Abstract
Helichrysum sanguineum is a traditional Palestinian medicinal plant used for the treatment of various diseases. The current investigation aimed to estimate H. sanguineum four fractions chemical compositions, antioxidant, anti-lipase, and anti-α-amylase activities. Phytochemical screening, total phenols, flavonoids, and tannins of H. sanguineum acetone, aqueous, methanol, and hexane fractions were carried out using standard analytical methods. In contrast, the free radical scavenging properties of these fractions were determined using the colorimetric DPPH method and Trolox as a positive control. Moreover, the anti-lipase activity was estimated using porcine pancreatic lipase enzyme inhibitory assay and Orlistat as a positive control. Also, the α-amylase inhibitory activity was established by utilizing the α-amylase porcine pancreatic enzyme and the 3,5-dinitrosalicylic acid (DNSA) method with Acarbose as a positive control. The obtained results showed that the H. sanguineum hexane fraction has the highest phenol and flavonoid contents and revealed the best antioxidant activity. Moreover, the aqueous fraction of H. sanguineum showed potential inhibitions against α-amylase and lipase porcine pancreatic enzymes in comparison with Acarbose and Orlistat, respectively. This study endorses the use of the H. sanguineum aqueous fraction for further in vivo studies to determine its potential against diabetes mellitus, obesity, and oxidative stress diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phytopharmaceuticals are an integral part of modern medicine and pharmacy, which have been utilized to treat many disorders and diseases throughout the world, including hypertension, obesity, cancer, diabetes, atherosclerosis, oxidative stress, and many other others [1]. The construction of oxygen-derived free radicals (oxidant) is balanced in the human body with antioxidant enzymes (e.g., superoxide dismutase). In the case of oxidative stress, a drastic imbalance between these two factors leads to potential oxidative damage; in response to this disturbance, these free radicals will affect many cellular structures, especially DNA. This can occur physiologically with aging but can also be present in the pathogenesis of many diseases such as diabetes mellitus, neurodegenerative disorders, cancer, and cardiovascular diseases [2]. However, several in vivo and in vitro investigations showed that the presence of high levels of antioxidants had been shown to prevent various types of free radical damage that have been associated with the development of cancer [3].
Despite the extensive efforts and researches to reduce the risk of obesity on health, its prevalence is increasing rapidly. It has been doubled over the past four decades in the USA. It is a multi-factorial disorder, involving genetic and environmental factors (such as diet and exercise), all of which are challenging to treat and prevent [4].
In fact, in the pharmaceutical markets, various types of herbal supplements have been utilized for the treatment of obesity and overweight in developed and developing countries, since these disorders have become common. However, obesity poses a global concern not only for the physiological harm it can cause but also because of its association with several diseases and disorders such as metabolic, endocrine, and cardiovascular diseases [5].
Recently, different strategies have been used globally to decrease hypertriglyceridemia, hyperlipidemia, and particularly to reduce body mass index in overweight and obese people. Moreover, several pharmaceutical agents available on the market have been approved for use hand-in-hand with the required exercises and diets to decrease body weight and lipid levels in the plasma [6].
Diabetes mellitus is considered the most prevalent and morbid disease, affecting millions of people around the world. According to the global burden of disease (GBD), its prevalence increased by approximately 30.6% from 2005 to 2015. Type 2 diabetes mellitus was first recognized as part of metabolic syndrome in 1988; it is characterized by insulin resistance, which leads to hyperglycemia and is caused by a combination of environmental, genetic, and behavioral factors. Obesity contributes to about 50% of DM type 2 cases [7]. The α-amylase is a primary metabolic enzyme found in pancreatic juice and saliva, which can hydrolyze homoglycan complex polysaccharide molecules of Amylum (starch) into absorbable di- and monosaccharide units. The inhibition of α-amylase can decrease postprandial blood glucose levels by delaying the digestion of starch in the small intestine [8].
Helichrysum sanguineum (L.) Kostel. is commonly known as the Red Everlasting and Blood of Maccabees. It is a perennial herbaceous plant belonging to the Compositae (Asteraceae) family, which is distributed in Mediterranean woodlands and in shrubby lands in Palestine. It has a collection of cylindrical yellow flowers, which are very close together, wrapped in red-straw like bracts [9].
H. sanguineum has been used in traditional medicine as a decoction in Turkey, Palestine, and other Mediterranean countries to treat respiratory problems, urogenital disorders, jaundice, skin inflammation, diarrhea, kidney stones, asthma, fever and tuberculosis [10,11,12].
The main chemical constituent of this and other species of Helichrysum (e.g., H. pamphylicum, H. orientale, and H. neoanum) are flavonoids, such as 5,7-dihydroxy-3,8-dimethoxy flavone, kaempferol, 3,5,7-trihydroxy-6,8-dimethoxy flavone, peonidin 3-glucoside anthocyanins, kaempferol 3-glucoside, quercetin 4′-glucoside, and pelargonidin 3-glucoside [13] which have antioxidant [11], anticancer, and antimicrobial properties [14].
This research aimed to investigate: (1) the phytochemical constituents of four solvent fractions of the Palestinian H. sanguineum plant and to evaluate its pharmacological activity against oxidative stress, obesity, and diabetes mellitus using in vitro models. (2) To evaluate the phytochemical characteristics of H. sanguineum, such as total phenols, tannins, and flavonoids.
2 Materials and Methods
2.1 Plant Material
The aerial parts of H. sanguineum were collected in May 2017 from the Jerusalem province of Palestine. The pharmacognosy’s conducted botanical characterization, Dr. Nidal Jaradat, in the Herbal products Laboratory at An-Najah National University and kept under the herbarium voucher specimen number: Pharm-PCT-1170.
The aerial plant parts were cleaned and dried in the shade at a controlled humidity (55 ± 5 RH) and temperature (25 ± 2 °C). After that, the dried aerial parts were powdered and kept in airtight containers for further use.
2.2 Instrumentation
A Spectrophotometer-UV/Visible (Jenway® 7135, Staffordshire, UK), filter papers (Whitman No. 1, Washington, USA), Shaker device (Memmert 531-25-1, Stockholm, Germany), rotavap apparatus (Heidolph-VV 2000, Schwabach, Germany), grinder (Aero Plus 500 W Mixer Grinder, I01, Wan Chai, China), electronic-balance (Radwag, AS 220/c/2, Toruńska, Poland) and Cryo-Desiccator (Mill-rock technology, BT85, Kingston, USA) were used.
2.3 Chemicals
Acetone, sodium hydroxide, n-hexane, and methanol were obtained from Loba Chemie (Mumbai, India), while Ninhydrin solution, Benedict’s and Millon’s reagents were purchased from Alfa Agar (Binfield, UK). Besides, iodine solution, sulfuric acid, and Molisch’s reagent were obtained from Alfa-Aesar (Lancaster, UK). Folin–Ciocalteu’s reagent, hydrochloric acid, aluminum chloride, potassium acetate, chloroform, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were bought from Sigma-Aldrich (Steinheim, Germany). Magnesium ribbon, acetic acid, ferric chloride, and dimethyl sulfoxide (DMSO) were obtained from Riedel-De-Haen (Teningen, Germany).
In addition, Trolox ((s)-(–)-6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid) and quercetin were purchased from Sigma-Aldrich (Søborg, Denmark). However, α-amylase was brought from Sigma (Mumbai, India). While DNSA (3,5-dinitrosalicylic acid) reagent, Acarbose, p-nitrophenyl butyrate, Orlistat, tris–HCl buffer, and Porcine pancreatic lipase type II were purchased from Sigma (St. Louis, USA).
2.4 The Four Solvent Fractionation Method
The powdered material of the H. sanguineum plant was sequentially fractionated by adding four solvents of increasing polarity: hexane (non-polar), acetone (polar-aprotic), methanol, and water (polar-protic solvents). About 25 g of the powdered plant material was steeped in 500 ml of acetone, n-hexane, methanol, and water separately, and each fraction was placed in a shaker for 72 h at room temperature, with 100 rounds/min. Then, each fraction was kept in a refrigerator for 6 days. After that, the hexane, acetone, and methanol fractions were filter-evaporated using a rotavapor under certain vacuum conditions. The aqueous fraction was lyophilized utilizing a Cryo-Desiccator. Finally, all crude plant fractions were stored in the refrigerator at 4 °C until further use [15].
2.5 Preliminary Phytochemical Assessment
The H. sanguineum plant methanol, water, n-hexane, and acetone fractions were screened for the presence of major natural phytochemical classes by utilizing the analytical tests of pharmacopeias [16, 17].
2.6 Antioxidant Activity
For the evaluation of four plant fractions and Trolox (positive control), a concentration of 1 mg/ml in methanol was initially prepared from the plant samples. The produced solution utilized to form 1, 2, 3, 5, 7, 10, 20, 30, 40, 50, and 80 µg/ml concentrations. Then, the DPPH reagent was dissolved in 0.002% w/v methanol and mixed with the prepared previously working concentrations in a 1:1:1 ratio. The pure methanol solution was utilized as a blank. All of the solutions were incubated in a dark chamber for 30 min at room temperature. Then, their absorbance values were estimated by utilizing a UV–visible spectrophotometer at a wavelength of 517 nm. The percentage of antioxidant potential of each plant fraction and the Trolox was estimated using the following formula:
where Z is the absorbance of the blank and Y is the absorbance of the sample.
The antioxidant half-maximal inhibitory concentration (IC50) of each plant fraction was calculated utilizing BioDataFit edition 1.02 [18].
2.7 Quantitative Total Phenol Content
The total phenolic contents of H. sanguineum four fractions were estimated colorimetrically utilizing the Folin–Ciocalteu procedure and Gallic acid as a reference molecule [19]. Briefly, from each plant fraction, 1 mg/ml aqueous solution was prepared for this analysis, while the reaction mixtures were prepared by taking 0.5 ml of the aqueous solution from each plant fraction, 2.5 ml of 10% Folin–Ciocalteu testing agent dissolved in water and 2.5 ml of 7.5% NaHCO3 aqueous solution. Thereafter, the produced mixtures were kept in a thermostat at 45 °C for 45 min. The absorbance was estimated utilizing a spectrophotometer at a wavelength of 765 nm. All of the studied samples were tested in triplicate for each plant fraction, and the mean values of absorbance obtained were reported. The same method was repeated for the standard molecule Gallic acid and then constructed the required calibration curve. Based on the measured absorbance, the concentration of the Gallic acid equivalent was determined in mg of GAEq/g for each working plant sample.
2.8 Quantitative Contents of Flavonoids
The quantitative contents of flavonoids in four H. sanguineum fractions were evaluated according to the methods of Chang et al. [20] with minor modifications. Ten mg of quercetin was mixed with 10 ml of distilled water and diluted to a final volume of 100 ml. However, a stock solution was subsequently diluted to concentrations of 10, 30, 40, 50, 70, and 100 mg/ml and 1 ml of each solution was mixed with 0.3 ml of 10% AlCl3 solution, 1 ml distilled water, 1 ml 2 M NaOH, and 0.3 ml NaNO2. These prepared samples were incubated for 30 min at room temperature. The colorimetrical absorbance values obtained were estimated by utilizing a UV–visible spectrophotometer at a wavelength of 510 nm. The evaluated total flavonoid content was obtained from quercetin’s calibration curve, which revealed mg of quercetin equivalent per gram for each plant fraction (mg QUE/g).
2.9 Determination of Total Tannin Content
The condensed tannins contents of four H. sanguineum fractions were assessed according to Sun et al.’s procedure, with minor modifications. Briefly, 0.5 ml of 10, 30, 50, 70, and 100 μg/ml concentrations of catechin were added to 3 ml of 4% vanillin-methanol solution and 1.5 ml of concentrated HCl. The obtained mixtures were allowed to stand for 15 min, and then absorption was measured at 500 nm against methanol as a blank. All samples were analyzed in triplicate, while the tannin content in each fraction was revealed in regards to catechin equivalents (mg of CAE/g of plant fraction) [21].
2.10 Porcine Pancreatic Lipase Inhibition Assay
The anti-lipase assay was conducted according to Bustanji et al.’s studies with minor corrections [22]. Briefly, a stock solution was prepared from 1 mg/ml of each H. sanguineum fraction with 10% dimethyl sulfoxide from which 50, 100, 200, 300, and 400 μg/ml concentrations were prepared. Moreover, a 1 mg/ml stock solution of pancreatic lipase was mixed with a Tris–HCl buffer solution. A stock solution of p-nitrophenyl butyrate was prepared by suspending 20.9 mg in 2 ml of acetonitrile. Then, 0.1 ml of porcine pancreatic lipase enzyme (1 mg/ml) was added to 0.2 ml of plant fraction. The resulting mixture was then made up to 1 ml by adding a Tri–HCl solution and kept at 37 °C for 15 min. After that, 0.1 ml of p-nitrophenyl butyrate was added to each working sample. These mixtures were incubated for 30 min at 37 °C. Pancreatic lipase activity was evaluated by calculating the hydrolysis of p-nitrophenolate to p-nitrophenol at 405 nm, utilizing a UV/visible spectrophotometer. The same method was conducted using Orlistat as a positive control [23]. Moreover, all of the studied samples were analyzed in triplicate.
2.11 α-Amylase Inhibitory Assay
This method was carried out by utilizing the procedure modified from McCue and Shetty [24]. A 200 μl aliquot of each plant fraction at concentrations of 10, 50, 70, 100, and 500 μg/ml was placed in a test tube with 200 μl of 0.02 M sodium phosphate buffer (pH 6.9) containing α-amylase solution (2 units/ml). This solution was kept for 10 min at 25 °C, after which 200 μl of 1% starch solution mixed with 0.02 M sodium phosphate buffer solution (pH 6.9) was added at timed intervals and then kept for 10 min at 25 °C. This reaction was stopped by adding 200 μl of dinitrosalicylic acid (DNS). These tubes were then incubated for 5 min in boiling water before being cooled to room temperature. The mixtures were then diluted with 5 ml distilled water, and the absorbance was calculated at 540 nm by utilizing a UV–visible spectrophotometer. A control sample was prepared using the same procedure and replacing the plant fraction with distilled water. The α-amylase inhibitory activity was calculated as a percentage of inhibition using the following equation [25]:
The concentrations of plant fractions resulting in a 50% inhibition of lipase enzyme activity (IC50) were estimated graphically. The same method was repeated for the utilized positive control of α-amylase inhibitory activity, which was Acarbose.
2.12 Statistical Analysis
All of the obtained results of the four studied plant fractions (antioxidant, anti-lipase, and anti-amylase activities) were expressed as mean ± SD standard deviation; the result was considered significant when the p value was < 0.05. Data were compared using unpaired t-tests.
3 Results
3.1 Phytochemical Screening
The results of the preliminary phytochemical tests on the H. sanguineum aqueous fractions showed the presence of saponin glycosides, phenols, tannins, and flavonoids, while volatile oils, steroids, phenols, tannins, and flavonoids were observed in the methanolic fraction. Simultaneously, phenols, flavonoids, steroids, volatile oils, and cardiac glycosides were identified in the acetone and hexane fractions, as shown in Table 1; the fractionation yields are shown in Table 2.
3.2 Quantitative Analysis of Phenols Contents
For the evaluation of total phenol content, the absorption values of several concentrations of the Gallic acid standard are listed in Table 3.
From the calibration curve of Gallic acid (Fig. 1), the following equation was calculated to estimate the total phenol content in the four H. sanguineum plant fractions.
where y is the absorbance at 765 nm, and x is the total phenol content of the plant fraction.
For the evaluation of total flavonoid contents in four H. sanguineum fractions, the absorbance of different concentrations was used, and the standard calibration curve was built to calculate the total flavonoid content in four H. sanguineum fractions. The absorption values of several concentrations of the standard quercetin are listed in Table 4.
According to the standard calibration curve of quercetin, as presented in Fig. 2, the equation y = 0.0004x + 0.0011, R2 = 0.995 was utilized to estimate total flavonoid contents in four H. sanguineum plant fractions.
Where Y is the absorbance at 510 nm, and X is the total flavonoids in the four studied plant fractions.
In addition, for the evaluation of total tannin content, the absorption values of several concentrations of the standard catechin were assessed, as listed in Table 5.
According to the standard calibration curve of catechin, as shown in Fig. 3, the equation y = 0.0009x + 0.011, R2 = 0.9596 was used to estimated total condensed tannins contents in the H. sanguineum four fractions.
Where Y is the absorbance at 500 nm and X is the total tannin contents in H. sanguineum plant four fractions.
The total phenol, flavonoid, and tannin contents of the hexane, acetone, methanol, and aqueous H. sanguineum fractions are presented in Table 6.
3.3 Antioxidant Activity
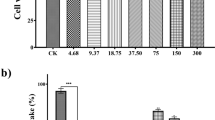
The DPPH method was utilized as an in vitro approach to assess the antioxidant activities of the H. sanguineum hexane, acetone, methanol, and aqueous fractions. The IC50 values were used to assess the ability of the examined samples to inhibit DPPH, which identified the amount of antioxidant potential required to inhibit the radical (DPPH) concentration by 50%; these were inversely linked to the antioxidant activities. The antioxidant IC50 values, percentage DPPH inhibition of H. sanguineum four fractions, and Trolox (positive control) are shown in Table 7 and Fig. 4.
3.4 α-Amylase Inhibition Assay
An in vitro assay of α-amylase inhibitory activity using starch as a substrate and Acarbose as a positive control was conducted on four H. sanguineum plant fractions. The results of IC50 α-amylase inhibitory activity values for the four fractions of H. sanguineum and Acarbose are illustrated in Table 8 and are obtained from Fig. 5.
3.5 Porcine Pancreatic Lipase Enzyme Inhibition Activity
The hydrolysis of p-nitrophenyl butyrate to p-nitrophenol was used to measure the influence of the four H. sanguineum fractions on the porcine pancreatic lipase enzyme. The assay worked by comparing a strong lipase inhibitory agent to Orlistat. The lipase enzyme inhibitory activity four H. sanguineum fractions and Orlistat also results in their IC50 values are shown in Table 9 and Fig. 6.
4 Discussion
Herbals have been used as essential sources of medicines since ancient times and are widely used as traditional medicines in many developing and developed countries; also, they are used in phytotherapy, which is an important branch of complementary and alternative medicine. In the last three decades, herbals have been used widely in drug discovery and utilized as pure active ingredients, such as morphine, papaverine, atropine. Also, it has been used as semisynthetic aminophylline and neostigmine among many other derivatives.
However, the current study preliminary phytochemical screening tests revealed the presence of saponin glycosides, phenols, tannins, and flavonoids in the H. sanguineum aqueous while volatile oils, steroids, phenols, tannins, and flavonoids were observed in the methanolic fraction. At the same time, phenol, flavonoids, steroids, volatile oils, and cardiac glycoside molecules were identified in the acetone and hexane fractions.
The quantitative phytochemical assessment of H. sanguineum hexane, acetone, methanol, and aqueous fractions showed that the hexane fraction displayed remarkably high levels of total phenol flavonoids contents among the other plant fractions: 63.24 ± 0.57 GAE/g and 26.3 ± 1.22 GAE/g, respectively, while the total tannin contents were not notable throughout of the studied plant four fractions.
In a study conducted by Alali et al., the total phenolic content of H. sanguineum aqueous and methanolic extracts was 41.3 GAE/g and 34.7 GAE/g, respectively, while the obtained total phenol contents in the current study for aqueous and methanolic fractions were 8.75 ± 0.34 GAE/g and 2.5 ± 0.07 GAE/g, respectively [26].
Another investigation established by Albayrak et al. for some Helichrysum species from Turkey found that the total phenolic content of H. sanguineum methanolic extract was 63.8 ± 0.6 GAE/g, and the antioxidant activity IC50 value was 12.90 µg/ml of the dry extract [27].
Moreover, the current investigation showed that the four fractions of H. sanguineum have antioxidant activity, while the hexane and aqueous fractions showed the highest antioxidant potentials with IC50 values of 8.36 ± 1.04 µg/ml and 10.7 ± 1.46 µg/ml, respectively.
The study by Alali et al. [26] revealed that the antioxidant activity of H. sanguineum aqueous and methanolic extracts was 0.5% and 3.3%, respectively, while the current antioxidant results were 20.84% and 7.33%, respectively.
These discrepancies can be explained by the fact that used methods of isolation were different, and the utilized method of isolation in the current study using various solvents fractions is sufficient than the conventional method of extract, and the studied H. sanguineum plant from Palestine is the best source for isolation of therapeutic active compounds.
To the best of the author’s knowledge, the total flavonoid and total tannins contents of H. sanguineum four fractions are also the antioxidant and total phenols of the hexane and acetone fractions have not been established before and the current study will be the first one.
Several previously conducted studies showed that the plants contained various classes of phenolic molecules, including flavonols, flavones, isoflavones, flavanones, flavanols, phenolic acids, anthocyanins, simple phenolics, and hydroxycinnamic acid derivatives. All received considerable attention due to their potential pharmacological activity, including antioxidant, anti-lipase, and anti-amylase properties. The antioxidant activity of phenolics is mainly due to their redox properties, which enable them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers. They may also have metal-chelating potential.
Hyperglycemia is a classical risk factor in the development of diabetes mellitus complications. Several therapeutic protocols have been utilized for the management of diabetes mellitus, one of which aims to alter the absorption of glucose via the inhibition of relevant carbohydrate-hydrolyzing enzymes, such as α-amylase; the inhibition of this enzyme has been considered a strong option in the prevention of hyperglycemia [28]. Therefore, inhibitors like Acarbose, Voglibose, and Miglitol are widely used in type 2 diabetic patients today. Besides, numerous studies have revealed that numerous medicinal plants have α-amylase enzyme inhibitory activity [25, 29, 30].
However, in a study conducted by Shaw et al. [31], it was shown that the world prevalence of diabetes among adults would increase to 7.7%, affecting 439 million adults by 2030. There will be a 69% increase in the number of adults with diabetes between 2010 and 2030 in developing countries.
As a consequence of this global health problem, the current study aimed to evaluate the α-amylase inhibitory activity of four H. sanguineum fractions. In brief, the results revealed that the aqueous fraction of H. sanguineum has the highest α-amylase inhibitory effect, which is even more potent than acarbose, with an IC50 value of 28.18 ± 1.04 µg/ml. In contrast, the hexane fraction showed a similar IC50 value (35.48 ± 0.62 µg/ml) as the commercial drug acarbose, with an α-amylase inhibitory activity IC50 value of 31.6 ± 1.22 µg/ml. However, the methanol and the acetone fractions showed weak α-amylase inhibitory activity.
Recently, one of the meaningful challenges for scientists has been investigating effective, safe, and low-cost antiobesity pharmaceutical dosage forms. Herbals and other natural products offered endless sources of the physiologically active compound, which can resolve this global health problem. The incidence of obesity has increased at an alarming rate and has become a significant public health concern, especially in the last 2 decades [32, 33].
Notably, the results of the current investigation revealed that the porcine pancreatic lipase enzyme inhibitory activity was best for the aqueous fraction of H. sanguineum, which has a pancreatic lipase inhibition IC50 value of 63.09 ± 0.3 µg/ml. The hexane and acetone fractions showed weak anti-lipase activity, with IC50 values 109.64 ± 1.12 µg/ml and 501.18 ± 0.69 µg/ml, respectively, compared with Orlistat, which is a commercial anti-lipase and antiobesity drug with an IC50 value of 12.3 ± 0.74 µg/ml.
The results demonstrate that the hexane fraction of H. sanguineum plant is rich in flavonoids and phenols contents and has the highest antioxidant activity, among other plant fractions. However, for many flavonoids and phenols, the potential antioxidant activity has been demonstrated in various in vitro and in vivo studies and considers the most important group of natural antioxidants [34,35,36].
However, flavonoids and phenols are oxidized by free radicals, resulting in a more stable and less-reactive radical. In other words, flavonoids and other phenols stabilize the reactive oxygen species by reacting with the radical reactive compound. Radicals are made inactive due to the high reactivity of the hydroxyl group of the flavonoids and phenols [37].
Furthermore, the aqueous fraction of the H. sanguineum plant showed powerful anti-lipase and anti-α-amylase activities. However, the phytochemical screening of this fraction revealed the presence of saponin glycosides. This secondary metabolic compound in different plant species has shown potential antidiabetic and hypolipidemic effects. Fenugreek seeds, Ginseng roots, and many other plant species containing saponins [38,39,40,41].
To the best of the authors’ knowledge, no previous studies have been published on the anti-lipase and α-amylase inhibitory activities of the four H. sanguineum solvents antioxidant effects of the hexane and acetone fractions. Also, the total phenol and tannin contents of the plant have not been previously investigated, meaning that the current study is the first to report these issues.
Further phytochemical, toxicological, and clinical investigations are required to isolate therapeutically active molecules from H. sanguineum, investigate the mechanism of action of the isolated compounds, evaluate the associated safety and toxicity issues and conduct severe clinical trials on human subjects for the treatment of oxidative stress, diabetes mellitus and obesity.
5 Conclusion
The data obtained from the current investigation imply that the hexane fraction of H. sanguineum plant is rich in flavonoids and phenols contents and has the highest antioxidant activity, among other plant fractions. Moreover, the aqueous fraction exerts inhibitory activity against α-amylase and lipase. The results exhibited by the obtained fractions are attracting sufficient interest to promote further in vivo studies and design suitable pharmaceutical dosage forms for the treatment of oxidative stress, diabetes mellitus, and obesity.
Data Availability
All the utilized data to support the findings of the current study are included within the article.
References
Al-Lahham, S.; Sbieh, R.; Jaradat, N.; Almasri, M.; Mosa, A.; Hamayel, A.; et al.: Antioxidant, antimicrobial and cytotoxic properties of four different extracts derived from the roots of Nicotiana tabacum L. Eur. J. Integr. Med. 33, 101039 (2020)
Jaradat, N.; Qadi, M.; Abualhasan, M.N.; Al-lahham, S.; Al-Rimawi, F.; Hattab, S.; et al.: Carbohydrates and lipids metabolic enzymes inhibitory, antioxidant, antimicrobial and cytotoxic potentials of Anchusa ovata Lehm from Palestine. Eur. J. Integr. Med. (2020). https://doi.org/10.1016/j.eujim.2020.101066
Carini, F.; Tomasello, G.; Jurjus, A.; Geagea, A.; Al Kattar, S.; Damiani, P.; et al.: Colorectal cancer and inflammatory bowel diseases: effects of diet and antioxidants. J. Biol. Regul. Homeost. Agents 31(3), 791–795 (2017)
Tylavsky, F.A.; Ferrara, A.; Catellier, D.J.; Oken, E.; Li, X.; Law, A.; et al.: Understanding childhood obesity in the US: the NIH environmental influences on child health outcomes (ECHO) program. Int. J. Obes. 44, 617–627 (2020)
Jaradat, N.; Zaid, A.N.; Hussein, F.; Zaqzouq, M.; Aljammal, H.; Ayesh, O.: Anti-lipase potential of the organic and aqueous extracts of ten traditional edible and medicinal plants in palestine; a comparison study with Orlistat. Medicines 4(4), 89–95 (2017)
Brauer, P.; Gorber, S.C.; Shaw, E.; Singh, H.; Bell, N.; Shane, A.R.; et al.: Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. Can. Med. Assoc. J. 187(3), 184–195 (2015)
Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B.: Type 2 diabetes mellitus: a review of current trends. Oman Med. J. 27(4), 269–274 (2012)
Kazeem, M.; Adamson, J.; Ogunwande, I.: Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth. leaf. Int. BioMed. Res. (2013). https://doi.org/10.1155/2013/527570
Boi, M.: The Ethnocultural Significance for the Use of Plants in Ancient Funerary Rituals and Its Possible Implications with Pollens Found on the Shroud of Turin. Valencia, Italy (2012)
Jaradat, N.; Al-Masri, M.; Zaid, A.N.; Hussein, F.; Shadid, K.A.; Al-Rimawi, F.; et al.: Assessment of the antimicrobial and free radical scavenging activities of Moluccella spinosa, Helichrysum sanguineum, and Styrax officinalis folkloric medicinal plants from Palestine. Orient. Pharm. Exp. Med. 18(2), 107–114 (2018)
Erolu, E.H.; Hamzaolu, E.; Aksoy, A.; Budak, Ü.; Özkul, Y.: In vitro genotoxic effects of four Helichrysum species in human lymphocytes cultures. Biol. Res. 43(2), 177–182 (2010)
Giovanelli, S.; De Leo, M.; Cervelli, C.; Ruffoni, B.; Ciccarelli, D.; Pistelli, L.: Essential oil composition and volatile profile of seven Helichrysum species grown in Italy. Chem. Biodivers. 15(5), e1700545 (2018)
Mericli, A.; Cubukcu, B.; Dortunc, T.: Flavoroids and anthocyanins of Helichrysum Sanguineum. Fitoterapia 55, 112–115 (1984)
Albayrak, S.; Sagdic, O.; Aksoy, A.; Hamzaoglu, E.: Antimicrobial and antioxidant activities of Helichrysum species from the Mediterranean region of Turkey. Asian J. Chem. 20(4), 3143 (2008)
Michel, C.; El-sherei, M.; Islam, W.; Sleem, A.; Ahmed, S.: Bioactivity-guided fractionation of the stem bark extract of Pterocarpus dalbergioides Roxb. ex Dc growing in Egypt. Bull. Fac. Pharm. Cairo Univ. 51(1), 1–5 (2013)
Trease, G.; Evans, W.: Pharmacognosy. Baillier Tindall, London (1983)
Cartwright, A.C.: The British Pharmacopoeia, 1864 to 2014: Medicines, International Standards and the State. Routledge, London (2016)
Jaradat, N.; Adwan, L.; K’aibni, S.; Shraim, N.; Zaid, A.N.: Chemical composition, anthelmintic, antibacterial and antioxidant effects of Thymus bovei essential oil. BMC Complement. Altern. Med. 16(1), 418–424 (2016)
Jaradat, N.; Hussen, F.; Al Ali, A.: Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata Decne. J. Mater. Environ. Sci. 6(6), 1771–1778 (2015)
Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C.: Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10(3), 178–182 (2002)
Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I.: Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 46(10), 4267–4274 (1998)
Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; et al.: Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 4(21), 2235–2242 (2010)
Drent, M.; Larsson, I.; William-Olsson, T.; Quaade, F.; Czubayko, F.; Strobel, W.; et al.: Orlistat (Ro 18-0647), a lipase inhibitor, in the treatment of human obesity: a multiple dose study. Int. J. Obes. Relat. Metab. Disorder 19(4), 221–226 (1995)
McCue, P.P.; Shetty, K.: Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia. Pac. J. Clin. Nutr. 13(1), 12–20 (2004)
Ojo, O.A.; Ojo, A.B.; Ajiboye, B.; Olayide, I.; Fadaka, A.: Helianthus annuus leaf Ameliorates postprandial hyperglycaemia by inhibiting carbohydrate hydrolyzing enzymes associated with type-2 diabetes. Iran. J. Toxicol. 10(5), 17–22 (2016)
Alali, F.Q.; Tawaha, K.; El-Elimat, T.; Syouf, M.; El-Fayad, M.; Abulaila, K.; et al.: Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: an ICBG project. Nat. Prod. Res. 21(12), 1121–1131 (2007)
Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E.: Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 119(1), 114–122 (2010)
Ajiboye, B.O.; Ojo, O.A.; Adeyonu, O.; Imiere, O.; Olayide, I.; Fadaka, A.; et al.: Inhibitory effect on key enzymes relevant to acute type-2 diabetes and antioxidative activity of ethanolic extract of Artocarpus heterophyllus stem bark. J. Acute Dis. 5(5), 423–429 (2016)
Ibrahim, M.A.; Koorbanally, N.A.; Islam, M.S.: Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (α-glucosidase and α-amylase) by Khaya senegalensis. Acta Pharm. 64(3), 311–324 (2014)
Ademiluyi, A.O.; Oboh, G.: Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 65(3), 305–309 (2013)
Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z.: Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87(1), 4–14 (2010)
Kim, G.-N.; Shin, M.-R.; Shin, S.H.: Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu peel. Int. BioMed Res. (2016). https://doi.org/10.1155/2016/1723042
Maiti, B.; Nagori, B.; Singh, R.: Recent trends in herbal drugs: a review. Int. J. Drug Res. Technol. 1(1), 20–31 (2017)
Huyut, Z.; Beydemir, Ş.; Gülçin, İ.: Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. (2017). https://doi.org/10.1155/2017/7616791
Ivanović, S.; Avramović, N.; Dojčinović, B.; Trifunović, S.; Novaković, M.; Tešević, V.; et al.: Chemical composition, total phenols and flavonoids contents and antioxidant activity as nutritive potential of roasted hazelnut skins (Corylus avellana L.). Foods 9(4), 430–437 (2020)
Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Hannachi, H.; Ayadi, L.; Elfalleh, W.: Comparison of three extraction protocols for the characterization of caper (Capparis spinosa L.) leaf extracts: evaluation of phenolic acids and flavonoids by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI–MS) and the antioxidant activity. Anal. Lett. 53, 1–12 (2020)
Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J.: Flavonoids as anticancer agents. Nutrients 12(2), 457–462 (2020)
Mowl, A.; Alauddin, M.; Rahman, M.; Ahmed, K.: Antihyperglycemic effect of Trigonella foenum-graecum (Fenugreek) seed extract in alloxan-induced diabetic rats and its use in diabetes mellitus: a brief qualitative phytochemical and acute toxicity test on the extract. Afr. J. Tradit. Complement. Altern. Med. 6(3), 255–261 (2009)
Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A.: Antidiabetic effect of fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J. Diabetes Res. (2019). https://doi.org/10.1155/2019/8507453
Molehin, O.R.; Elekofehinti, O.O.; Oyeyemi, A.O.: Antihyperlipidemic, antiperoxidative and hypoglycemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. FASEB J (2020). https://doi.org/10.1096/fasebj.2020.34.s1.00510
Yang, R.; Jiang, X.; He, X.; Liang, D.; Sun, S.; Zhou, G.: Ginsenoside Rb1 improves cognitive impairment induced by insulin resistance through Cdk5/p35-NMDAR-IDE pathway. Biomed. Res. Int. (2020). https://doi.org/10.1155/2020/3905719
Acknowledgements
The authors would like to acknowledge the Faculty of Medicine and Health Sciences at An-Najah National University for facilitating the accomplishment of the current study.
Author information
Authors and Affiliations
Contributions
The current research done by the authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jaradat, N., Qneibi, M., Hawash, M. et al. Chemical Composition, Antioxidant, Antiobesity, and Antidiabetic Effects of Helichrysum sanguineum (L.) Kostel. from Palestine. Arab J Sci Eng 46, 41–51 (2021). https://doi.org/10.1007/s13369-020-04707-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04707-z