Abstract
The object of this study is to experimentally evaluate the inhibition properties of Acacia concinna (A. concinna) extract in a 0.5 M sulphuric acid media for mild steel. The protective film-formation study was done using UV–Vis spectroscopy. Surface film composition and morphology were examined by scanning electron microscope and atomic force microscope. Electrochemical impedance spectroscopy (EIS) and potentiodynamic spectroscopy (PDS) were carried out to explain the combined (cathodic and anodic) nature and cathodic dominance of A. concinna. Results of electrochemical analyses showed that 250 mg/L inhibitor concentration had the highest performance and efficiency (proved to be 94% by EIS and 92% by PDS results). The formed film in this sample showed the highest hydrophobicity and literally no obvious forms of corrosion. The theoretical results showed the comparative adsorption of phytochemicals on the steel. All acquired outcomes confirmed that A. concinna extract can develop an efficient protective layer and resist the corrosion procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
We lose almost 75,000 billion USD per year due to corrosion across the world, which equals to a 3.4% global GDP. In India, around 1670 billion USD is lost due to corrosion per year, which equals to 4.2% GDP of the country [1]. Steel is effectively accessible with its exceptional mechanical properties in manufacturing procedure and construction [2]. The principal issue with utilizing steel is its lower corrosion-resistance nature, as it gets rapidly corroded when comes into contact with an acidic medium, for example, sulphuric acid [3]. It is notable that the acidic solutions are broadly utilized in different industrial processes for pickling, descaling, and cleaning. The inhibitor forms a protective film on the surface of the steel using their π-electrons framework and heteroatoms, for example, O, P, S, N [4,5,6,7]. The inhibitor adsorption on MS surface is either physical (physisorption) or chemical (chemisorption).The plant-extract corrosion inhibitor is eco-friendly, biodegradable, and non-toxic. It is easily accessible from our surrounding, hence cost-effective [8,9,10,11]. Reason to choose 0.5 M sulphuric acid was due to this being the lowest concentration with a significant corrosion rate which does not damage the electrochemical workstation. A few investigations were reported by different scientists utilizing such materials as an efficient corrosion inhibitor for various metals in different media. However, majority of corrosion inhibitors gave good inhibition performance at higher inhibitor concentration. On the other hand, synthetic inhibitors can significantly inhibit corrosion, but are costly.
A. concinna pod is a climbing shrub, belonging to the Mimosaceae family and commonly known as Shikakai. These are usually available all around India and East Asian continent. A. concinna contains Acacia acid, Acacidiol, and Acacigenin-B as its main phytochemical constituents [12, 13]. Figure 1 represents A. concinna and its main secondary metabolites.
The present work is aimed to examine the extract of A. concinna for corrosion inhibition behaviour in 0.5 M sulphuric acid corrosive medium which relies on electrochemical methods followed by weight-loss estimates. Adsorption of A. concinna extract and protective film formation on the surface of steel were examined using AFM and SEM which give important information about the surface morphology. UV–Vis spectroscopy was used to examine the adsorption. The theoretical study is an add-on to compare the film-formation activeness of the main phytochemical. Using A. concinna is an eco-friendly, cost-effective corrosion inhibitor.
2 Experimental Studies
2.1 Preparation of Extract and Electrochemical Cell
Dry A. concinna, as verified by Dr. A. A. Bhatt at Lovely Professional University, Punjab (INDIA), were thoroughly washed with tap water followed by sterile distilled water and then dried under shade. Their leaves were crushed to a powdered form. Static extraction was performed using approximately 350 g of dry A. concinna in methanol (350 ml) in a round-bottom flask connected to a Soxhlet apparatus for 94 h at 69 °C. The filtrate was gathered and concentrated utilizing the rotary evaporator at controlled conditions. From this technique, around 9% of yield was obtained, with a pH value of 7.5. 1 cm × 1 cm × 0.03 cm steel coupons were utilized in weight-loss estimates. The chemical composition of steel coupon was recorded as (wt%) Ni = 0.27, Si = 0.39, Cr = 0.45, Cu = 0.43, P = 0.12, C = 0.08, Mn = 0.43, and balance Fe. The steel coupons were abraded with different grades of emery papers (100–1200) and then washed with acetone followed by distilled water, dried in oven, and then placed in a desiccator. The corrosive solution was prepared by diluting sulphuric acid to 0.5 M. The corrosive media were prepared at different concentrations (50–250 mg/L) by diluting A. concinna extract with 0.5 M sulphuric acid.
2.2 Weight-Loss Measurement
This measurement was taken according to the ASTM standard G 31–72 for 24 h [14]. All evaluations were carried out at 298 ± 0.5 K thermostat. The average value was taken by repeating the experiment three times. The following formula was employed to obtain the corrosion rate:
The effectiveness of the inhibition was obtained by applying the following formulas:
where the surface coverage values denoted by θ, \( C_{\text{R}}^{i} \), and \( C_{R}^{0} \) are used for the corrosion rate of the MS coupons in the inhibitor and blank solutions, respectively. Adsorption isotherms between C and C/θ are shown in Fig. 4. The value of slope and regression coefficient (R2) close to 1 suggests a monolayer adsorption on the MS surface [15]. This can be described by following equation:
where C represents inhibitor concentration, \( K_{\text{ads}} \) represents the equilibrium adsorption constant, and θ represents the surface coverage.
2.3 CH-Instrumental Studies
The electrochemical techniques (Tafel: polarization estimations and EIS: impedance spectroscopy) were performed by using the CHI760C electrochemical workstation. MS coupons (1 cm2) were immersed in 0.5 M sulphuric acid solution along with a mixture of different concentrations of A. concinna extract for 60 min. At 298 ± 0.5, the values of open circuit potential (OCP) with respect to the saturated calomel electrode (SCE) were noted. Corrosion inhibition measurements through EIS techniques were applied in a cell with SCE as the reference electrode, steel as a working electrode, and platinum as the counter electrode. Scanning frequencies from 100 kHz to 0.01 Hz are used for obtaining the Nyquist and Bode plots. A 5 mV signal amplitude perturbation at OCP was considered in EIS measurement. The polarization plots were obtained in the potential range of − 250 to + 250 mV with respect to corrosion potential (Ecorr) and a scan rate of 1 mV/s. EIS and polarization tests were repeated three times to get the average values. For the calculation of inhibition efficiency, following equations were used:
The efficiency of corrosion inhibition (IE %) in the electrolytes was calculated by Eq. 6 on the basis of EIS test results [16].
2.4 Surface Investigations
For the surface examination, AFM, followed by SEM, micrographs of the pre-treated surfaces of MS coupons in 0.5 M sulphuric acid, in the presence and absence of inhibitor extract (500 mg/L), at 298 ± 0.5 K were taken.
2.5 UV–Visible Spectroscopic Studies
Shimadzu UV-1800 spectrophotometer with a range of 200–400 nm was used for recording the absorption spectra. The UV–Vis measurement was taken at 298 K.
2.6 Theoretical Studies
Theoretical studies were done for a deeper understanding of the adsorption mechanism. Quantum chemical calculations were performed as theoretical studies. It is a well-known fact that the plant extracts have several phytochemical constituents. For theoretical examinations, we followed the existence of three main phytochemicals in A. concinna extract. For theoretical studies, density functional theory (DFT) at a B3LYP function was used 6-31G+(d,p) basis set utilizing Hyperchem 8.0 software. Followings equations have been used to calculate different quantum chemical parameters [17].
where \( \chi_{\text{inh}} \) and \( \eta_{\text{inh}} \) represent the electronegativity and hardness of inhibitor molecule, whereas \( \chi_{\text{inh}} \) and \( \eta_{\text{Fe}} \) mean the electronegativity and hardness of iron, respectively.
3 Results and Discussion
3.1 Spectroscopic Studies
UV–Vis absorption spectrum (Fig. 2) was obtained for solutions containing the extract of A. concinna in 0.5 M sulphuric acid, before and after the corrosion test, to detect the presence of organic compounds and identify the possible adsorption of phytochemicals on the steel surface. Maximum values of intense absorption peaks recorded for solution containing A. concinna extract and 0.5 M sulphuric acid appeared at 278 nm. The peak assigned at 278 nm corresponded to the n − π* transitions attributed to the functional groups of phytochemicals present in the A. concinna extract, for example, C=O. These results reveal some clear evidences about the possibility of complex formation between iron and phytochemicals of A. concinna extract [18].
3.2 Weight-Loss Experiment and Adsorption Parameters
The weight-loss technique is the important and easiest procedure to calculate corrosion-inhibition efficiency of the natural corrosion inhibitor at different concentrations.
Table 1 shows that with the increment of inhibitor concentration the corrosion rate gradually decreases and the inhibition ability increases. This happens because of the adsorption of phytochemicals on the MS surface. When inhibitor concentration is zero, the highest corrosion rate (11.33 mm year−1) was observed; on the other hand, when the concentration of inhibitor was highest (250 mg/L), the lowest corrosion rate (1.32 mm year−1) was observed. The highest corrosion inhibition effectiveness of 91.13% was achieved at 250 mg/L. For further confirmation of adsorption mechanism of A. concinna, Langmuir isotherms were used.
In the previous study, M. fragrans showed 83.27% inhibitory effect at 500 mg/L with Kads (9.95 Lg−1) [19] whereas A. concinna showed a 94% inhibitory effect at 250 mg/L with Kads (41.09 Lg−1), which confirms its better performance in the same corrosive medium (Fig. 3).
3.3 Potentiodynamic Polarization Technique (Tafel)
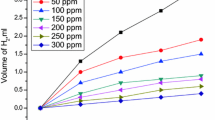
The corrosion rates of the inhibitor concentrations and polarization curves are provided after 1 h immersion time of the MS coupons (Fig. 4). The lowest corrosion current density was recorded at 250 mg/L concentration of A. concinna extract which confirmed its highest inhibitory effect. The slight shift of Tafel slope towards cathodic side shows the predominant cathodic inhibitory action of the A. concinna extract. Cationic and anion reaction rates at the surface of steel are explained by the curves of different concentrations of sample. It is found that the cathodic reaction was highly affected, that is, the rate of releasing hydrogen gas at cathode decreased resulting in a reduced rate of corrosion. The displacement of corrosion potential of inhibitor with respect to the blank is within the range of 0–85 mV, indicating that the inhibitor behaved as a mixed type of inhibitor. Tafel provided various parameters such as corrosion current density (icorr), corrosion potential (Ecorr), anodic Tafel slope (βa), and cathodic Tafel slopes (βc) [20,21,22,23,24,25,26].
Table 2 shows that at 250 mg/L inhibitor concentration (C) maximum decrement in icorr. The changes in the position of anodic and cathodic sides explain the types of inhibition effects. The change in the value of βa and βc significantly explains the combined cathodic and anodic effects of inhibitor. This indicates that both iron dissolution and hydrogen evolution reactions were affected by adsorption of active components of S. chirata extract. In the previous study, M. fragrans showed 87.42% inhibitory effect at 500 mg/L with icorr (112.00 μA cm−2) [19] whereas S. chirata showed 92.73% inhibitory effect at 250 mg/L with icorr (68.35 μA cm−2), which confirms its better performance towards the same corrosive medium.
3.4 Electrochemical Impedance Spectroscopy (EIS)
For impedance spectroscopy, MS coupons were exposed to the corrosive solution protected by A. concinna extract [27,28,29,30,31,32]. Nyquist and Bode plots were provided by EIS analysis and are presented in Fig. 5. Figure 5 shows that the solution inhibited with 250 mg/L A. concinna extract provided capacitive loops with larger diameter. The enlargement of capacitive loop using the A. concinna extract was greater than the blank. A concentration of 250 mg/L A. concinna extract leads to the highest enlargement in the capacitive loop, implying maximum inhibition effect of corrosion natural corrosion inhibitor. Electrochemical parameters include polarization resistance (Rp), solution resistance (Rs), double-layer capacitance (Cdl), and phase shift (n) of inhibitor layer.
According to Fig. 5 and Table 3, the use of a mixture of 250 mg/L A. concinna extract gives the maximum corrosion inhibition efficiency of 94% and the highest enlargement of Rp [33]. The surface corrosion diminished significantly in the presence of aggressive solution. Hence, it can be deduced that blocking the active sites via extract components protected the metal surface from corrosion attacks, and because shape of semicircles is same the mechanism of corrosion does not change during the process. Another result that can be perceived from Table 3 is the decrement of Cdl value in the presence of A. concinna extract. This shows that the molecules of inhibitor successfully adsorbed on the metal surface and decreased the local dielectric constant.
By enhancing the extract concentration, the surface coverage was improved based on suitable physisorption and chemisorption of phytochemicals of A. concinna extract [34, 35]. The replacement of water molecules with inhibitive molecules of A. concinna extract is the main reason for the Cdl decrement, and therefore, corrosion inhibition effect increases. The obtained results show chemisorption of A. concinna extract on the MS surface with the help of coordination bond formation. This is due to sharing of heteroatomic non-bonding electrons of A. concinna phytochemical to the vacant d orbital of Fe. The physisorption process is due to electrostatic interaction between protonated phytochemical and anionic steel surface. These results confirm the synergic inhibition effect of A. concinna extract on steel in 0.5 M sulphuric acid solution [36,37,38]. In a previous study, M. fragrans showed 87.88% inhibitory effect at 500 mg/L with Rct (128.88 Ω cm2) [39] whereas A. concinna showed 94% inhibitory effect at 250 mg/L with Rct (313.00 Ω cm2), which confirms its better performance towards the same corrosive medium.
3.5 AFM and SEM Studies
Figure 6a displays the AFM images along with SEM micrograph of polished MS with a smooth surface and minimum surface roughness (2.99 nm). Figure 6b displays the AFM and SEM images of MS coupon dipped in a solution of 0.5 M sulphuric acid for 24 h. The SEM and AFM images of MS surface were badly corroded by the sulphuric acid. In this case, maximum surface roughness (138.81 nm) appears due to formation of iron hydroxide and oxide on the metal surface, while Fig. 6c displays the AFM and SEM images of the MS specimens dipped for the same period of time interval in 0.5 M sulphuric acid solution containing 250 mg/L of A. concinna extract with a lower surface roughness (32.37 nm), because of blocking of active site and protecting metal from oxide and hydroxide formation or corrosion. The surface roughness value of steel was drastically reduced after adding extract in aggressive media. This result supports the weight-loss data, where, in the presence of 250 mg/L inhibitor concentration, highest inhibition efficiency was observed [16, 39,40,41].
4 Computational Study Explanation
Plant extracts have lots of phytochemical components. There are three main components of A. concinna selected for the theoretical study as per literature. A. concinna pod contains Acacia acid, Acacidiol, and Acacigenin-B as the main phytochemical components. Figure 7 shows the optimized, HOMO, and LUMO orbitals of the Acacia acid, Acacidiol, and Acacigenin-B.
Table 4 shows a high value of EHOMO for Acacigenin-B (− 0.19 eV) indicating its high capacity to donate the electrons to steel. Acacigenin-B has the lowest values of ELUMO (− 4.73 eV) showing the highest capacity to accept electrons from Fe. Acacigenin-B (4.54 eV) shows the lower value of ΔE indicating its strong influence of the [Fe-Acacigenin-B] complex. Figure 8 shows the suggested mechanism for the adsorption procedure on the MS surface.
5 Conclusion
-
The anti-corrosive film of A. concinna extract on MS surface in 0.5 M sulphuric acid was analysed by UV adsorption studies.
-
SEM micrographs revealed that almost no obvious corrosion products formed at 250 mg/L of A. concinna extract sample on the surface of the steel.
-
AFM observations show that 250 mg/L of A. concinna extract sample on steel had the lowest roughness in comparison with blank.
-
The electrochemical investigation revealed that 250 mg/L had the highest corrosion inhibition in the 0.5 M sulphuric acid solution. Efficiencies of about 94% through EIS and 92% through PDS technique were calculated for this sample.
References
Koch, G.; et al.: International measures of prevention, application, and economics of corrosion technologies study. NACE 1–226 (2013)
Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadi, S.: Enhanced corrosion resistance of steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros. Sci. 114, 133–145 (2017)
Deng, S.; Li, X.: Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 55, 407–415 (2012)
Rose, K.; Kim, B.S.; Rajagopal, K.; Arumugam, S.; Devarayan, K.: Surface protection of steel in acid medium by Tabernaemontana divaricata extract: physicochemical evidence for adsorption of inhibitor. J. Mol. Liq. 214, 111–116 (2016)
Garai, S.; Garai, S.; Jaisankar, P.; Singh, J.K.; Elango, A.: A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of steel in acid solution. Corros. Sci. 60, 193–204 (2012)
Patel, N.; Rawat, A.; Jauhari, S.; Mehta, G.: Inhibitive action on Bridelia retusa leaves extract on corrosion of steel in acidic media. Eur. J. Chem. 1, 129–133 (2010)
Ibrahim, T.; Alayan, H.; Al-Mowaqet, Y.: The effect of thyme leaves extract on corrosion of steel in HCl. Prog. Org. Coat. 75, 456–462 (2012)
Al-Otaibi, M.S.; et al.: The effect of temperature on the corrosion inhibition of steel in 1 M HCL solution by Curcuma longa extract. Int. J. Electrochem. Sci. 5, 847–859 (2013)
Oguzie, E.E.; Chidiebere, M.A.; Oguzie, K.L.; Adindu, C.B.; Momoh-Yahaya, H.: BioLC-MS extracts for materials protection: corrosion inhibition of steel in acidic media by Terminalia chebula extracts. Chem. Eng. Commun. 201, 790–803 (2014)
Garai, S.; Garai, S.; Jaisankar, P.; Singh, J.K.; Elango, A.: A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of steel in acid solution. Corros. Sci. 60, 193–204 (2012)
Qing, Y.; Zang, S.; Tan, B.; Chen, S.: Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 133, 6–16 (2018)
Anjanetulu, A.S.R.; Row, L.R.; Singh, A.: Acacidiol, a new nor-triterpene from the sapogenins of Acacia concinna. Phytochemistry 18, 1199–1201 (1979)
Anjaneyulu, A.S.R.; Bapuji, M.; Row, L.R.; Sree, A.: Structure of acacigenin-B, a novel triterpene ester isolated from Acacia concinna. Phytochemistry 18, 463–466 (1979)
Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R.: Musa paradisica peel extract as green corrosion inhibitor for steel in HCl solution. Corros. Sci. 90, 107–117 (2015)
Saxena, A.; Prasad, D.; Haldhar, R.: Investigation of corrosion inhibition effect and adsorption activities of Achyranthes aspera extract for steel in 0.5 M H2SO4. J. Fail. Anal. Prev. 18, 957–968 (2018)
Zahra, S.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.: Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: a complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 69, 18–31 (2019)
Haldhar, R.; Prasad, D.; Saxena, A.; Kumar, A.: Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for steel in 0.5 M H2SO4 solution. Sustain. Chem. Pharm. 9, 95–105 (2018)
Haldhar, R.; Prasad, D.; Saxena, A.: Armoracia rusticana as sustainable and eco-friendly corrosion inhibitor for steel in 0.5 M sulphuric acid: experimental and theoretical investigations. J. Environ. Chem. Eng. 6, 5230–5238 (2018)
Haldhar, R.; Prasad, D.; Saxena, A.: Myristica fragrans extract as an eco-friendly corrosion inhibitor for steel in 0.5 M H2SO4. J. Environ. Chem. Eng. 6, 2290–2301 (2018)
Bhardwaj, N.; Prasad, D.; Haldhar, R.: Study of the Aegle marmelos as a green corrosion inhibitor for steel in acidic medium: experimental and theoretical approach. J. Bio-and Tribo-Corros. 4, 1–10 (2018)
Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M.: Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 255, 185–198 (2018)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M.: Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: electrochemical and theoretical studies. J. Mol. Liq. 277, 895–971 (2019)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M.: A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 279, 603–624 (2019)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.: A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 282, 366–384 (2019)
Bahlakeh, G.; Ramezanzadeh, B.; Dehghani, A.; Ramezanzadeh, M.: Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala seed extract for mild steel corrosion in HCl solution: detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq. 283, 174–195 (2019)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.: Green Eucalyptus leaf extract: a potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 130, 107339 (2019)
Haldhar, R.; Prasad, D.; Saxena, A.; Singh, P.: Valeriana wallichii root extract as a green and sustainable corrosion inhibitor for steel in acidic environments: experimental and theoretical study. Mater. Chem. Front. 2, 1225–1237 (2018)
Saxena, A.; Prasad, D.; Haldhar, R.: Investigation of corrosion inhibition effect and adsorption activities of Cuscuta reflexa extract for steel in 0.5 M H2SO4. Bioelectrochemistry 124, 156–164 (2018)
Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M.: Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 255, 185–199 (2018)
Saxena, A.; Prasad, D.; Haldhar, R.: Use of Asparagus racemosus extract as green corrosion inhibitor for steel in 0.5 M H2SO4. J. Mater. Sci. 53, 8523–8535 (2018)
Deng, S.; Li, X.: Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 55, 407–415 (2012)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M.: Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: electrochemical and theoretical studies. J Mol Liq. 277, 895–911 (2019)
Kumar, R.; Yadav, O.S.; Singh, G.: Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for steel in acidic media: a cumulative study. J. Mol. Liq. 237, 413–427 (2017)
Joseph, O.O.; Fayomi, O.S.I.; Joseph, O.O.; Adenigba, O.A.: Effect of Lecaniodiscus cupaniodes extract in corrosion inhibition of normalized and annealed steels in 0.5 M HCl. Energ. Proce. 119, 845–851 (2017)
Najmeh, A.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.: Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1 M HCl solution: a detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Taiwan Inst Chem Eng 95, 252–272 (2019)
Mohammad, R.; Bahlakeh, G.; Sanaei, Z.; Ramezanzadeh, B.: Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl. Surf. Sci. 463, 1058–1077 (2019)
Qiang, Y.; Zhang, S.; Wang, L.: Understanding the adsorption and anticorrosive mechanism of DNA inhibitor for copper in sulfuric acid. Appl. Surf. Sci. 492, 228–238 (2019)
Qiang, Y.; Zhang, S.; Zhao, H.; Tan, B.; Wang, L.: Enhanced anticorrosion performance of copper by novel N-doped carbon dots. Corros. Sci. 161, 108–193 (2019)
Haldhar, R.; Prasad, D.; Saxena, A.; Kaur, A.: Corrosion resistance of steel in 0.5 M H2SO4 solution by plant extract of Alkana tinctoria: experimental and theoretical studies. Eur. Phys. J. Plus 133, 1–18 (2018)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M.: Electronic/atomic level fundamental theoretical evaluations combined with electrochemical/surface examinations of Tamarindus indiaca aqueous extract as a new green inhibitor for mild steel in acidic solution (HCl 1 M). J. Taiwan Inst. Chem. E. 102, 349–377 (2019)
Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M.: Detailed macro-/micro-scale exploration of the excellent active corrosion inhibition of a novel environmentally friendly green inhibitor for carbon steel in acidic environments. J. Taiwan Inst. Chem. E. 100, 239–261 (2019)
Acknowledgement
The authors are grateful to Dr. Gurmeet Singh, Ex-HOD of Department of Chemistry at University of Delhi, Delhi, India, for offering the laboratory resource for impedance workstation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interests.
Rights and permissions
About this article
Cite this article
Haldhar, R., Prasad, D. & Bhardwaj, N. Surface Adsorption and Corrosion Resistance Performance of Acacia concinna Pod Extract: An Efficient Inhibitor for Mild Steel in Acidic Environment. Arab J Sci Eng 45, 131–141 (2020). https://doi.org/10.1007/s13369-019-04270-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-019-04270-2