewater. Sediment samples were collected from 6 various points at the discharge point. The samples were sent to a laboratory with ISO 9001:2000 accreditation for analysis. They were analyzed by ICP/MS (ICP/MS-Perkin-Elmer ELAN 9000) in the laboratory. Also, translocation factors (TFs) and bioconcentration factors (BCFs) were calculated to evaluate the mobility of metals.
2.3 Bioconcentration and Translocation Factor
Bioconcentration factor is calculated from (Eq. 1)
where Cplant is metal value in the P. australis and Cenv is metal value in the environment [6].
The values of bioconcentration are calculated by the following equations:
where Csed is the value of the metal in sediment, Cr is the value of the metal in root, Cs is the value of the metal in stem, and Cw is the value of the metal in water.
Translocation factor is calculated by the following equations:
where Cl, Cr and Cs are values of metal in leaves, roots and stems, respectively.
2.4 Statistical Analysis
Results were analyzed using the IBM SPSS Statistics 21 program (SPSS Inc., Chicago IL, USA), and values shown were the means of three replicates (n = 3). Results were analyzed by a two-tailed Pearson correlation test to determine the relationship among the metals.
3 Results and Discussion
3.1 The Concentrations of Some Metals Detected in P. australis
The concentrations of some metals detected in organs of P. australis are given in Fig. 2. As the concentrations determined for each metal differ, they are shown in the graphs separately. To explain the accumulation mechanism of metals accumulated by P. australis, metal concentrations were determined separately in root, stem, and leaves of the P. australis.
Due to its antimicrobial and growth-stimulating effects, it is common practice to add minerals such as copper (Cu), zinc (Zn), and arsenic (As) to animal feed through mineral additives [7,8,9]. Arsenic compounds are generally used to improve the weight gain, the feed efficiency and the pigmentation of animals [10, 11]. Cang et al. [12] reported average arsenic content of feed of chickens in Jiangsu Province as .02 mg/kg and As the content of chicken feeds as 6.7%. Li et al. [13] reported mean Cd in feeds of chicken as 8.13 mg/kg in China. In addition to the high cadmium level in animal feeds, cadmium accumulation in animal edible offal has been reported to exceed food hygiene criteria of cadmium occurrences in animal production, which may be closely related to animal feed [9]. Therefore, it is possible to encounter these metals in poultry slaughterhouse wastewater.
In this study, the highest Fe, Al, and Mn metals, followed by Cu, Zn, Cr, Ba and Ti metals, and then Mo, As, Cd and Se metals were detected in the root, stem and leaves, respectively. The maximum metal value in the roots of the plant was 3940 ± 19.7 mg/kg for Fe, and the minimum metal value was .1 ± .05 mg/kg for Se. The total value of Mo in the plant was 2.13 ± .1 mg/kg (Fig. 2). 17.84% of this value was found in the roots (.38 ± .02 mg/kg), 7.51% in the stems (.16 ± .01 mg/kg) and 74.65% in the leaves (1.59 ± .08 mg/kg). For Mo, the best accumulation was seen in the leaves. It has been suggested that the ability of the plant to transport metals to aboveground parts is one of the main factors affecting heavy metal accumulation in the aboveground tissues of macrophytes [14,15,16]. Gupta et al. [17] found that metal accumulation/gr.dw is more in leaves than in roots. El-Nahhal et al. [18] found a high concentration of heavy metals in the leaves of Chinese cabbage and corn [19].
The total arsenic concentration in P. australis was 2.2 ± .1 mg/kg (Fig. 2). 54.54% of this value was determined in the roots (1.2 ± .06 mg/kg), 4.54% in the stems (.1 ± .05 mg/kg) and 40.92% in the leaves (.9 ± .05 mg/kg). It was determined that the best accumulation for arsenic took place in the roots, unlike Mo metal. Total Cd concentration in P. australis plant was found to be .17 ± .01 mg/kg (Fig. 2). 64.70% of this value was determined in the roots (.11 ± .01 mg/kg), 5.88% in the stems (.01 ± .05 mg/kg) and 29.42% in the leaves (.05 ± .02 mg/kg). It was determined that the best accumulation for Cd was in the roots as in As metal. Vymazal [20] reported concentrations of Cd in Phalaris arundinacea belowground/aboveground biomass were 150/80 μg/kg. Total Se concentration in the plant was .5 ± .01 mg/kg (Fig. 2). 20% of this value was determined in the roots (.1 ± .01 mg/kg), 40% in stem (.2 ± .02 mg/kg) and the remaining 40% in leaves (.2 ± .02 mg/kg). The total Cu value in the plant was determined as 21.78 ± 1.1 mg/kg (Fig. 2). 57.98% of this value was determined in the roots (12.63 ± .6 mg/kg), 11.20% in the stem (2.44 ± .13 mg/kg) and 30.82% in the leaves (6.71 ± .8 mg/kg). It was determined that the best accumulation for Cu took place in the roots such as Cd and As metal. The total Zn concentration in P. australis was 68 ± 3.2 mg/kg (Fig. 2). 70.14% of this value was determined in the roots (47.7 ± 2.3 mg/kg), 6.32% in the stem (4.3 ± .2 mg/kg) and 23.54% in the leaves (16 ± .8 mg/kg). It was determined that the best accumulation for Zn was found in the roots such as Cd, As and Cu metals. The total Cr value in P. australis plant was determined as 34.8 ± 1.7 mg/kg (Fig. 2). 39.08% of this value was determined in the roots (13.6 ± .7 mg/kg), 9.48% in the stem (3.3 ± .16 mg/kg) and 51.44% in the leaves (17.9 ± .9 mg/kg). It was determined that the best accumulation for Cr took place in leaves such as Mo metal. Vymazal [20] reported concentrations of Cr in Phalaris arundinacea belowground/aboveground biomass were 5420/228 μg/kg. The total Ba concentration in P. australis was 80.4 ± 4.1 mg/kg (Fig. 2). 14.30% of this value was determined in the roots (11.5 ± .6 mg/kg), 20.27% in the stem (16.3 ± .8 mg/kg) and 65.43% in the leaves (52.6 ± 2.5 mg/kg). It was determined that the best accumulation for Ba took place in leaves such as Mo and Cr metal. The total Ti concentration in P. australis was 137 ± 6.5 mg/kg (Fig. 2). 59.12% of this value was determined in the roots (81 ± 4.0 mg/kg), 2.19% in the stem (3 ± .2 mg/kg) and 38.69% in the leaves (53 ± 2.5 mg/kg). It was determined that the best accumulation for Ti was in the roots such as Cd, As, Cu, and Zn metals. The total Fe concentration in P. australis was 6470 ± 32 mg/kg (Fig. 2). 60.89% of this value was determined in the roots (3940 ± 19.7 mg/kg), 2.16% in the stem (140 ± 7.0 mg/kg) and 36.95% in the leaves (2390 ± 11 mg/kg). It was determined that the best accumulation for Fe was observed in the roots such as Cd, As, Cu, Zn, and Ti metals. The total Al concentration in the plant was determined as 4800 ± 24 mg/kg (Fig. 2). 56.25% of this value was determined in the roots (2700 ± 13 mg/kg), 2.08% in the stem (100 ± 5.0 mg/kg), and 41.67% in the leaves (2000 ± 10 mg/kg). It was determined that the best accumulation for Al took place in roots such as Cd, As, Cu, Zn, Ti, and Fe metals. The total Mn concentration in P. australis was 267 ± 13 mg/kg (Fig. 2). 42.69% of this value was determined in the roots (114 ± 5.5 mg/kg), 7.86% in the stem (21 ± 1.1 mg/kg), and 49.45% in the leaves (132 ± 6.0 mg/kg). It was determined that the best accumulation for Al took place in roots such as Cd, As, Cu, Zn, Ti, and Fe metals.
The distribution of the metal concentrations determined in P. australis plants exposed to PSW according to their presence in organs is summarized in Table 1.
3.2 The Concentration of Metals Detected in PSW
Mo, As, Cd, Se, Cu, Zn, Cr, Ba Ti, Fe, Al, and Mn were determined in poultry slaughterhouse wastewater. The metal concentrations determined in the poultry slaughterhouse wastewaters are given in Fig. 3.
According to Fig. 3, the highest Mn concentration detected in the slaughterhouse wastewater was 83.22 ± 4.2 mg/L, while the lowest Cd concentration was .05 ± .01 mg/L. The metal concentrations in the poultry slaughterhouse wastewaters were as follows: Mn: 83.22 ± 4.2 mg/L, Ti: 38 ± 2.0 mg/L, Cr: 24.1 ± 1.2 mg/L, Ba: 21.81 ± 1.1 mg/L, Zn: 15.5 ± .8 mg/L, Al: 8 ± .4 mg/L, Cu: 3.3 ± .16 mg/L, As: 2.4 ± .12 mg/L, Se: 1.6 ± .08 mg/L, Mo: .2 ± .01 mg/L and Cd: .05 ± .01 mg/L, respectively.
3.3 Concentrations of Metals Detected in Sediment Samples
Since P. australis is a rooted plant, sediment samples were taken from the poultry slaughterhouse wastewaters and metal concentrations were determined. The concentrations of metals determined in the sediments (Hinsor stream) are shown in Fig. 4.
According to Fig. 4, the highest metal concentration was determined as Fe in sediment samples taken from P. australis area. Fe concentration in sediment samples was determined as 26,500 ± 132 mg/kg. Fe metal was followed by Al, Mn, Ti, Zn, Ba, Cu, Cr, As, Mo, Se, and Cd, respectively. The lowest metal concentration in sediment samples was observed as .16 ± .08 mg/kg for Cd. The reason for the higher occurrence of Fe and Al metals in sediment samples compared to other metals is the immediate deposition of Al and Fe metals and their accumulation in sediment.
3.4 BCF and TF
BCF and TF values were calculated to evaluate the accumulation mechanism of plants exposed to poultry slaughterhouse wastewaters, and the values obtained are summarized in Table 2.
Bioconcentration factor and translocation factor values > 1 have been used to evaluate the potential of plant species for phytoremediation [21,22,23]. BCF values are significantly > 1. This shows efficient metal accumulation to plant.
According to Table 2, the BCFa value for Mo was calculated as 1.02. This shows that Mo metal is bioaccumulated from sediment to roots. When BCFb values were examined, the highest BCFb value was calculated for Se metal. It shows that Se metal accumulates well from sediment to the stem. The highest BCFc value was calculated as 12,500 for Al. This showed that Al metal is good bioaccumulation from poultry slaughterhouse wastewater to the stem. The BCFd value was the highest for Al metal, like the BCFc value (337,500). According to BCFd value, Al metal was well accumulated from poultry slaughterhouse wastewater to roots.
Plants with high TF values are suitable for phytoextraction, which is based on the translocation of heavy metals in easily harvestable plant parts [23, 24]. Low TF indicates the immobilization of trace elements from root to shoot or vacuole [25]. The highest TFa value for P. australis was 4.57 for Ba metal. This shows that Ba metal in poultry slaughterhouse wastewater is transported well from roots to leaves. Metals carried from roots to leaves in terms of TFa values followed. Ba > Mo > Se > Cr > Mn > As > Al > Ti > Fe > Cu > Cd. The metal with the highest TFb value was determined as Se. This showed that Se metal was well transported from the roots to the stem. Metals carried from roots to stem in terms of TFb values followed: Se > Ba > Mo > Cr > Cu > Mn > Cd = Zn > As > Ti > Fe > Al. When TFc was examined, the maximum TFc value was calculated as 20 for Al metal. This showed that Al metal had good transport from the stem to the leaves. For other metals, the order of transport followed Ti > Fe > Mo > As > Mn > Cr > Cd > Zn > Ba > Cu > Se.
Table 3 shows the comparison of the metal concentrations detected in plants exposed to the PSW with the metal concentrations of the reference plant [26].
According to Table 3, the highest metal concentration in P. australis plant was determined as 6470 mg/kg for Fe and Fe concentration in the reference plant was 150 mg/kg. In terms of Fe metal, 4213% uptake of the plant was determined. The lowest metal concentration detected in P. australis plant was 0,17 mg/kg for Cd. Cd concentration in the reference plant was .05 mg/kg. The uptake percentage of the plant in terms of Cd metal was calculated as 240%. When % values were compared, it was determined that the highest uptake percentage was calculated for Al metal. This shows that the P. australis plant preferred Al more than other metals.
3.5 Statistics of the Metals in P. australis
Correlation coefficients showing the relationship between Mo, Se, Cu, Zn, Cr, Ba, Ti, Fe, Al, Mn, As and Cd concentrations detected in P. australis are given in Table 4.
There was important correlation between Fe–Ti (p = .05), As–Al (p = .01), Cd–Cu (p = .05) and Cu–Ba (p = .05). Correlations between Cu–Se, Zn–Mo, Zn–Se, Cr–Se, Ba–Mo, Ba–Se, Ti–Se, Fe–Se, Al–Se, Mn–Se, As–Se and Cd–Se were observed as negative and not significant. It was observed that there was a significant correlation between Al–As (R2 = 1, p = .01) and Cd–Cu (R2 = 1, p = .05). This may show that elements have similar input sources or are precipitated in the same environment similarly reported by Oyebamiji et al. [27].
4 Conclusions
When the ability of P. australis exposed to PSW to accumulate metals was investigated, Al, Fe, Cu, and Mo metals were found to be well accumulated. This was controlled by calculating BCF and TF values. According to BCF value, P. australis was a bioaccumulator for Mo, Se, and Al. Also, in the study, it was found that the best metal accumulation was obtained for Al metal when the metals detected in plant exposed to poultry slaughterhouse wastewater and the metal concentrations in the reference plant were compared. It was determined that P. australis plant can be used as a bioaccumulator plant in areas contaminated with poultry slaughterhouse wastewater. As a result, this study showed that P. australis performs well for bioaccumulative pollutants and can be used in phytoremediation technology. Furthermore, the answer was given to the question of how fragmentation accumulates various metals and other pollutants from wastewater and flow, and how new this study is to investigate the bioaccumulative properties.
References
Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; et al.: Heavy metals removal from aqueous environments by electrocoagulation process—a systematic review. J. Environ. Health Sci. Eng. 13, 74 (2015)
Del Nery, V.; De Nardi, I.R.; Damianovic, M.H.R.Z.; Pozzi, E.; Amorim, A.K.B.; Zaiat, M.: Long-term operating performance of a poultry slaughterhouse wastewater treatment plant. Resour. Conserv. Recycl. 50(1), 102–114 (2007)
Avula, R.Y.; Nelson, H.M.; Singh, R.K.: Recycling of poultry process wastewater by ultrafiltration. Innov. Food Sci. Emerg. Technol. 10(1), 1–8 (2009)
Debik, E.; Coskun, T.: Use of the Static Granular Bed Reactor (SGBR) with anaerobic sludge to treat poultry slaughterhouse wastewater and kinetic modeling. Bioresour. Technol. 100(11), 2777–2782 (2009)
Rinquest, Z.; Basitere, M.; Ntwampea, S.K.O.; Njoya, M.: Poultry slaughterhouse wastewater treatment using a static granular bed reactor coupled with single stage nitrification–denitrification and ultrafiltration systems. J. Water Process Eng. 29, 100778 (2019)
Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A.: Phytoremediation of industrial wastewater potentiality by Typha domingensis. Int. J. Environ. Sci. Technol. 8(3), 639–648 (2011)
Nicholson, F.A.; Chambers, B.J.; Williams, J.R.; Unwin, R.J.: Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresour. Technol. 70, 23–31 (1999)
Li, Y.X.; Li, W.; Wu, J.; Xu, L.C.; Su, Q.H.; Xiong, X.: Contribution of additive Cu to its accumulation in pig feces: study in Beijing and Fuxin of China. J. Environ. Sci. 19, 610–615 (2007)
Zhang, F.; Li, Y.; Yang, M.; Li, W.: Content of heavy metals in animal feeds and manures from farms of different scales in northeast China. Int. J. Environ. Res. Public Health 9, 2658–2668 (2012). https://doi.org/10.3390/ijerph9082658
Jackson, B.P.; Bertsch, P.M.; Cabrera, M.L.; Camberato, J.J.; Seaman, J.C.; Wood, C.W.: Trace element speciation in poultry litter. J. Environ. Qual. 32, 535–540 (2003). https://doi.org/10.2134/jeq2003.0535
Li, Y.X.; Chen, T.B.: Concentrations of additive arsenic in Beijing pig feeds and the residues in pig manure. Res. Conserv. Recycl. 45, 356–367 (2005)
Cang, L.; Wang, Y.J.; Zhou, D.M.; Dong, Y.H.: Heavy metals pollution in poultry and livestock feeds and manures under intensive farming in Jiangsu Province, China. J. Environ. Sci. 16, 371–374 (2004)
Li, Y.X.; Xiong, X.; Lin, C.Y.; Zhang, F.S.; Li, W.; Han, W.: Cadmium in animal production and its potential hazard on Beijing and Fuxin farmlands. J. Hazard. Mater. 177, 475–480 (2010)
Rauser, W.E.: Structure and function of metal chelators produced by plants. The case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochem. Biophys. 31, 19–48 (1999)
Yeh, T.Y.; Chou, C.C.; Pan, C.T.: Heavy metal removal within pilot-scale constructed wetland receiving river water contaminated by confined swine operations. Desalination 249, 368–373 (2009)
Vymazal, J.; Březinová, T.: Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: a review. Chem. Eng. J. 290, 232–242 (2016)
Gupta, S.; Satpati, S.; Nayek, S.; Garai, D.: Effect of wastewater irrigation on vegetables in relation to bioaccumulation of heavy metals and biochemical changes. Environ. Monit. Assess. 165, 169–177 (2010)
El-Nahhal, Y.; Tubail, K.; Safi, M.; Safi, J.: Effect of treated waste water irrigation on plant growth and soil properties in Gaza Strip, Palestine. Am. J. Plant Sci. 4, 1736–1743 (2013)
Khaliq, S.J.A.; Al-Busaidi, A.; Ahmed, M.; Al-Wardy, M.; Agrama, H.; Choudri, B.S.: The effect of municipal sewage sludge on the quality of soil and crops. Int. J. Recycl. Org. Waste Agric. 6(4), 289–299 (2017)
Vymazal, J.: Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci. Total Environ. 544(2016), 495–498 (2016)
Baker, A.J.M.; Brooks, R.R.; Pease, A.J.; Malaisse, F.: Studies on copper and cobalt tolerance in three closely related taxa within the genus Silene L. (Caryophyllaceae) from Zaïre. Plant Soil 73, 377–385 (1983)
McGrath, S.P.; Zhao, F.J.: Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 14, 277–282 (2003)
Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X.: Bamboo: an untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 246, Article 125750 (2020)
Malik, R.N.; Husain, S.Z.: Classification and ordination of vegetation communities of the Lohibehr reserve forest and its surrounding areas, Rawalpindi, Pakistan. Pak. J. Bot. 38, 543–558 (2006)
Batool, A.; Saleh, T.A.: Removal of toxic metals from wastewater in constructed wetlands as a green technology; catalyst role of substrates and chelators. Ecotoxicol. Environ. Saf. 189, Article 109924 (2020)
Markert, B.: Establishing of reference plant for inorganic characterization of different plant species by chemical fingerprinting. Water Air Soil Pollut. 64, 533–538 (1992)
Oyebamiji, A.; Amanambu, A.; Zafar, T.; Adewumi, A.J.P.; Akinyemi, D.S.: Expected impacts of active mining on the distribution of heavy metals in soils around Iludun-Oro and its environs, Southwestern Nigeria. J. Cogent Environ. Sci. 4(1), 1–21 (2018)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Topal, M. Investigation of Some Metal Accumulation Ability of Phragmites australis from Poultry Slaughterhouse Wastewaters. Arab J Sci Eng 46, 115–122 (2021). https://doi.org/10.1007/s13369-020-04825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04825-8