Abstract
Epoxidation of uncommon vegetable oil like Citrullus lanatus seed oil (CLO) offers an inherent biodegradable and vastly applicable novel product. In the present study, CLO was epoxidized in the presence of acetic acid, sulphuric acid and hydrogen peroxide. Temperature, sulphuric acid loading and stirring speed were considered as significant process parameters. Response surface methodology-based central composite design was employed to statistically optimize these parameters. Response surface graphs displayed the effect of parameters on the conversion of iodine value and relative conversion to oxirane oxygen. Epoxidized product with the maximum conversion of iodine value of 86.7% (predicted, 87.8%) and relative conversion to oxirane oxygen of 84.2% (predicted, 85.2%) was attained at 3% of sulphuric acid loading, 65 °C and 1200 rpm within 9 h of reaction time. FTIR analysis revealed the characteristic peak of the epoxide group with disappeared peak for double bond. 1H-NMR and 13C-NMR analyses confirmed the maximum conversion of the double bond to oxirane group of an epoxide. The present study demonstrates a promising epoxide from Citrullus lanatus seed oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Modified vegetable oils have captivating and desirable applications in the chemical industry. The ready availability and obtainability from renewable resources at a reasonably low cost make them a better alternative for conventional products. Epoxidation is one of the cost-effective techniques used to modify the vegetable oils to explore their effectiveness for various applications. In epoxidation reactions, oxygen is added to a carbon–carbon double bond to form a carbon–oxygen bond [1, 2]. Epoxidized vegetable oils can be used as stabilizers and plasticizers in polymers, as additives in lubricants, as components in plastics and urethane foams and as intermediates for various products [3, 4]. Different vegetable oils are developed as epoxides on an industrial scale. Epoxidized soybean oil is produced worldwide for one of the major applications as a plasticizer and a stabilizer for polymers like polyvinyl chloride (PVC) [5]. Okieimen et al. [6] have performed in situ epoxidation of rubber seed oil by peroxyacetic acid. Lligadas et al. [7] reported formic acid catalysed epoxidation of linseed oil. Chavan and Gogate [8] synthesized epoxidized sunflower oil in an ultrasound reactor. The easy and cheap availability of vegetable oil decides the profitability of the epoxidation process. Citrullus lanatus (watermelon) seeds are mostly discarded either as cheap animal feed or just thrown away. The vegetable oil obtained from these seeds is one of the unusual vegetable oils, and its epoxidation will explore its competency as a plasticizer and stabilizer.
Citrullus lanatus is tropical or subtropical species of fruits with high water content. Seeds obtained from them are used for biodiesel production at the insignificant level in few African countries and in the Middle East, and hence, the industrial-scale consideration of their oil will deliver a productive utilization of seeds [9, 10]. Due to its moisturizing characteristics, Citrullus lanatus seed oil (CLO) is also rarely used in skin care products. It is typically comprised of polyunsaturated fatty acids along with sterols, diglycerides, monoglycerides and phospholipids. It shows high iodine value indicating high unsaturation of fats and oils [11]. Therefore, CLO will be a cost-effective possibility rather than the wastage of seeds.
Epoxidized Citrullus lanatus seed oil might reduce the demand for phthalates, which are disparaged for their toxic effects. The most widely used method for epoxidation is in situ synthesis with appropriate catalysts like sulphuric acid or the acidic ion exchange resin [12, 13]. Among them, sulphuric acid exhibits a very effective catalytic activity [14,15,16,17]. In this method, process parameters like temperature, stirring speed and catalyst concentration have effects during epoxidation procedure [18, 19]. Therefore, the determination of their optimum levels is a necessity for an epoxidation process to deliver a potential epoxide.
The objective of this study was to synthesize epoxidized product from the renewable resource, Citrullus lanatus seed oil. Further objectives were to study the effect of process parameters like temperature, stirring speed and catalyst concentration on epoxidation using a statistical approach; characterize the epoxidized product by FTIR, 1H-NMR and 13C-NMR analyses.
2 Materials and Methods
2.1 Materials
Citrullus lanatus seed oil (CLO) was purchased from a local store at Rourkela, India. Hydrogen peroxide (50%), sulphuric acid (98%) and glacial acetic acid (99%) were obtained from Merck Chemicals, India. All other analytical-grade chemicals were procured from Merck chemicals, India, and Fischer scientific, India.
2.2 Experimental Set-up
The epoxidation reactions were performed in a reactor comprising a magnetically stirred three-neck round-bottom flask of 250 ml capacity, reflux condenser, constant pressure-equalizing funnel and thermometer. The experimental set-up is presented in Fig. 1. A water bath was prepared in crystallizing dish with temperature control of ± 1.5 °C, and the reactor was placed in a water bath. The water bath and reactor temperature were maintained at the desired level so that both were consistent.
2.3 Experimental Procedure
The requisite quantities of Citrullus lanatus seed oil, acetic acid with mole ratio to double bond 0.5:1 and the sulphuric acid were added to the reactor. The mixture was magnetically stirred for half an hour using a magnetic stirrer. Then, the calculated amount of 50% aqueous hydrogen peroxide with mole ratio to double bond 1.5:1 was added dropwise using a constant pressure-equalizing funnel. It was finished within 30 min, and this was considered as the start of the reaction (0 h). Subsequently, the reaction was carried out for 9 h as preliminary experiments based on one factor a time method suggested the maximum conversion of double bonds without further cleavage of oxirane ring occur at 9 h of reaction time. The samples were withdrawn at each 3-h interval and were extracted with diethyl ether and washed with water in a separating funnel until the pH of aliquot reached 7. They were analysed for iodine value and oxirane oxygen content. During operation, reaction mixture might become explosive since peracetic acid is potentially unstable in nature. Therefore, the constant stirring speed was maintained throughout the experiment. The final reaction mixture was cooled and washed in a separating funnel until mixture with the pH 7 was obtained.
2.4 Design of Experiments Using RSM
The process parameters have a substantial effect during the epoxidation process and need to be optimized to attain the maximum yield [20]. The effect of temperature, stirring speed and loading of sulphuric acid on the rate of reaction was taken into account for epoxidation of Citrullus lanatus seed oil. The full factorial face-centred central composite design was exercised to explore the effect of factors on the reaction and to identify their optimum levels. A set of 20 experiments and their experimental design are given in Table 1. Conversion of iodine value and relative conversion to oxirane oxygen were considered as responses. The second-order polynomial equation was used to estimate the correlation between the process parameters, and the response is as follows:
where Y denotes the response, Xi and Xj are input variables, β0 is a model intercept, βi is factor estimates, βii is the ith quadratic coefficient, and βij is the ijth interaction coefficient. The statistical software Design-Expert 7.0.0 (Stat-Ease Inc., Minneapolis, USA) (trial version) was used for designing experiments and analysing the interaction between factors using the three-dimensional response surface graphs.
2.5 Analytical Techniques
The iodine value was determined in terms of percentage of the total conversion of unsaturation based on AOCS Official Method Tg 1-64. The percentage of oxirane oxygen was derived by a direct method involving hydrobromic acid solution in glacial acetic acid [21]. Relative conversion to oxirane (RCO) was calculated as per the following equation:
where OOe is the experimental oxirane oxygen; OOt is the theoretical maximum oxirane oxygen, which was calculated from the equation:
where Ai (126.9) and A0 (16.0) are the atomic weights of iodine and oxygen, respectively, and IV0 is the initial iodine value of the Citrullus lanatus seed oil.
3 Results and Discussion
3.1 RSM-Based Optimization of Process Parameters
The effect of temperature, stirring speed and loading of sulphuric acid on the rate of reaction was investigated using response surface methodology (RSM). Experimentally obtained results of conversion of iodine value (%) and relative conversion to oxirane oxygen (%) are given in Table 1. The second-order polynomial equation that predicts the epoxidation of CLO within the scope of present process conditions is as follows:
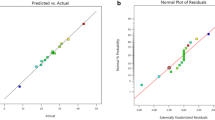
where Y1 and Y2 are the conversion of iodine value (%) and relative conversion to oxirane oxygen (%), respectively. X1, X2 and X3 are stirring speed, H2SO4 loading and temperature, respectively. The adequacy of the model was assessed based on the diagnostic plots of the normal residuals, internally studentized residuals versus predicted, internally studentized residuals versus run number and predicted versus actual (experimental) as shown in Figs. 2 and 3 for responses Y1 and Y2, respectively. The obtained data were analysed to evaluate the normality of the residuals (Figs. 2a and 3a). Liu et al. [22] elucidated that the experimental responses fitting derived model should follow a straight line at a normal distribution. The present data formed a straight line, and the normal plot of residuals for the conversion of iodine value and relative conversion to oxirane oxygen were normally distributed. It designates no deviation of the variance and suggests the obtained data delivered appropriate analysis.
The residuals versus run number and residuals versus predicted plot (Figs. 2b, c and 3b, c) show scattered patterns with consistently close to zero-axis. It confirmed the initial assumption of the absence of constant variance [23]. The predicted versus actual plots showed excellent agreement (Figs. 2d and 3d). The R2 (correlation coefficient) value for the regression model was 0.9659 and 0.9705 for responses Y1 and Y2, respectively, indicating experimental results were best fitted by the quadratic models. Hence, the adequacy of the obtained models was well established.
Analysis of variance (ANOVA) for responses is given in Tables 2 and 3 for responses Y1 and Y2, respectively. The lack of fit F value for the conversion of iodine value (%) and relative conversion to oxirane oxygen (%) were 3.92 and 3.51, respectively, and found non-significant. A coefficient of variation and adequate precision measures specified that the performed experiments were highly consistent.
Response surface graphs describing the effect of two parameters on the response (Y), keeping the residual parameter at their zero levels, were plotted (Fig. 4). Figure 4a exhibits the effect of stirring speed and H2SO4 loading on epoxidation reaction. It was observed that at low stirring speed (1000 rpm) and sulphuric acid loading (2.5%), the conversion of iodine value was less and it was increased as levels of parameters were increased further. At stirring speed (1200 rpm) and sulphuric acid loading (3%), maximum conversion of iodine value was obtained. Similarly, Fig. 4b shows the effect of stirring speed and H2SO4 loading on conversion to oxirane oxygen. At stirring speed (1200 rpm) and sulphuric acid loading (3%), maximum conversion to oxirane oxygen (84.2%) was obtained. This indicates that the given conditions of stirring speed and catalyst loading have an effect on mass transfer and subsequently on an epoxidation reaction. Chavan and Gogate [8] and Goud et al. [18] also concluded that the stirring speed and catalyst loading have a substantial effect on epoxidation.
3-D surface plot: effect of a stirring speed and H2SO4 loading on iodine value, b stirring speed and H2SO4 loading on oxirane oxygen, c stirring speed and temperature on iodine value, d stirring speed and temperature on oxirane oxygen e temperature and H2SO4 loading on iodine value, f temperature and H2SO4 loading on oxirane oxygen
Figure 4c demonstrates the effect of stirring speed and temperature on the reaction. It was perceived that an increase in temperature and stirring speed exhibited a promising influence on the formation of peracetic acid. Maximum conversion of iodine value was obtained at 65 °C and 1200 rpm. Similarly, at these levels, maximum conversion to oxirane oxygen was observed depicting that these are optimum levels (Fig. 4d). At higher temperature (75 °C), oxirane ring might be unstable, resulting in the less conversion to iodine value and oxirane oxygen. Goud et al. [9] suggested that better epoxidation could be in the reaction temperature range of 55–65 °C. de Haro et al. [24] also concluded that the epoxidation reaction performance differs with temperature and at moderate to low reaction temperature, the better yield would be attained as the conversion to oxirane oxygen increased with the reaction time. Figure 4e and f illustrates the effect of temperature and H2SO4 loading on epoxidation reaction. The increase in catalyst (H2SO4) loading and temperature leads to an intensification in the conversion of iodine value and oxirane cleavage up to an optimum level of 3% and 65 °C, respectively. Further, an increase in parameter levels decreased responses significantly. Dinda et al. [25] reported that the increase in loading of sulphuric acid beyond optimum values might lead to cleavage in oxirane ring. This demonstrates that the optimum loading of sulphuric acid (3%) leads to higher oxirane content in shorter reaction time.
3.2 Validation of Experimental Design
Epoxidation of CLO was performed at obtained optimum levels of process parameters: 3% of sulphuric acid loading, 65 °C and 1200 rpm for the duration of 9 h. It was observed that the maximum conversion of iodine value was 86.7% (predicted, 87.8%) and relative conversion to oxirane oxygen was 84.2% (predicted, 85.2%) (Fig. 5). These results validated the obtained models, and design as experimental results are close to predicted ones.
3.3 FTIR Analysis
Citrullus lanatus seed oil (CLO) and its epoxide (ECLO) were analysed by FTIR spectroscopy (PerkinElmer, USA). The obtained FTIR spectra for both the compounds were compared as depicted in Fig. 6. The characteristic peak of C=C around 3008.58 cm−1 was observed in the spectra for CLO. Okieimen et al. [6] observed the characteristic peak of C=C at 3009 cm−1 for rubber seed oil. Yang et al. [26] also observed C=C peak (3008 cm−1) for non-epoxidized microbial oil. In the ECLO spectra, C=C peak disappeared, and the characteristic peak of the epoxide group (C–O–C) was observed around 823.28 cm−1. This indicates that most of the double bond was transformed in the formation of the epoxy groups. Vleck and Petrovic [27] confirmed the occurrence of epoxy groups at 822–833 cm−1. Turco et al. [28] also observed a peak defining epoxy ring at 819 cm−1. Thus, the present study is consistent with the earlier reports and it emphasizes the effective epoxidation of CLO.
3.4 1H-NMR and 13C-NMR Spectra Analyses
1H-NMR and 13C-NMR spectra of CLO and epoxidized product (ECLO) in deuterated chloroform (CDCl3) were determined by nuclear magnetic resonance (NMR) spectroscopy (Bruker Biospin International, Switzerland). Figure 7 depicts a 1H-NMR spectra of CLO and ECLO. The characteristic peak of C=C was detected at 5.3–5.4 ppm (i) along with the peak for methylene protons of glycerine unit at 4.1–4.3 ppm (g) in the CLO sample [29]. In the spectra of ECLO, C=C peak almost disappeared but not absolutely indicated that the C=C bonds from unsaturated fatty acid were converted and still reaction was not complete as weak C=C bonds might be present which is supported by the results obtained for conversion of iodine value and oxirane oxygen for ECLO. Yang et al. [26] also reported the presence of weak double bonds in the epoxidized product. Meanwhile, the peak for the epoxy ring (C–O–C) at 2.8–3.1 ppm (i′) was observed in the ECLO sample, while no peak was detected in the CLO sample. 13C-NMR spectra of CLO and ECLO are shown in Fig. 8. It the spectra for CLO, carbon attached to C=C was designated at the peak of 130 ppm (i), while for ECLO, it became weaker in intensity which exemplified that most of the C=C bonds were oxidized. The new peaks at 55 and 58 ppm (i′) were observed for C–O–C group. 1H-NMR and 13C-NMR spectra were found to be consistent with each other. In addition, these results agree well with the earlier reports [30, 31]. It confirms the formation of epoxide (ECLO) resulting from the epoxidation reaction. The proposed structure of CLO and ECLO based on the NMR spectra is shown in Fig. 9 with the NMR labels.
4 Conclusion
The epoxidation of Citrullus lanatus seed oil was carried out in the magnetically stirred reactor. The central composite design was exercised to find optimum levels of process parameters. The iodine value and relative conversion to oxirane oxygen were determined for a total reaction time of 9 h. ECLO with the maximum conversion of iodine value (86.7%) and relative conversion to oxirane oxygen (84.2%) were obtained at 3% of sulphuric acid loading, 65 °C and 1200 rpm. The characterization of ECLO by FTIR, 1H-NMR and 13C-NMR analyses confirmed the transformation of double bonds of fatty acid into the epoxy group. Eventually, it is concluded that using the conventional chemical process of epoxidation, Citrullus lanatus seed oil was effectively transformed in the product (ECLO), which could be employed in the form of value-added chemicals.
References
Chua, S.C.; Xu, X.; Guo, Z.: Emerging sustainable technology for epoxidation directed toward plant oil-based plasticizers. Process Biochem. 47(10), 1439–1451 (2012). https://doi.org/10.1016/j.procbio.2012.05.025
Zhang, H.; Zhu, F.; Fu, Q.; Zhang, X.; Zhu, X.: Mechanical properties of renewable plasticizer based on ricinoleic acid for PVC. Polym. Test. 76, 199–206 (2019). https://doi.org/10.1016/j.polymertesting.2019.03.020
Goud, V.V.; Patwardhan, A.V.; Dinda, S.; Pradhan, N.C.: Kinetics of epoxidation of jatropha oil with peroxyacetic and peroxyformic acid catalysed by acidic ion exchange resin. Chem. Eng. Sci. 62(15), 4065–4076 (2007). https://doi.org/10.1016/j.ces.2007.04.038
Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Wärnå, J.; Leveneur, S.; Marchant, T.; Salmi, T.: Kinetic modelling of Prileschajew epoxidation of oleic acid under conventional heating and microwave irradiation. Chem. Eng. Sci. 199, 426–438 (2019). https://doi.org/10.1016/j.ces.2019.01.035
Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R.: Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 71, 91–143 (2017). https://doi.org/10.1016/j.progpolymsci.2016.12.009
Okieimen, F.E.; Pavithran, C.; Bakare, I.O.: Epoxidation and hydroxylation of rubber seed oil: one-pot multi-step reactions. Eur. J. Lipid Sci. Technol. 107(5), 330–336 (2005). https://doi.org/10.1002/ejlt.200401126
Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V.: Bionanocomposites from renewable resources: epoxidized linseed oil-polyhedral Oligomeric Silsesquioxanes hybrid materials. Biomacromol 7(12), 3521–3526 (2006). https://doi.org/10.1021/bm060703u
Chavan, A.P.; Gogate, P.R.: Ultrasound assisted synthesis of epoxidized sunflower oil and application as plasticizer. J. Ind. Eng. Chem. 21, 842–850 (2015). https://doi.org/10.1016/j.jiec.2014.04.021
Baboli, Z.M.; Safe Kordi, A.A.: Characteristics and composition of watermelon seed oil and solvent extraction parameters effects. J. Am. Oil Chem. Soc. 87(6), 667–671 (2010). https://doi.org/10.1007/s11746-010-1546-5
Albishri, H.; Almaghrabi, O.; Moussa, T.A.: Characterization and chemical composition of fatty acids content of watermelon and muskmelon cultivars in Saudi Arabia using gas chromatography/mass spectroscopy. Pharmacogn. Mag. 9, 58–66 (2013). https://doi.org/10.4103/0973-1296.108142
Asokan, M.A.; Senthur Prabu, S.; Kamesh, S.; Khan, W.: Performance, combustion and emission characteristics of diesel engine fuelled with papaya and watermelon seed oil bio-diesel/diesel blends. Energy 145, 238–245 (2018). https://doi.org/10.1016/j.energy.2017.12.140
Jia, L.K.; Gong, L.X.; Ji, W.J.; Kan, C.Y.: Synthesis of vegetable oil based polyol with cottonseed oil and sorbitol derived from natural source. Chin. Chem. Lett. 22, 1289–1292 (2011). https://doi.org/10.1016/j.cclet.2011.05.043
Saurabh, T.; Patnaik, M.; Bhagat, S.L.; Renge, V.C.: Studies on synthesis of biobased epoxide using cottonseed oil. J. Adv. Eng. Res. Stud. 1, 279–284 (2012)
Fogassy, G.; Ke, P.; Figueras, F.; Cassagnau, P.; Rouzeau, S.; Courault, V.; Gelbard, G.; Pinel, C.: Catalyzed ring opening of epoxides: application to bioplasticizers synthesis. Appl. Catal. A Gen. 393, 1–8 (2011). https://doi.org/10.1016/j.apcata.2010.11.020
Shagal, M.H.; Barminas, J.T.; Aliyu, B.A.; Osemeahon, S.A.: Epoxidation of Ximenia americana seed oil. J. Pet. Technol. Altern. Fuels 4, 72–77 (2013). https://doi.org/10.5897/JPTAF2013.0092
Musik, M.; Milchert, E.: Selective epoxidation of sesame oil with peracetic acid. Mol. Catal. 433, 170–174 (2017). https://doi.org/10.1016/j.mcat.2017.02.012
Vianello, C.; Piccolo, D.; Lorenzetti, A.; Salzano, E.; Maschio, G.: Study of soybean oil epoxidation: effects of sulfuric acid and the mixing program. Ind. Eng. Chem. Res. 57, 11517–11525 (2018). https://doi.org/10.1021/acs.iecr.8b01109
Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C.: Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide. Bioresour. Technol. 97(12), 1365–1371 (2006). https://doi.org/10.1016/j.biortech.2005.07.004
Sun, S.; Yang, G.; Bi, Y.; Liang, H.: Enzymatic epoxidation of corn oil by perstearic acid. JAOCS, J. Am. Oil Chem. Soc. 88, 1567–1571 (2011). https://doi.org/10.1007/s11746-011-1820-1
Milchert, E.; Smagowicz, A.; Lewandowski, G.: Optimization of the epoxidation of rapeseed oil with peracetic acid. Org. Process Res. Dev. 14, 1094–1101 (2010). https://doi.org/10.1021/op900240p
Durbetaki, A.: Direct titration of oxirane oxygen with hydrogen bromide in acetic acid. Anal. Chem. 28(12), 5–6 (1956). https://doi.org/10.1021/ac60120a055
Liu, M.; Jing, H.; Zhang, J.; Che, G.; Zhou, M.; Gao, Z.; Li, S.; Ren, Z.; Hao, L.; Liu, Y.; Jia, L.: Optimization of mycelia selenium polysaccharide extraction from Agrocybe cylindracea SL-02 and assessment of their antioxidant and anti-ageing activities. PLoS ONE 11, 1–15 (2016). https://doi.org/10.1371/journal.pone.0160799
Lewis, G.A.: Optimization methods. In: Swarbrick, J., Boylan, J.C. (eds.) Encyclopedia of Pharmaceutical Technology, 2nd edn, pp. 1922–1937. Marcel Dekker, New York (2002)
de Haro, J.C.; Izarra, I.; Rodriguez, J.F.; Perez, A.; Carmona, M.: Modelling the epoxidation reaction of grape seed oil by peracetic acid. J. Clean. Prod. 138, 70–76 (2016). https://doi.org/10.1016/j.jclepro.2016.05.015
Dinda, S.; Patwardhan, A.V.; Goud, V.V.; Pradhan, N.C.: Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 99(9), 3737–3744 (2008). https://doi.org/10.1016/j.biortech.2007.07.015
Yang, D.; Xiong, L.; Wang, B.; Chen, X.; Wang, C.; Huang, C.; Zhang, H.; Guo, H.; Chen, X.: Preparation and characterization of epoxidized microbial oil. Korean J. Chem. Eng. 33(3), 964–971 (2016). https://doi.org/10.1007/s11814-015-0216-6
Vlček, T.; Petrović, Z.S.: Optimization of the chemoenzymatic epoxidation of soybean oil. J. Am. Oil Chem. Soc. 83(3), 247–252 (2006). https://doi.org/10.1007/s11746-006-1200-4
Turco, R.; Tesser, R.; Vitiello, R.; Russo, V.; Andini, S.; Serio, M.Di: Synthesis of biolubricant basestocks from epoxidized soybean oil. Catalysts 7, 309–320 (2017). https://doi.org/10.3390/catal7100309
Cheikhyoussef, N.; Bo, M.K.R.; Koning, C.; Cheikhyoussef, A.; Hussein, A.A.: Characterization of Acanthosicyos horridus and Citrullus lanatus seed oils: two melon seed oils from Namibia used in food and cosmetics applications. 3 Biotech 7, 297–306 (2017). https://doi.org/10.1007/s13205-017-0922-3
Chen, J.; Liu, Z.; Jiang, J.; Nie, X.; Zhou, Y.; Murray, R.E.: A novel biobased plasticizer of epoxidized cardanol glycidyl ether: synthesis and application in soft poly(vinyl chloride) films. RSC Adv. 5(69), 56171–56180 (2015). https://doi.org/10.1039/c5ra07096a
Jia, P.Y.; Bo, C.Y.; Zhang, L.Q.; Hu, L.H.; Zhang, M.; Zhou, Y.H.: Synthesis of castor oil based plasticizers containing flame retarded group and their application in poly (vinyl chloride) as secondary plasticizer. J. Ind. Eng. Chem. 28, 217–224 (2015). https://doi.org/10.1016/j.jiec.2015.02.017
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Patil, S.S., Jena, H.M. Synthesis of Epoxidized Citrullus lanatus Seed Oil: Experimental Investigation and Statistical Optimization. Arab J Sci Eng 44, 9965–9976 (2019). https://doi.org/10.1007/s13369-019-04077-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-019-04077-1