Abstract
Japanese Encephalitis remains a significant global health concern, contributing to millions of deaths annually worldwide. Microglial cells, as key innate immune cells within the central nervous system (CNS), exhibit intricate cellular structures and possess molecular phenotypic plasticity, playing pivotal roles in immune responses during CNS viral infections. Particularly under viral inflammatory conditions, microglial cells orchestrate innate and adaptive immune responses to mitigate viral invasion and dampen inflammatory reactions. This review article comprehensively summarizes the pathophysiology of viral invasion into the CNS and the cellular interactions involved, elucidating the roles of various immune mediators, including pro-inflammatory cytokines, in neuroinflammation. Leveraging this knowledge, strategies for modulating inflammatory responses and attenuating hyperactivation of glial cells to mitigate viral replication within the brain are discussed. Furthermore, current chemotherapeutic and antiviral drugs are examined, elucidating their mechanisms of action against viral replication. This review aims to provide insights into therapeutic interventions for Japanese Encephalitis and related viral infections, ultimately contributing to improved outcomes for affected individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese Encephalitis virus [JE virus] was initially isolated in Japan in 1935. It is a family member of flaviviridae it included the dengue virus, west nile virus, tick-borne encephalitis virus, and as well zika virus. The global distribution of JE virus is spans both tropical and temperate climates region. JE virus is one of the leading causes of death in worldwide, it is highly persistent in large part of the Indian region cause encephalitis in the pediatric population with a high morbidity rate (Campbell et al. 2011; Misra and Kalita 2010). JE Virus is a one of the leading causes of severe encephalitis in children of Asia region an estimated 68,000 clinical case annually. Recently JE virus has been was detected in Angola in the course of a yellow fever outbreak in 2016 (Parida et al. 2006; Halstead and Thomas 2011). The Genomic organization of the virus is linear, + ve Single Stranded [single-stranded RNA] Isohederal, Envelope, Containing and Non-Segmented virus. JE virus mainly affects the brain neuronal cell and CNS, JE virus is Neurotropic in nature that can invade the blood–brain barrier and direct infect Neuronal cells, and cause inflammation in the Brain and neck stiffness, disorientation, coma, seizure, spastic Paralysis, movement disorder [motor impairment], and death (Pfeffer and Dobler 2010; van den Hurk et al. 2009). JE Virus still highly endemic in the Asian subcontinent with a high mortality rate in paediatric population (Dwibedi et al. 2015; Das et al. 2011) Viral infection in central nervous system [CNS] is one of the most challenging tasks for the Neurotropic virus to invade due presence of the robust biophysical barrier including Blood–brain barrier [BBB] these anatomical barriers restrict the viruses to invades and infect the Brain. CNS has consist of the variety of highly specialized Immune cells which have potential to encounter the various viral infections and provide the cellular defence. For the understanding of the developmental trajectory of different cell types within the central nervous system (CNS) is essential for comprehending their responses during neuroinvasion by the Japanese Encephalitis (JE) virus. Each cell type has a distinct origin, maturation process, and functional specialization, which influences their susceptibility to viral infection and their response to the virus.
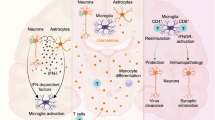
The brain are enriched with different type of the resident immune cell including Microglial cell, astrocytes, oligodendrocyte etc. all these cells have performed the various functions including they have served as a neuroprotective role in the CNS, as well they make the BBB to impermeable by supporting the growth of the endothelial cells also maintaining the Ionic balance and nurture the developing neurons form the infections. The CNS has consisted of astrocytes almost 20% to 40% glial cell at a different region of the brain. Astrocytes are also known as astroglia. Its star-shaped characteristics make it to differentiate from other immune cells. Astrocyte has performed the many important biological functions, including they perform the biochemical support of the endothelial cell of BBB, nutritional support of neurons, ionic balance, tissue repair in infection and traumatic brain injury. These cells contribute to defence against the invading viral pathogen by elimination. Microglia is one of the major brain residents innate immune cell especially in the 10–15% found brain it presents throughout within CNS in which some region of they heavily populated than other region.
The glial distribution is mainly very high in gray matter, while white matter is poorly populated with glial cells. Microglial cells perform major neuroprotective function in the brain from immune challenges like- scavenging Amyloid β- plaque in gray matter, degenerative neuronal structure, damage unnecessary neurons, synapse, and infectious agent. It is extremely highly sensitive to the ionic imbalance, even small pathophysiological changes in the CNS because of existence of a unique potassium ion channel. It will respond very fast to small ionic changes in the extracellular environment which is called the [microglial sensome]. Microglia cells are highly neuroplastic in nature it found in the ramified state in the specific region of the brain and spinal cord in absence of immune response. When any viral infection, traumatic brain injury, occurs then ramified microglia cells will transform into activated microglia cells and phagocytosed the infectious agents (Hickman et al. 2013; Chen et al. 2010).
Astrocytes
Astrocytes are another type of glial cell in the CNS and originate from radial glial cells during embryonic development. They migrate and differentiate into mature astrocytes during late embryogenesis and early postnatal development. Astrocytes provide structural support, regulate neurotransmitter levels, and modulate synaptic transmission. While astrocytes can be activated in response to viral infection, they may not be the first cell type to become activated during neuroinvasion by the JE virus. Astrocytes are another types of glial cell mainly found in the central nervous system (CNS), alongside microglia. The name often referred to as "star-shaped" cells due to their morphology, astrocytes play diverse and crucial roles in maintaining the health and functionality of the nervous system under stress conditions to nourish and assist the microglial cells functions. Astrocytes are multifunctional cells that play essential roles in maintaining CNS homeostasis, supporting neuronal function, regulating synaptic activity, and contributing to neuroinflammation. Their diverse functions highlight their importance in both normal brain function and neurological disease. Astrocytes provide structural support to neurons and other cells in the CNS. They form a network of processes that ensheath synapses and blood vessels, contributing to the formation of the blood–brain barrier (BBB), which regulates the exchange of molecules between the bloodstream and the brain. In the context of central nervous system (CNS) inflammation, astrocytes exhibit multifaceted roles influenced by their intrinsic neurotoxic properties, the activation of resident microglia, and the recruitment of peripheral inflammatory cells (Linnerbauer et al. 2020). Typically, immune pathways within astrocytes are triggered following various insults, such as brain trauma, ischemia, and different neuroinfections, notably viral infections (Farina et al. 2007; Lindqvist et al. 2016a, b). Viruses access the CNS via diverse routes, including traversal of brain capillary endothelial cells, penetration through the meningeal barrier, and infiltration via infected peripheral and olfactory neurons (Potokar et al. 2019; Ludlow et al. 2016; Mandl 2005. Among the initial cell types to host and replicate invading CNS viruses are astrocytes (Potokar et al. 2019; Jorgačevski et al. 2019; Palus et al. 2014). Upon viral entry, these cells promptly release immunomodulatory molecules, thereby contributing to the innate immune response, the foremost defense against viral invasion (Li et al. 2016). The dual role of astrocytes in the neurotoxic and neuroprotective aspects of the CNS innate immune response is increasingly recognized. During the early stages of viral infection, the antiviral response of astrocytes manifests in a dualistic manner, potentially benefiting either the host or the virus. Astrocytes may aid the host by producing antiviral agents that hinder virus replication and dissemination (Li et al. 2021; Vonderstein et al. 2018). Conversely, they might facilitate virus propagation between CNS cells, serving as a reservoir to sustain long-term viral presence within the tissue (Jorgačevski et al. 2019, Li et al. 2016). The persistence of viral infections within the CNS is an underestimated and underexplored facet of viral pathogenesis. Recent research indicates certain viruses, including TBEV, ZIKV, HIV-1, JEV, CCHFV, and RABV, possess the capability to establish persistent infections within astrocytes (Potokar et al. 2014 Jorgačevski et al. 2019; Palus et al. 2014; Anderson et al. 2003; Spengler et al. 2017; Li et al. 2021; Knap et al. 2012; Ray et al. 1997). For instance, in the case of ZIKV, infected activated astrocytes have been identified as reservoirs capable of sustaining viral replication within specific brain regions in mice for over a year (Ireland et al. 2020). Moreover, human fetal astrocytes have been shown to support chronic ZIKV infection and continuous viral shedding for at least one month (Limonta et al. 2018). Speculation surrounds the potential contribution of persistent ZIKV replication and associated CNS inflammation to neurological deficits and long-term prognosis, though the precise impact of persistent inflammation on limiting viral spread within the CNS parenchyma warrants further investigation (Ireland et al. 2020). Similarly, the persistence of EBV within astrocytes remains a subject for further study, including its potential implications for CNS function (Jakhmola and Jha 2021; Mameli et al. 2012).
Advances in understanding the immune functions of astrocytes indicate their involvement in both acute and persistent viral infections of various etiologies. However, the specific role of astrocytes in infections caused by individual viruses requires detailed investigation. Of particular interest is the persistent infection of astrocytes, as it may have long-term repercussions on CNS function. Abortive infection of astrocytes and the induction of innate immune responses are believed to play pivotal roles during infection and in regulating subsequent adaptive immune pathways. Evidence of abortive infection and persistent chronic infection arises from studies involving human astrocytes infected with HIV-1, rat astrocytes infected with TBEV, and the detection of CCHFV and ZIKV in astrocytes within infected mouse brains for varying durations, up to 22 weeks (Spengler et al. 2017; Ireland et al. 2020; Chauhan et al. 2014). Persistent inflammation within the CNS resulting from viral infection may lead to tissue damage due to viral tropism in specific brain regions, and neurological symptoms may persist long after viral clearance, underscoring the lasting impact of viral infections. In all forms of viral infection, be it acute or persistent, astrocytes are intricately involved in immune responses. They detect viral presence through a variety of receptors, initiating signaling cascades that activate the innate immune response. Astrocytes are known to express and release a wide array of cytokines, including type I interferons (IFNs), which are continuously produced at low levels in various organs, including the central nervous system (CNS). Working in tandem with interferon regulatory factors (IRFs), IFNs play crucial roles in modulating immune responses following pathogen invasion. Astrocytes exhibit high basal expression levels of type I IFNs (IFN-α and IFN-β) along with proteins encoded by interferon-stimulated genes (ISGs), enabling them to mount a rapid IFN response to contain viral spread. Particularly, astrocytes emerge as primary producers of IFN-β in the CNS following acute infections by various neurotropic viruses and during abortive infection instances. (Gough et al. 2012; Li et al. 2017; Negishi et al. 2018).
Endothelial cells
Endothelial cells line the blood vessels in the CNS and originate from angioblasts during embryonic development. They form the blood–brain barrier (BBB), which regulates the exchange of molecules between the bloodstream and the brain parenchyma. Endothelial cells can become activated in response to inflammatory signals released by infected cells, leading to BBB disruption and immune cell infiltration into the CNS.
Ueually Endothelial cells are play key role in brain microvasculature it provides the structural and functional integrity to BBB. The interaction of neurovasculature cells with each other and their cooperation with endothelial cells is also play vital role for the regulation of the tight junctions within BBB integrity (Villabona-Rueda et al. 2019; Keaney and Campbell 2015). The neuro invasion mechanisms JE virus to crosses the BBB are supported by four possible modes: first is proliferation of virus within endothelial cells without affecting the cell viability, followed by passive transport of viral particles across the endothelial cells, second is diapedesis of virus-infected leukocytes between endothelial cell junctions, third is transport of viruses via the peripheral nervous system, and fourth is disruption of the BBB through the release of virus-induced inflammatory mediators from the cells of apical (blood) and basolateral (brain) sides of the BBB, which is considered to be the most common mode of invasion (Miner and Diamond 2016; Hsieh et al. 2020). usually the JE virus infection it up-regulate the expression of cathepsin L and endoglycosidase heparanase in endothelial cells, and subsequantly leading to the degradation of glycocalyx-like layer (EGL) components and ultimately leads to the enhanced the permeability of the Blood brain barrier (Puerta-Guardo 2019; Glasner et al. 2017).
Neurons
Neurons are the functional units of the CNS and originate from neural stem cells during embryonic development. They undergo extensive migration, differentiation, and synaptogenesis to form neural circuits. Neurons are highly susceptible to viral infection and can undergo apoptosis or necrosis following viral invasion. However, neuronal activation in response to JE virus infection may occur secondary to the activation of microglia and astrocytes.
Identifying the first cell type to become activated during neuroinvasion of the JE virus depends on various factors, including the route of viral entry, viral tropism, and the host immune response. However, microglia are often among the earliest responders due to their role as sentinel immune cells in the CNS. Upon detection of viral particles or viral RNA, microglia rapidly become activated and release pro-inflammatory cytokines and chemokines, initiating the immune response to contain viral spread and recruit other immune cells to the site of infection.
Japanese encephalitis virus pathogenesis
Transmission of the JE virus is mainly occurs in the rainy [Monsoon] season in Southeast Asian region; however, it can happens in throughout of the year in sub-tropical and tropical regions. The disease distribution is also found in the temperate regions of the Korean, China, peninsula, and some eastern parts of the, and Japan. JE virus transmission mainly depends on the density of vector, precipitation of rainfall, and temperature and these meteorological factors will determine the Bio-burden of JE Virus infection. Japanese Encephalitis disease principally occurs in agricultural regions of the Asian subcontinent where rice field is the prime habitat for JE Virus vector mosquitoes breed close vicinity (Pfeffer and Dobler 2010). The natural transmission of JE virus cycle are involves a various species of the genus Culex mosquito as a vector in the zoonotic cycle. whereas, Aquatic birds and bats are the more susceptible reservoir, and pigs are one of the main virus amplifying hosts and human are considering as a dead-end host for JE virus because lower viremia will not allow to further amplification. Neuro invasion of the JE virus is done through the hematogenous route and infection is spread all over the brain (Misra and Kalita 1997; Endy and Nisalak 2002). Pathophysiology of the JE virus is established through the disruption of the barrier between peripheral and CNS. It also enhanced the BBB permeability by reduction of the expression of the barrier proteins such as Occludin, claudin5 and ZO1 within CNS (Chen et al. 2010; Mishra et al. 2009; Lai et al. 2012). These all proteins will maintain the integrity of the BBB and JE virus infection cause the secretion of inflammatory cytokines they can regulate BBB permeability (Al-Obaidi et al. 2017; Plesner et al. 1998; Liu et al. 2008).
Glial plasticity and its activation
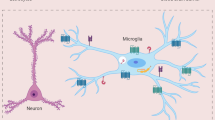
Microglia are the resident immune cells of the CNS and originate from myeloid progenitor cells in the yolk sac during embryonic development. They colonize the brain early during embryogenesis and undergo further maturation postnatally. Microglia play crucial roles in immune surveillance, synaptic pruning, and neuroinflammation. In the context of JE virus infection, microglia are among the first cells to respond to viral invasion due to their rapid activation upon detection of viral particles or viral RNA in the brain parenchyma. Our [CNS] system is protected by the [BBB] from the infectious pathogen, Neuro invasive virus, bacteria, fungus, etc. It’s also separated from the peripheral circulatory system for the lower infection purpose. Inflammation is an innate immune defense mechanism for the elimination of foreign Pathogens from the body with the help of the co-coordinating with the adaptive immune system and localizes the immune cells from our peripheral system to Trans endothelial migration of the cell in CNS and provides the immunoregulation (Endy and Nilasak 2002; Lai et al. 2012). Neuroinflammatory responses are useful for the short time for the Microbial clearance [acute response] as well as it is harmful to the chronic response because of the secretion of inflammatory cytokines. Neuroinflammation is the inflammation of the CNS, including the brain and spinal cord, followed by traumatic brain injury, invasion of neurotropic virus [Including JE, WNV], toxic metabolites, spinal cord injury, etc. (German et al. 2006; Plesner et al. 1998; Chen et al. 2012) Fig. 1.
Microglial cells are generally derived the from yolk sac progenitors and they further migrate into CNS at early embryogenesis. Microglial cells are highly dynamic in nature they can proliferate & differentiate in CNS. Generally, have found the two alternate state one is classical state is M1 which has shown the pro-inflammatory response and other alternative state is M2 which it has to perform the tissue repair by anti-inflammatory response (Dudvarski et al. 2016; Fekete et al. 2018; Chen et al. 2010). M2 has further divides into the In CNS microglia were performing the neuroprotective role against the infection and maintain cellular homeostasis in brain. Glial activation has play pivotal role in the generation of the various pro-inflammatory cytokines, chemokine’s. It polarized into the two states M1 a first line of immune defence against the infection and produced the pro-inflammatory cytokines including [TNF-α, IFN-ϒ, IL-1, IL-6, IL-12, IL-1α and IL-1β] and they are causer significantly kill the offending invaders within brain (Mishra et al. 2008; Man et al. 2007). In the brain, the major outcome of the M1 polarization of microglia has enhanced the various immunological responses, including production of the Reactive oxygen species [ROS], iNOS which subsequently caused the viral clearance from the Brain. Polarization of the Microglia from M1/M2 are dynamic process which perform more immunoregulatory function, it minimizes the extensive neuronal damage by secretion of the anti-pro-inflammatory [IL-4, IL-13, IL-10, and TGF-β] cytokines (Mathur et al. 1992).
Alternative activation of glial has many important functions in the brain it causes the inhibition of the inflammatory response, angiogenesis, dampening, and tissue damaging and enhanced the remodelling, immunoregulation. Activated M2 microglial cells have caused the NF-kB bio signalling induced by the M1 state of microglial cells. Microglia are also activated by the various form it can be M2A, M2B, M2C, M2D depends upon the cellular receptor. M2 state of the microglial cells causes the deactivate the inflammatory immune response and re-established the Cellular homeostasis. During Neuro inflammatory response, it will activate the brain resident Innate Immune cell Microglial cell to change their phenotype and morphology in response to neuronal injury in the brain. Rapid activation of a microglial cell typically characterizes it to minimize the infection in the brain (Hickman et al. 2013; Kim et al. 2016a, b).
Neuro inflammatory mediators cause neurodegeneration
The invasion of the central nervous system (CNS) by the JE virus leads to a cascade of inflammatory responses, where microglial cells play a pivotal role. Microglia, as resident immune cells of the CNS, release a variety of cytokines and chemokines upon activation, influencing the immune response to the viral invasion. During acute inflammation, there is without peripheral immune response involved but over the time chronic inflammation it leads to cause the degradation of blood–brain barrier tissue and provide the access of Neuro invasion and resulting CNS is not well protected by the BBB and microglial cell will lead to produce reactive oxygen species and it will then lead to release of cytokines like IL-1, IL-4, IL-6, IL-18, TNF-α, IL- 1β. It produced these pro-inflammatory cytokines from activated microglial cells, astrocyte endothelial cells at the site of infection or injury in the brain (Huppert et al. 2010;Chen et al. 2014; Chen et al. 2012; Kim et al. 2016a, b). These cytokines act as neuromodulators. They regulate the inflammation and they coordinate with the peripheral immune cell for the pathogen clearance from the brain and provide Neuro protection for developing neurons. In the brain, during Japanese encephalitis virus infects the neurons by the Trojan horse mechanism and caused the degeneration of the neurons. Activation of glial cells results in the production of many pro-inflammatory cytokines. Neuroinflammation sustained release of cytokines from immune cells affects the blood–brain barrier, allows the penetration of peripheral systems and causes cellular damage (Al-Obaidi et al. 2017; Chen et al. 2014; Singh et al. 2000).
Tumor necrosis factor-alpha (TNF-α)
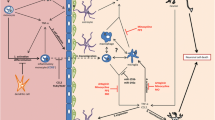
TNF-α is released by activated microglia and contributes to neuroinflammation during JE virus infection. It promotes the recruitment of immune cells to the site of infection and activates other glial cells, such as astrocytes. However, excessive TNF-α production can lead to neuronal damage and exacerbate neuroinflammation. TNF-α Induced Neuronal cytotoxicity although, pro- inflammatory cytokines may cause cell death as well as it also caused the secondary tissue damage in the brain. After the BBB Becomes Permeable to Neutrophils, Monocytes, macrophages, T- cell, enter the brain and innate and adaptive immune response excavate the inflammatory response in the brain and contribute to chronic Neuroinflammation and Neurodegeneration and affect the brain Functionality. These Inflammatory responses will lead to affect the degeneration of neurons, and finally cause motor impairment, other neurological complications (Al-Obaidi et al. 2017; Kim et al. 2016a, b). Because Neuro inflammatory response produced both arms of the immune system cell will excavate the inflammatory response and it will lead to increase the activated immune cell density monocytes, neutrophils, macrophage, etc. that will produce the various immunoregulatory cytokine. Each cytokine released by microglial cells during JE virus infection exerts its effects through specific signaling pathways and interactions with other immune cells within the CNS. For example, IFNs signal through the JAK-STAT pathway to induce the expression of antiviral proteins and modulate immune responses. TNF-α activates NF-κB signaling, leading to the production of pro-inflammatory mediators and the upregulation of adhesion molecules on endothelial cells, facilitating immune cell recruitment. ILs signal through various pathways, including the JAK-STAT, MAPK, and NF-κB pathways, to regulate immune cell activation and cytokine production. Chemokines bind to their respective receptors on immune cells and activate signaling pathways that control cell migration and infiltration into the CNS. Japanese Encephalitis virus is Neurotropic and it will mainly affect the neurons and caused neuronal death. Recent studies Rodent models show JE virus has a high tropism for neuronal precursors Neurons and developing neurons, affecting their proliferation and development due to own cytotoxicity and indirectly it will Affect the Neurons by Inflammatory responses that produced the various type of the immunoregulatory chemokine, pro-inflammatory cytokine that will disrupt endothelial architecture and allow to invade form peripheral system to enter CNS and caused the Neurodegeneration (Kalita et al. 2010; Zhang et al. 2008). Post infection of JE virus the number of the various immune cell types increase in within various including the lymph nodes, spleen, and blood cells. Several studies has shown that numbers of macrophages, inflammatory monocytes, granulocytes, and pDC increase in lymphoid tissues, such as spleen and lymph nodes although not dramatically, natural killer cell population decreased in the spleen of JE virus-infected mice in vivo study (Srivastava et al. 2012; Christensen et al. 2004). TNF-α is a pro-inflammatory cytokine released by activated immune cells, including microglia and macrophages, in response to viral infection. TNF-α can induce the expression of adhesion molecules on endothelial cells and promote the recruitment of immune cells to the site of infection. While TNF-α primarily contributes to the inflammatory response, its role in directly influencing viral entry into the CNS is less clear. However, excessive TNF-α production may disrupt the integrity of the BBB, facilitating viral entry into the brain parenchyma. (Choi et al. 2017; Lannes et al. 2017a, b) Fig. 2.
Neuroinvasion of japanese encephaletitis virus within brian. After JE virus infection, activated Astrocytes, microglial, and endothelial cells are secrete chemo attractant that attract the immune cell from peripheral system to CNS. (1] TNF-α are mainly involved in the activation/differentiation of the monocytes (2] due impairment of the BBB cause the transmigration of the cells within Brain. (3] Trans migration of the inflammatory monocytes occurs through the impaired (BBB]. (4] Then after microglia cells contribute to viral propagation and Neuroinflammation. (5] TNF-α/CCL2 leads to cause the microglial activation/expansion. (6] Ultimately, neuronal cell death due to direct cytotoxic effect of JE virus mediated inflammatory mediators
Interferon (IFNs)
Type I IFNs, such as IFN-α and IFN-β, play a critical role in restricting viral replication and spread by inducing an antiviral state in infected cells. Upon sensing viral invasion, cells release type I IFNs, which activate signaling pathways that upregulate the expression of antiviral proteins. These proteins inhibit various stages of the viral life cycle, including viral entry, replication, and assembly. Additionally, type I IFNs modulate the expression of adhesion molecules on endothelial cells, potentially affecting the ability of the virus to breach the blood–brain barrier (BBB) and invade the CNS (Kalita et al. 2010).
Interleukins (ILs)
Various interleukins, such as IL-1β, IL-6, and IL-12, are released by immune cells in response to viral infection and inflammation. These cytokines can modulate endothelial cell function and BBB permeability, potentially affecting viral entry into the CNS. For example, IL-1β and IL-6 have been implicated in increasing BBB permeability by promoting the expression of matrix metalloproteinases (MMPs) and disrupting tight junction proteins between endothelial cells. IL-12, on the other hand, can enhance the activation of immune cells, such as natural killer (NK) cells and cytotoxic T lymphocytes, which may contribute to the clearance of virus-infected cells in the CNS.
Interleukin-1 (IL-1)
IL-1 is a pro-inflammatory cytokine that promotes BBB disruption and increases endothelial cell permeability. Elevated levels of IL-1 have been associated with increased BBB permeability, facilitating the entry of viruses and immune cells into the CNS. Additionally, IL-1 can induce the expression of adhesion molecules on endothelial cells, promoting leukocyte migration, which may inadvertently facilitate viral transport across the BBB.
Interleukin-6 (IL-6)
IL-6 is a pleiotropic cytokine involved in inflammation and immune regulation. It can induce the production of acute-phase proteins and enhance leukocyte recruitment to sites of inflammation. IL-6 may indirectly contribute to viral entry into the CNS by promoting BBB disruption and increasing vascular permeability. Moreover, IL-6 can activate astrocytes and microglia, leading to the production of additional inflammatory mediators that contribute to neuroinflammation and facilitate viral spread.
Chemokines
Chemokines are small cytokines that regulate the migration and recruitment of immune cells to sites of infection. During viral invasion, chemokines are released by infected cells and immune cells to attract leukocytes, such as monocytes, neutrophils, and lymphocytes, to the site of infection. Chemokines can also influence BBB permeability by promoting the transendothelial migration of immune cells. While chemokines primarily regulate immune cell trafficking, they may indirectly influence viral entry by modulating the inflammatory milieu within the CNS. Overall, cytokines released during neuroinvasion by the JE virus can influence viral entry into the CNS by modulating BBB permeability, promoting immune cell recruitment, and inducing antiviral responses in infected cells. Further research is needed to elucidate the specific mechanisms by which individual cytokines contribute to viral entry and CNS invasion, as this may provide insights into potential therapeutic targets for limiting viral spread and neuroinflammation (Kim et al. 2015; Larena et al. 2013; Lindqvist et al. 2016a, b).
Immunoregulatory role of Chemokines in CNS during JE virus infection
Chemokines is a small Immunomodulatory Protein; they play a major role in JE virus infection. It was found that inflammation induced by JE virus infection triggered BBB disruption in the CNS. And resultant it causes the production of the Chemokine and cytokines were produced in the CNS, including TNF-α, IL-6, CCL2, CCL3 CXCL10, CCL4, CCL5, and in mice model (14, 19, Lai et al. 2012; Chen et al. 2012; Kalita et al. 2010). During viral infection these Chemokine are allowing the migration of peripheral innate immune cell monocytes, neutrophils, NK cells, etc. in CNS and provide neuroprotection (Liu et al. 2000; Klein et al. 2005; Christensen et al. 2009). Chemokine has found short acidic N-terminals with seven helical trans membrane domain-like [GPCR] with three intracellular/extracellular loops and an intracellular C-terminal ser/threonine residue for phosphorylation for the receptor regulation.
CCR-2
CCR-2 is the chemokine receptor that regulates the extravasations and transmigration of neutrophils from bone marrow to the inflammatory site. Chemokine/chemokine receptor has shown a pivotal role in organizing and coordinating the complex immune function during viral infection. CCR-2 is a G-protein-coupled receptor that is activated by binding of the CCL-2 Ligand. Functional Ligand of CCR-2 is CCL-2, [MCP-1], CCL-7[MCP-3], CCL-8[MCP-2], CCL-12[MCP-5], CCL-13[MCP-4], CCL-16[MCP-4]. CCR-2 act as both as a pro-inflammatory mediated by [APC, T-cell] and Anti-Inflammatory mediated by regulatory T-cell [Christensen et al. 2009; Liu et al. 2001]. CCR-2 predominantly expressed in both hematopoietic cells like a macrophage, and hematopoietic cells such as fibroblast, mesenchymal cell, astrocyte, a microglial cell in the brain. Whenever any neurotropic virus likes JE, WNV infects the CNS then they induced the expression of the [CCR-2/CCL-2] on the surface of the immune cell and they allow the extravasations of neutrophils from the peripheral blood circulatory system and promote the inflammatory response in the brain and limit the viral replication (Singh et al. 2020; Liu et al. 2018). CCR-2 regulates transmigration of the Monocyte and other immune cells to restrict the viral titer in CNS. It also performs as stimulatory role within CNS and cause the activation of the glial cell to maintain neuronal survival and proliferation by scavenging the virus from the brain. During the JE infection, the expression of the various chemokine will high and it maintains the BBB integrity and lowers the viral effect on the endothelial cell (Lai et al. 2012; Chen et al. 2014). The Brains of JE virus-infected mice have increased levels of various chemokine ligand and receptors such as CCL-2, CXCL-10, CCL-3, CCL-4 and CCL-5 (Christensen et al. 2009; Singh et al. 2020; Liu et al. 2018). Expressions of these chemokine’s are varied from region to region but cortex, thalamus, hippocampus, and midbrain have found to higher levels of CCL2. It enhanced mRNA expression of CXCR2, CXCR-3, CCR1, CCR2, CCR4, and CCR5 in the brain of JE virus-infected mice (Glass et al. 2005; Chen et al. 2012).
CCR-5
CCR-5 receptor belongs to β Chemokine receptor family of integral membrane protein. It is the type of [C–C] Chemokine group.CCR-5 receptor found on the surface of the white blood cells (Liu et al. 2018; Chowdhury and Khanet al. 2019). CCR-5 expressed predominantly on T- cell, macrophage, DC, Eosinophils. CCR-5 cognate Ligand-induced CCL-3, CCL-4, [MIP-1α, MIP-1β], CCL-3L1 this Ligand is highly up-regulated during JE virus infection and they will provide the neuroprotective role by their expression.CCR-5 have found a critical role during Japanese encephalitis virus infection when JE infection occurs in the brain it will finally allow the expression of the CCL-3, CCL-4, CCL-5 Receptor on the immune cells of the body, and then further it will be caused the recruitment of NK cell T- cell and the will be caused the clearance of the virus (Liu et al. 2007). If CCR-5 receptor deficiency occurs then we found the high mortality/viral titer load in the brain during JE virus infection that’s why CCR-5 is critical for controlling both Leukocyte migration and effecter immune function for the clearance of the virus in the brain. CCR-5 performs a protective role against the JE it will control the viral replication in the brain. JE virus activated astrocytes play major role source of CCL-5 and CXCL-10 and important for the migration of NK cells and monocytes into the CNS (Lannes et al. 2017a, b; Zhang et al. 2019; Lim et al. 2008). The CCL-5-CCR-5 axis is involved in recovery and it regulates the level of inflammation within brain. In JE patients the level of the CCL-5 is the key determinant for the survival of the being human, levels of CCL-5 has found higher in CSF of the non-survivors’ patient as compared to the survivors.
Recent in vitro study has shown the neutralization of CCL-5, produced by JE Virus-infected murine glial cells that significantly inhibit the attraction of peripheral immune cells, including monocytes/macrophages. However, a previous study points out the CCR-5 knock-out [KO] mice has higher chance to lethal JE Virus infection upon both intravenous and as well as intraperitoneal injection, although only intravenous JE virus infection leads to higher viral burden shown in the brain as compared to control (Winter et al. 2004; Charo and Ransohoff 2006). It is already reported that the CCR-5 Knockout mice are shows the higher numbers of brain infiltrating monocytes and granulocytes, as well as activated brain immune cell microglia and macrophages. Moreover, CCR-5 KO mice show higher levels of the mediators IL-1β, IL6, CCL-2, CCL-3, CCL-4, and CCL-5. The levels of the CCL2-CCR2 are axis plays a crucial role within brain inflammatory environment and caused severe Neuronal death, and destroys neuronal signalling (Klein et al. 2005; Liu et al. 2001). Upon intradermal injection of mice with JE Virus CCL2 deficiency leads to cause the higher mortality and morbidity of animals which presented higher viral loads in the brain and spinal cord as compared to control animals due to lower transmigration of the blood immune cell (Murdoch and Finn 2000; Muse et al. 2008). Neutralization of the CCL2 produced by JE virus-infected glial cells lower the attraction of monocytes/macrophages cell line in vitro, monocytes and granulocytes accumulate in the brain of JE virus infected CCL-2-deficient mice (Lau et al. 2004). Higher expression levels of the chemokine ligands CXCL-9, CCL-3, CCL-4, and CCL-5 as well as the receptors CCR-1, CCR-2, CCR-4, and CCR-5 are detected in the brain of these animals in the thalamic region Interestingly, CCR-2 deficiency in mice will leads to decreased susceptibility against lethal infection by JE Virus (Sorce et al. 2010; Kim et al. 2016a, b). Because CCR-2 deficiency will reduced the accumulation of monocytes cells from the peripheral system, but do not restrict the migration of the granulocytes in JE Virus-infected mice model. Another mouse model study has confirmed that when the DC was ablated, CCR-2 deficiency increases the migration rate of monocytes within the CNS. JE Virus-infected CCR-2deficient mice has also lower the expression of CCL-3, CCL-4, CCL-5, and CCR-1 within brain (Kim et al. 2016a, b; Glass et al. 2004).
Prevention of Japanese Encephalitis virus infection
Control of JE There is still very challenging because unavailability of a specific curative chemo therapeutic drug for the treatment for JE infection. Some of the alternate drugs are shown anti-viral potential that used in JE treatment. These drugs have shown promising anti-viral/anti-inflammatory effects during a JE Virus infection and lower the disease severity and inhibiting the viral replication, and reduced the inflammatory response in the brain.
Antiviral agents used for Japanese Encephalitis virus infection
Therapeutic interventions for Japanese Encephalitis (JE) pose a challenge due to the absence of specific treatment options. Currently, supportive therapy remains the mainstay for managing JE, aiming to alleviate clinical symptoms and aid patients in overcoming the infection. Despites of numerous research endeavors, only a handful of therapies have advanced to randomized clinical trials, including dexamethasone (an anti-inflammatory agent), IFNα2a, ribavirin (both antiviral drugs), intravenous immunoglobulins (with virus-neutralizing and anti-inflammatory properties), and minocycline (another anti-inflammatory agent). However, none of these therapies have demonstrated significant efficacy in altering the course of JE or improving patient outcomes when compared to placebo controls. Additionally, several therapeutic candidates have exhibited potential effectiveness against JE in preclinical studies using mouse models of JE Virus infection.
Minocycline
Minocycline is lipophilic, semi-synthetic tetracycline analogue (Sooryanarain et al. 2012). It has shown the broad-spectrum, therapeutic efficacy against the MDR viral, bacterial infection. It has found an excellent tissue penetration/absorption; higher bioavailability half-life will makes them a suitable drug that can use for the JE treatment. Therapeutic role of the minocycline to reduces the action of the kinases such as PI3K, p38 MAPK and Akt, and, also NF-κB factor. Minocycline, lower the production of pro-inflammatory cytokines [TNF-α, IL-6, IL-1, IL-12, IFN-γ] and [CCL-2/CCR-2] in the brain. Because of the low level of these cytokines, they will inhibit microglial activation (Trifilo et al. 2004; Mishra, et al. 2008). Minocycline is also reduced the infiltration of immune cells from the blood, that subsequently cause the lower the distribution of the infectious viral particle from blood to brain and restrict the viral replication (Hasegawa et al. 1990; Dutta et al. 2010; Wang et al. 2018).
Arctigenin
Arctigenin is a polyphenolic compound that is derived from lignin [lignin is plant secondary metabolites] founds in the Asteraceae plant family. Arctigenin cause the inhibition of the kinases such as Akt, p38 MAPK, ERK, and abrogates the microglial activation subsequently it lowers the cytokines production (Swarup et al. 2008). Arctigenin is also reduces JE virus-induced neuronal cell death within CNS and provide neuroprotection.
Acyclovir [Zovirax]
Acyclovir is a potent synthetic nucleoside analogue of purine. It is an antiviral drug that is widely used in the treatment of the JE virus infection. Acyclovir is phosphorylating mainly the inhibiter of viral-induced DNA polymerase (Richards et al. 1983). It has found a cyclic carbohydrate moiety that will block the premature chain termination and provide protection against the JE virus infection.
Ganciclovir [Cytovene]
Ganciclovir is an anti-viral nucleoside analogue; it is a homolog of acyclovir. It is prominently used in the treatment of JE virus infection. Mechanisms of the action of the Ganciclovir inhibit the viral replication and pathogenesis and limit the viral load in the brain and provide protection against JE infection (Murayama et al. 2006).
[PP2]
It also known as acetamide and it is a synthetic anilido quinolone derivative compound. PP2 mainly target the NF-κB signalling pathway, and subsequently lower the production of inflammatory cytokines (Ghosh et al. 2008). Although PP2 mainly caused the reduction of the phosphorylation of the viral protein NS3 it leads subsequently caused reduction of the virus particles from neuron/glia release.
Vivo-morpholinos [MOs]
This is another therapeutic drug that used it is a synthetic uncharged drug, it has found the anti-sense oligomer that is analogues of DNA or RNA targeting specific genomic regions. MOs targets the genome of the JE virus as well as its also lower the kinases phosphorylation the p38 MAPK and ERK and downstream NF-κB signalling pathways. MOs inhibit the production of inflammatory mediators such as TNF-α, IL-6, IFN-γ, within brain and inhibit microglial activation (Nazmi et al. 2010; Ghoshal et al. 2007). MOs cause the abrogation of the inflammatory mediated neuronal death.
Etanercept
JE virus is directly caused the neuronal damage as well as also causes intense micro-gliosis and astrogliosis through over activation of astrocytes and microglial cells leads to secretion of many pro- inflammatory cytokines such as [TNF-α], interleukin 1β [IL1β], and [IL-6] (Ye et al. 2014; Takeuchi et al. 2015). TNF-α is play an important role in CNS immunopathology. Etanercept is a lipid soluble TNF-α–binding inhibitor Protein; it directly binds to TNF-α, lower the biological effect in viral pathogenesis (Ye et al. 2014). Etanercept declines microglial cell activation as well as also reduce through inflammatory response induced neuronal death. etanercept is one the anti-viral agent that will used against the JE virus infection. Etanercept regulates the viral induced [BBB] disintegration and reduces viral load in brain..
Drug Name | Therapeutic action against JE Virus | References |
|---|---|---|
Cilnidipine,FGIN-1–27 Niclosamide | It has shown a potent Anti JE activity in -vivo study | Fang et al. 2013 |
Quercetin | It caused the inhibition of the viral replication in vitro | Johari et al. 2012 |
Ribavirin | inhibits viral replication in -vitro | Kumar et al. 2009 |
Dexamethasone | Anti-inflammatory agent used for anti Viral infection | Hoke et al. 1992 |
Pentoxifylline | TNF-α inhibitor inhibit inflammation mediated Neuronal cell death within CNS | Sebastian et al. 2009 |
Fenofibrate | Cause inhibition of the NF-kβ, lower the inflammatory mediated Neuronal cell death | Sehgal et al. 2012 |
Manidipine | Cause viral replication inhibition and inhibit the viral entry within CNS | Klein et al. 2005 |
Nitazoxanide | NTZ cause significant inhibition of viral replication of virus in cultured cells in a dose dependent manner | Shi et al. 2014 |
rosmarinic acid | Inhibit GP78 viral replication within mice brain | Swarup et al. 2007 |
tilapia hepcidin | Cause inhibition of the viral load and viral replication within the mice brain, inhibit neuronal death Inhibit secondary inflammation and microglial activation within CNS | Huang et al. 2011 |
These potential therapies include monoclonal antibodies targeting specific JEV proteins (such as JEV E and NS1 proteins), inhibitors of TNF-α and NF-κB signaling pathways (such as pentoxifylline and fenofibrate), as well as compounds with anti-inflammatory and/or antiviral properties (like arctigenin, rosmarinic acid, and nitazoxanide). Of note, arctigenin demonstrated 100% efficacy in JEV-challenged mice, while others exhibited variable mortality rates, with survival rates ranging from 40 to 80%. Furthermore, certain compounds, such as ivermectin, decanoyl-Arg-Val-Lys-Arg-chloromethylketone, FGIN-1–27, niclosamide, and cilnidipine, have shown anti-JEV activity in vitro. RNA-based therapeutics have emerged as promising candidates for JE treatment. Antagomirs specific for certain microRNAs have shown efficacy in ameliorating acute JE symptoms and improving survival rates in JEV-infected mice. Additionally, recombinant JEV strains engineered to incorporate neuron-specific miRNA recognition sequences have elicited protective immunity against subsequent JEV challenges. Furthermore, single miRNA-like polycistrons expressing siRNAs targeting conserved regions of JEV strains have demonstrated potent anti-JEV activity in vitro. Nanostructures, including metallic or silica nanoparticles, graphene oxide, nanostructured glycans, and carbon dots, have also shown promise as antiviral agents against various viruses. These nanoparticles can interfere with different stages of the virus life cycle, including viral attachment, RNA synthesis, and budding. Specifically, non-cytotoxic carbon dots derived from benzoxazine monomers have demonstrated the ability to block JEV infection in vitro by interacting with viral particles and preventing their entry into host cells. However, further experiments are needed to assess the prophylactic or therapeutic potential of nanoparticles against JEV in vivo. Given the challenges associated with novel drug discovery, drug repurposing has emerged as a viable strategy for identifying potential treatments for JE. By repurposing existing drugs with known safety profiles in humans, this approach offers a cost-effective and expedited alternative to traditional drug discovery methods. Identifying repurposed drugs with validated targets may lead to the development of specific therapies for JE.
Conclusion
JE virus presents a significant morbidity and mortality with no specific treatment currently available. Despite this, recent advancements in understanding JEV-induced neuroinflammation and neuronal damage have uncovered new therapeutic targets for JE treatment. Several promising therapeutic candidates identified through preclinical studies warrant further investigation in clinical trials. Given the multifaceted role of the innate immune system in JE progression, combination therapies targeting both inflammation and viral replication may offer a synergistic approach to treating JE in the future. In this review article we briefly explained the therapeutic role of the various important immune mediators such as cytokine/chemokine and their biological role in Japanese encephalitis infection. These mediators are played key role in transmigration of the innate immune cell and chemokine receptor in response to viral infection in brain (Endy and Nisalak 2002). It provides the basic Understanding the mechanisms of the viral pathogenesis and also about the inflammation-induced BBB disruption in JE infection may provide a potential target for therapeutic intervention in CNS diseases that are driven or exacerbated by a compromised BBB. It is very important for the development of the novel immunotherapeutic for treating Japanese encephalitis and we can target the chemokine for modulation of the inflammatory response and regulate the glial cell activation and we target the Immunomodulatory role of chemokine and reduced the viral infection replication in the brain.
Data Availability
The data generated during the current study are available from the corresponding author on reasonable request.
Change history
10 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13365-024-01220-z
References
Al-Obaidi M, Bahadoran A, Har LS, Mui WS, Rajarajeswaran J, Zandi K, Manikam R, Sekaran SD (2017) Japanese encephalitis virus disrupts blood-brain barrier and modulates apoptosis proteins in THBMEC cells. Virus research 233:17–28. https://doi.org/10.1016/j.virusres.2017.02.012
Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, Simmonds P, Bell JE (2003) Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol Appl Neurobiol 29:378–388
Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS (2011) Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 89(10):766-774E. https://doi.org/10.2471/BLT.10.085233
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354(6):610–621. https://doi.org/10.1056/NEJMra052723
Chauhan A, Mehla R, Vijayakumar TS, Handy I (2014) Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology 456–457:1–19
Chen CJ, Ou YC, Lin SY, Raung SL, Liao SL, Lai CY, Chen SY, Chen JH (2010) Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol 91:1028–1037. https://doi.org/10.1099/vir.0.013565-0
Chen ST, Liu RS, Wu MF, Lin YL, Chen SY, Tan DT, Chou TY, Tsai IS, Li L, Hsieh SL (2012) CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PLoS Pathog 8(4):e1002655. https://doi.org/10.1371/journal.ppat.1002655
Chen CJ, Ou YC, Li JR, Chang CY, Pan HC, Lai CY, Liao SL, Raung SL, Chang CJ (2014) Infection of pericytes in vitro by Japanese encephalitis virus disrupts the integrity of the endothelial barrier. J Virol 88(2):1150–1161. https://doi.org/10.1128/JVI.02738-13
Choi JY, Kim JH, Patil AM, Kim SB, Uyangaa E, Hossain FMA et al (2017) Exacerbation of Japanese Encephalitis by CD11c(hi) Dendritic Cell Ablation Is Associated with an Imbalance in Regulatory Foxp3(+) and IL-17(+)CD4(+) Th17 Cells and in Ly-6C(hi) and Ly-6C(lo) Monocytes. Immune Netw 17(3):192–200. https://doi.org/10.4110/in.2017.17.3.192. PMID:28680381;PubMedCentralPMCID:PMC5484650
Chowdhury P, Khan SA (2019) Differential Expression Levels of Inflammatory Chemokines and TLRs in Patients Suffering from Mild and Severe Japanese Encephalitis. Viral Immunol 32(1):68–74. https://doi.org/10.1089/vim.2018.0103
Christensen JE, Nansen A, Moos T, Lu B, Gerard C, Christensen JP, Thomsen AR (2004) Efficient T-cell surveillance of the CNS requires expression of the CXC chemokine receptor 3. The Journal of neuroscience : the official journal of the Society for Neuroscience 24(20):4849–4858. https://doi.org/10.1523/JNEUROSCI.0123-04.2004
Christensen JE, Simonsen S, Fenger C, Sørensen MR, Moos T, Christensen JP, Finsen B, Thomsen AR (2009) Fulminant lymphocytic choriomeningitis virus-induced inflammation of the CNS involves a cytokine-chemokine-cytokine-chemokine cascade. J Immunol 182(2):1079–1087. https://doi.org/10.4049/jimmunol.182.2.1079
Das S, Dutta K, Kumawat KL, Ghoshal A, Adhya D, Basu A (2011) Abrogated inflammatory response promotes neurogenesis in a murine model of Japanese encephalitis. PloS One 6(3):e17225. https://doi.org/10.1371/journal.pone.0017225
Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MHH (2016) Microglia–blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol 131:347–63
Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A (2010) Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology 215(11):884–893. https://doi.org/10.1016/j.imbio.2009.12.003
Dwibedi B, Mohapatra N, Rathore SK, Panda M, Pati SS, Sabat J, Thakur B, Panda S, Kar SK (2015) An outbreak of Japanese encephalitis after two decades in Odisha, India. Indian J Med Res 142(Suppl 1):S30–S32. https://doi.org/10.4103/0971-5916.176609
Endy TP, Nisalak A (2002) Japanese encephalitis virus: ecology and epidemiology. Curr Top Microbiol Immunol 267:11–48. https://doi.org/10.1007/978-3-642-59403-8_2
Fang J, Sun L, Peng G, Xu J, Zhou R, Cao S, Chen H, Song Y (2013) Identification of three antiviral inhibitors against Japanese encephalitis virus from library of pharmacologically active compounds 1280. PloS One 8(11):e78425. https://doi.org/10.1371/journal.pone.0078425
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145
Fekete R, Cserép C, Lénárt N, Tóth K, Orsolits B, Martinecz B et al (2018) Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol 136:461–482
German AC, Myint KS, Mai NT, Pomeroy I, Phu NH, Tzartos J et al (2006) A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg 100(12):1135–45. https://doi.org/10.1016/j.trstmh.2006.02.008. PMID: 16814333
Ghosh J, Swarup V, Saxena A, Das S, Hazra A, Paira P, Banerjee S, Mondal NB, Basu A (2008) Therapeutic effect of a novel anilidoquinoline derivative, 2-[2-methyl-quinoline-4ylamino]-N-[2-chlorophenyl]-acetamide, in Japanese encephalitis: correlation with in vitro neuroprotection. Int J Antimicrob Agents 32(4):349–354. https://doi.org/10.1016/j.ijantimicag.2008.05.001
Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, Koli P, Sen E, Basu A (2007) Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55(5):483–496. https://doi.org/10.1002/glia.20474
Glasner DR, Ratnasiri K, Puerta-Guardo H et al (2017) Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog 13:e1006673
Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM (2005) Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 202(8):1087–1098. https://doi.org/10.1084/jem.20042530
Glass WG, Hickey MJ, Hardison JL, Liu MT, Manning JE, Lane TE (2004) Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol [Baltimore, Md. : 1950] 172(7):4018–4025. https://doi.org/10.4049/jimmunol.172.7.4018
Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE (2012) Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36:166–174
Halstead SB, Thomas SJ (2011) New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines 10(3):355–364. https://doi.org/10.1586/erv.11.7
Hasegawa H, Satake Y, Kobayashi Y (1990) Effect of cytokines on Japanese encephalitis virus production by human monocytes. J Microbiol Immunol 34(5):459–466. https://doi.org/10.1111/j.1348-0421.1990.tb01028.x
Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J (2013) The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16(12):1896–1905. https://doi.org/10.1038/nn.3554
Hoke CH Jr, Vaughn DW, Nisalak A, Intralawan P, Poolsuppasit S, Jongsawas V, Titsyakorn U, Johnson RT (1992) Effect of high-dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. J Infect Dis 165(4):631–637. https://doi.org/10.1093/infdis/165.4.631
Hsieh JT, St John AL, Evans MJ (2020) Japanese encephalitis virus and its mechanisms of neuroinvasion. PLoS Pathog 16(4):e1008260
Huang HN, Rajanbabu V, Pan CY, Chan YL, Hui CF, Chen JY, Wu CJ (2011) Modulation of the immune-related gene responses to protect mice against Japanese encephalitis virus using the antimicrobial peptide, tilapia hepcidin 1–5. Biomaterials 32(116)
Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR (2010) Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J 24:1023–1034. https://doi.org/10.1096/fj.09-141978
Ireland DDC, Manangeeswaran M, Lewkowicz AP, Engel K, Clark SM, Laniyan A, Sykes J, Lee HN, McWilliams IL, Kelley-Baker L et al (2020) Long-term persistence of infectious Zika virus: Inflammation and behavioral sequela in mice. PLoS Pathog 16:e1008689
Jakhmola S, Jha HC (2021) Glial cell response to Epstein-Barr Virus infection: A plausible contribution to virus-associated inflammatory reactions in the brain. Virology 559:182–195
Johari J, Kianmehr A, Mustafa MR, Abubakar S, Zandi K (2012) Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int J Mol Sci 13(12):16785–16795. https://doi.org/10.3390/ijms131216785
Jorgačevski J, Korva M, Potokar M, Lisjak M, Avšič-Županc T, Zorec R (2019) ZIKV Strains Differentially Affect Survival of Human Fetal Astrocytes versus Neurons and Traffic of ZIKV-Laden Endocytotic Compartments. Sci Rep 9:8069
Kalita J, Srivastava R, Mishra MK, Basu A, Misra UK (2010) Cytokines and chemokines in viral encephalitis: a clinicoradiological correlation. Neurosci Lett 473(1):48–51. https://doi.org/10.1016/j.neulet.2010.02.017
Keaney J, Campbell M (2015) The dynamic blood-brain barrier. Febs J 282(21):4067–4079
Kim JH, Choi JY, Kim SB, Uyangaa E, Patil AM, Han YW et al (2015) CD11c(hi) Dendritic Cells Regulate Ly-6C(hi) Monocyte Differentiation to Preserve Immune-privileged CNS in Lethal Neuroinflammation. Sci Rep 5:17548. https://doi.org/10.1038/srep17548. PMID:26626303;PubMedCentralPMCID:PMC4667186
Kim JH, Hossain FM, Patil AM, Choi JY, Kim SB, Uyangaa E, Park SY, Lee JH, Kim B, Kim K, Eo SK (2016a) Ablation of CD11c[hi] dendritic cells exacerbates Japanese encephalitis by regulating blood-brain barrier permeability and altering tight junction/adhesion molecules. Comparative immunology, microbiology and infectious diseases 48:22–32. https://doi.org/10.1016/j.cimid.2016.07.007
Kim JH, Patil AM, Choi JY, Kim SB, Uyangaa E, Hossain FM, Park SY, Lee JH, Kim K, Eo SK (2016b) CCL2, but not its receptor, is essential to restrict immune privileged central nervous system-invasion of Japanese encephalitis virus via regulating accumulation of CD11b[+] Ly-6C[hi] monocytes. Immunology 149(2):186–203. https://doi.org/10.1111/imm.12626
Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS (2005) Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol 79(17):11457–11466. https://doi.org/10.1128/JVI.79.17.11457-11466.2005
Knap N, Korva M, Dolinšek V, Sekirnik M, Trilar T, Avšič-Županc T (2012) Patterns of tick-borne encephalitis virus infection in rodents in Slovenia. Vector Borne Zoonotic Dis 12:236–242
Kumar R, Tripathi P, Baranwal M, Singh S, Tripathi S, Banerjee G (2009) Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 48(4):400–406. https://doi.org/10.1086/596309
Lai CY, Ou YC, Chang CY, Pan HC, Chang CJ, Liao SL, Su HL, Chen CJ (2012) Endothelial Japanese encephalitis virus infection enhances migration and adhesion of leukocytes to brain microvascular endothelia via MEK-dependent expression of ICAM1 and the CINC and RANTES chemokines. J Neurochem 123(2):250–261. https://doi.org/10.1111/j.1471-4159.2012.07889.x
Lannes N, Summerfield A, Filgueira L (2017a) Regulation of inflammation in Japanese encephalitis. J Neuroinflammation 14(1):158-. https://doi.org/10.1186/s12974-017-0931-5. PMID: 28807053
Lannes N, Neuhaus V, Scolari B, Kharoubi-Hess S, Walch M, Summerfield A, Filgueira L (2017b) Interactions of human microglia cells with Japanese encephalitis virus. Virol J 14(1):8. https://doi.org/10.1186/s12985-016-0675-3
Larena M, Regner M, Lobigs M (2013) Cytolytic effector pathways and IFN-gamma help protect against Japanese encephalitis. Eur J Immunol 43(7):1789–1798. https://doi.org/10.1002/eji.201243152. Epub 2013/04/10. PMID: 23568450
Lau EK, Allen S, Hsu AR, Handel TM (2004) Chemokine-receptor interactions: GPCRs, glycosaminoglycans and viral chemokine binding proteins. Adv Protein Chem 68:351–391. https://doi.org/10.1016/S0065-3233[04]68010-7
Li GH, Henderson L, Nath A (2016) Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr HIV Res 14:373–381
Li W, Hofer MJ, Songkhunawej P, Jung SR, Hancock D, Denyer G, Campbell IL (2017) Type I interferon-regulated gene expression and signaling in murine mixed glial cells lacking signal transducers and activators of transcription 1 or 2 or interferon regulatory factor 9. J Biol Chem 292:5845–5859
Li L, Acioglu C, Heary RF, Elkabes S (2021) Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav Immun 91(740–755):53
Lim JK, Louie CY, Glaser C, Jean C, Johnson B, Johnson H, McDermott DH, Murphy PM (2008) Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. The Journal of infectious diseases 197(2):262–265. https://doi.org/10.1086/524691
Limonta D, Jovel J, Kumar A, Airo AM, Hou S, Saito L, Branton W, Ka-Shu Wong G, Mason A, Power C et al (2018) Human Fetal Astrocytes Infected with Zika Virus Exhibit Delayed Apoptosis and Resistance to Interferon: Implications for Persistence. Viruses 10:646
Lindqvist R, Mundt F, Gilthorpe JD, Wölfel S, Gekara NO, Kröger A, Överby AK (2016a) Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflammation 13:277
Lindqvist R, Mundt F, Gilthorpe JD, Wolfel S, Gekara NO, Kroger A et al (2016b) Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J Neuroinflammation 13(1):277. https://doi.org/10.1186/s12974-016-0748-7. Epub 2016/10/26. PMID:27776548; PubMed Central PMCID: PMC5078952
Linnerbauer M, Wheeler MA, Quintana FJ (2020) Astrocyte Crosstalk in CNS Inflammation. Neuron 108:608–622
Liu H, Nakayama EE, Theodorou I, Nagai Y, Likanonsakul S, Wasi C, Debre P, Iwamoto A, Shioda T (2007) Polymorphisms in CCR5 chemokine receptor gene in Japan. Int J Immunogenet 34(5):325–335. https://doi.org/10.1111/j.1744-313X.2007.00694.x
Liu TH, Liang LC, Wang CC, Liu HC, Chen WJ (2008) The blood-brain barrier in the cerebrum is the initial site for the Japanese encephalitis virus entering the central nervous system. J Neurovirol 14(6):514–521. https://doi.org/10.1080/13550280802339643
Liu K, Xiao C, Wang F, Xiang X, Ou A, Wei J, Li B, Shao D, Miao D, Zhao F, Long G, Qiu Y, Zhu H, Ma Z (2018) Chemokine receptor antagonist block inflammation and therapy Japanese encephalitis virus infection in mouse model. Cytokine 110:70–77. https://doi.org/10.1016/j.cyto.2018.04.022
Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, Lane TE (2000) The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease [Baltimore, Md. : 1950]. J Immunol 165(5):2327–2330. https://doi.org/10.4049/jimmunol.165.5.2327
Liu MT, Keirstead HS, Lane TE (2001) Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol [Baltimore, Md. : 1950] 167(7):4091–4097. https://doi.org/10.4049/jimmunol.167.7.4091
Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, Trebst C, Griffin DE, Brindle HE, Solomon T, Brown AS et al (2016) Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol 131:159–184
Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, Manetti R, Dolei A (2012) Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: Inference for multiple sclerosis. PLoS ONE 7:e44991
Man S, Ubogu EE, Ransohoff RM (2007) Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol 17:243–250. https://doi.org/10.1111/j.1750-3639.2007.00067.x
Mandl C (2005) Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis. Virus Res 111:161–174
Mathur A, Khanna N, Chaturvedi UC (1992) Breakdown of blood-brain barrier by virus-induced cytokine during Japanese encephalitis virus infection. Int J Exp Pathol 73:603–611
Miner JJ, Diamond MS (2016) Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr Opin Immunol 38:18–23
Mishra MK, Kumawat KL, Basu A (2008) Japanese encephalitis virus differentially modulates the induction of multiple proinflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol Int 32:1506–1513. https://doi.org/10.1016/j.cellbi.2008.08.020
Mishra MK, Dutta K, Saheb SK, Basu A (2009) Understanding the molecular mechanism of blood-brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent. Neurochem Int 55(8):717–723. https://doi.org/10.1016/j.neuint.2009.07.006
Misra UK, Kalita J (1997) Movement disorders in Japanese encephalitis. J Neurol 244(5):299–303. https://doi.org/10.1007/s004150050090
Misra UK, Kalita J (2010) Overview: Japanese encephalitis. Prog Neurobiol 91(2):108–120. https://doi.org/10.1016/j.pneurobio.2010.01.008
Murayama T, Yamaguchi N, Iwamoto K, Eizuru Y (2006) Inhibition of ganciclovir-resistant human cytomegalovirus replication by Kampo [Japanese herbal medicine]. Antivir Chem Chemother 17(1):11–16. https://doi.org/10.1177/095632020601700102
Murdoch C, Finn A (2000) Chemokine receptors and their role in inflammation and infectious diseases. Blood 95(10):3032–3043
Muse M, Kane JA, Carr DJ, Farber JM, Lane TE (2008) Insertion of the CXC chemokine ligand 9 [CXCL9] into the mouse hepatitis virus genome results in protection from viral-induced encephalitis and hepatitis. Virology 382(2):132–44. https://doi.org/10.1016/j.virol.2008.09.032
Nazmi A, Dutta K, Basu A (2010) Antiviral and neuroprotective role of octaguanidinium dendrimer-conjugated morpholino oligomers in Japanese encephalitis. PLOS Negl Trop Dis 4(11):e892. https://doi.org/10.1371/journal.pntd.000089
Negishi H, Taniguchi T, Yanai H (2018) The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb Perspect Biol 10:a028423
Palus M, Bílý T, Elsterová J, Langhansová H, Salát J, Vancová M, Růžek D (2014) Infection and injury of human astrocytes by tick-borne encephalitis virus. J Gen Virol 95:2411–2426
Parida M, Dash PK, Tripathi NK, Sannarangaiah Ambuj S, Saxena P, Agarwal S, Sahni AK, Singh SP, Rathi AK, Bhargava R, Abhyankar A, Verma SK, Rao PV, Sekhar K (2006) Japanese Encephalitis Outbreak, India, 2005. Emerg Infect Dis 12(9):1427–1430. https://doi.org/10.3201/eid1209.060200
Pfeffer M, Dobler G (2010) Emergence of zoonotic arboviruses by animal trade and migration. Parasites Vectors 3(1):35. https://doi.org/10.1186/1756-3305-3-35
Plesner AM, Arlien-Soborg P, Herning M (1998) Neurological complications to vaccination against Japanese encephalitis. Eur J Neurol 5(5):479–485. https://doi.org/10.1046/j.1468-1331.1998.550479.x
Potokar M, Korva M, Jorgačevski J, Avšič-Županc T, Zorec R (2014) Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS ONE 9:e86219
Potokar M, Jorgacevski J, Zorec R (2019) Astrocytes in Flavivirus Infections. Int J Mol Sci 20:691
Puerta-Guardo H, Glasner DR, Espinosa DA et al (2019) Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep 26:1598–1613
Ray NB, Power C, Lynch WP, Ewalt LC, Lodmell DL (1997) Rabies viruses infect primary cultures of murine, feline, and human microglia and astrocytes. Arch Virol 142:1011–1019
Richards DM, Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS (1983) Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs 26(5):378–438. https://doi.org/10.2165/00003495-198326050-00002
Sebastian L, Desai A, Madhusudana SN, Ravi V (2009) Pentoxifylline inhibits replication of Japanese encephalitis virus: a comparative study with ribavirin. Int J Antimicrob Agents 33(2):168–173. https://doi.org/10.1016/j.ijantimicag.2008.07.013
Sehgal N, Kumawat KL, Basu A, Ravindranath V (2012) Fenofibrate reduces mortality and precludes neurological deficits in survivors in murine model of Japanese encephalitis viral infection. PloS one 7(4):e35427. https://doi.org/10.1371/journal.pone.0035427
Shi Z, Wei J, Deng X, Li S, Qiu Y, Shao D, Li B, Zhang K, Xue F, Wang X, Ma Z (2014) Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol J 11:10. https://doi.org/10.1186/1743-422X-11-10
Singh A, Kulshreshtha R, Mathur A (2000) Secretion of the chemokine interleukin-8 during Japanese encephalitis virus infection. J Med Microbiol 49(7):607–612. https://doi.org/10.1099/0022-1317-49-7-607
Singh S, Singh G, Tiwari S, Kumar A (2020) CCR2 Inhibition Reduces Neurotoxic Microglia Activation Phenotype After Japanese Encephalitis Viral Infection. Front Cell Neurosci 14:230. https://doi.org/10.3389/fncel.2020.00230
Sooryanarain H, Sapkal GN, Gore MM (2012) Pathogenic and vaccine strains of Japanese encephalitis virus elicit different levels of human macrophage effector functions. Arch Virol 157(10):1905–1918. https://doi.org/10.1007/s00705-012-1386-8
Sorce S, Bonnefont J, Julien S, Marq-Lin N, Rodriguez I, Dubois-Dauphin M, Krause KH (2010) Increased brain damage after ischaemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol 160(2):311–321. https://doi.org/10.1111/j.1476-5381.2010.00697.x
Spengler JR, Kelly Keating M, McElroy AK, Zivcec M, Coleman-McCray JD, Harmon JR, Bollweg BC, Goldsmith CS, Bergeron É, Keck JG et al (2017) Crimean-Congo Hemorrhagic Fever in Humanized Mice Reveals Glial Cells as Primary Targets of Neurological Infection. J Infect Dis 216:1386–1397
Srivastava R, Kalita J, Khan MY, Misra UK (2012) Status of proinflammatory and anti-inflammatory cytokines in different brain regions of a rat model of Japanese encephalitis. Inflamm Res : Official Journal of the European Histamine Research Society … [et al.] 61(4):381–389. https://doi.org/10.1007/s00011-011-0423-5
Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A (2007) Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother 51(9):3367–3370. https://doi.org/10.1128/AAC.00041-07
Swarup V, Ghosh J, Mishra MK, Basu A (2008) Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J Antimicrob Chemother 61(3):679–688. https://doi.org/10.1093/jac/dkm503
Takeuchi T, Miyasaka N, Kawai S, Sugiyama N, Yuasa H, Yamashita N, Sugiyama N, Wagerle LC, Vlahos B, Wajdula J (2015) Pharmacokinetics, efficacy and safety profiles of etanercept monotherapy in Japanese patients with rheumatoid arthritis: review of seven clinical trials. Mod Rheumatol 25(2):173–186. https://doi.org/10.3109/14397595.2014.914014
Trifilo MJ, Montalto-Morrison C, Stiles LN, Hurst KR, Hardison JL, Manning JE, Masters PS, Lane TE (2004) CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J Virol 78(2):585–594. https://doi.org/10.1128/jvi.78.2.585-594.2004
van den Hurk AF, Ritchie SA, Mackenzie JS (2009) Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol 54:17–35. https://doi.org/10.1146/annurev.ento.54.110807.090510
Villabona-Rueda A, Erice C, Pardo CA et al (2019) The evolving concept of the Blood Brain Barrier (BBB): From a single static barrier to a heterogeneous and dynamic relay center. Front Cell Neurosci 13:405
Vonderstein K, Nilsson E, Hubel P, Nygård Skalman L, Upadhyay A, Pasto J, Pichlmair A, Lundmark R, Överby A.K. Viperin (2018) Targets Flavivirus Virulence by Inducing Assembly of Noninfectious Capsid Particles. J Virol 92
Wang X, Zhang K, Yang F, Ren Z, Xu M, Frank JA, Ke ZJ, Luo J (2018) Minocycline protects developing brain against ethanol-induced damage. Neuropharmacology 129:84–99. https://doi.org/10.1016/j.neuropharm.2017.11.019
Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu I, House D, White NJ, Farrar JJ, Hart CA, Solomon T (2004) Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis 190(9):1618–1626. https://doi.org/10.1086/423328
Ye J, Jiang R, Cui M, Zhu B, Sun L, Wang Y, Zohaib A, Dong Q, Ruan X, Song Y, He W, Chen H, Cao S (2014) Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis 210(6):875–889. https://doi.org/10.1093/infdis/jiu179
Zhang B, Chan YK, Lu B, Diamond MS, Klein RS (2008) CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol [Baltimore, Md 1950] 180(4):2641–2649. https://doi.org/10.4049/jimmunol.180.4.2641
Zhang F, Qi L, Li T, Li X, Yang D, Cao S, Ye J, Wei B (2019) PD1+CCR2+CD8+ T Cells Infiltrate the Central Nervous System during Acute Japanese Encephalitis Virus Infection. Virol Sin 34(5):538–548. https://doi.org/10.1007/s12250-019-00134-z
Acknowledgements
I extend my heartfelt gratitude to the IIRC-3 Immunobiochemistry Lab, Department of Biosciences, Integral University Lucknow, for their invaluable support and assistance. I am also deeply thankful to the Department of Clinical Immunology & Rheumatology at SGPGIMS and the Department of Neurology for their kind support.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Firoz Ahmad]; Methodology: [Firoz Ahmad], Formal analysis and investigation: [Firoz Ahmad, Adil Husain, Shad Ahmad], Writing - original draft preparation: [Firoz Ahmad, Shad Ahmad]; Writing - review and editing: [Firoz Ahmad, Adil Husain, Niharika Pandey], Supervision: [Mohd Khubaib, Rolee Sharma]
Corresponding author
Ethics declarations
Competing interests
not any conflict of interest within article all authors read write and agree to submit the article for the publication.
Conflict of interest
Author has declared no conflict of interest within manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, F., Ahmad, S., Husain, A. et al. Role of inflammatory cytokine burst in neuro-invasion of Japanese Encephalitis virus infection: an immunotherapeutic approaches. J. Neurovirol. 30, 251–265 (2024). https://doi.org/10.1007/s13365-024-01212-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-024-01212-z