Abstract
Herpes simplex encephalitis relapses have been rarely reported, with only few cases occurring after neurosurgical interventions. A young man presented a late herpes simplex encephalitis relapse after left antero-mesial temporal resection for his refractory temporal lobe epilepsy. Eight days after surgery, he developed fever and aphasia. CSF PCR revealed more than 12,000 copies/ml of HSV-1 DNA. Intravenous acyclovir was immediately started with a complete recovery. Postoperative herpes simplex encephalitis can occur as primary infection or as relapse of previous infection. Surgical manipulation of brain parenchyma in the site of a previous infection can act as a trigger for viral reactivation. Early onset of antiviral therapy is fundamental and it is a strong predictor of clinical outcome. Despite no studies on prophylactic treatment with acyclovir in patients with previous herpes simplex encephalitis candidate to neurosurgery are available, we suggest that prophylactic treatment should be recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herpes simplex encephalitis (HSE) is the commonest fatal sporadic encephalitis caused by the herpes simplex virus (HSV) 1-2 (Kennedy and Steiner 2013). Typically, HSE involves the limbic network in the frontal and temporal lobes with personality changes, cognitive impairment, fever, aphasia, seizures, and focal neurological/neuropsychological deficits (Kennedy and Chaudhuri 2002).
Late relapses, defined as a recurrence more than 3 months after the first episode, are very uncommon in adulthood (Rigamonti et al. 2013) with only few cases occurring after neurosurgical interventions (Fearnside and Grant 1972; Ochsner 1981; Perry et al. 1998; Spuler et al. 1999; Bourgeois et al. 1999; Molloy et al. 2000; Lellouch-Tubiana et al. 2000; Sheleg et al. 2001; Aldea et al. 2003; Filipo et al. 2005; Ploner et al. 2005; Kwon et al. 2008; Ihekwaba and Battersby 2009; Jalloh et al. 2009; Gong et al. 2010; Lund 2011; Raper et al. 2011; Mallory et al. 2012; Kim et al. 2013; Prim et al. 2013; Uda et al. 2013; Vik-Mo et al. 2014; Lo Presti et al. 2015; Monteiro de Almeida et al. 2015; Jaques et al. 2016; Alonso-Vanegas et al. 2016). We report the case of a young man who presented a late HSE relapse after antero-mesial temporal lobectomy.
Case report
A 27-year-old right-handed man, without any personal or familial antecedent, came to our observation in May 2015 with a 3-day history of partial loss of consciousness and falls. He described forced thoughts, and olfactory hallucinations (smell of lemon), sweating, pallor, and confused speech were described by witnesses.
A brain CT scan and a basal electroencephalography (EEG) were normal; the neurological examination revealed only a mild confusion.
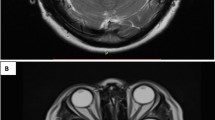
Two days later, he presented with low-grade fever and a partial epileptic seizure with right head deviation followed by secondary generalization. EEG showed a theta-delta activity in the left temporal region. At neurological re-evaluation, he presented with expressive aphasia with anomia, semantic and phonemic paraphasias, and right-sided hemiparesis. Brain MRI showed a bulging lesion involving the head of the left hippocampus, the amygdala, and the uncus, hyperintense in T2-weighted and FLAIR sequences, hypointense in T1-weighted sequences, without contrast enhancement (Fig. 1a, b).
Brain coronal plane FLAIR–weighted (a) and axial plane T2–weighted (b) scan of May 2015 showing hyperintensity involving the head of the left hippocampus, the amygdala, and uncus; coronal plane FLAIR–weighted (c) and axial plane T1 post–gadolinium (d) scan of February 2017 showing recent craniotomy with temporal lobe resection; the residual temporal lobe is edematous. After gadolinium, presence of linear superficial corticopial enhancement in the left temporal pole is noted
Cerebrospinal fluid (CSF) examination revealed normal count of cell, protein, and glucose. In the suspicion of encephalitis, intravenous acyclovir (10 mg/kg 3 daily) and ceftriaxone were immediately started, with antiepileptic treatment (levetiracetam 1000 mg bid).
A few days later, PCR examination on CSF demonstrated 262 copies/ml of HSV-1 DNA. After 10 days of intravenous acyclovir, neurological examination and EEG were normal. The patient was discharged with oral acyclovir (750 mg 3 daily for 2 weeks) and levetiracetam (1000 mg bid).
During the following months, the patient developed drug-resistant epilepsy. In July 2015, levetiracetam was stopped and valproate (500 mg bid and then 750 mg bid) was introduced without improvement. The ictal symptomatology included a “jamais vu” sensation, autoscopy, associated with a raising epigastric feeling, disgusting olfactory hallucination, and scarce oroalimentary automatisms. Neuropsychological examination was normal.
Five months later, the patient was referred to an epilepsy surgery center for presurgical evaluation. Brain MRI findings were unchanged; 11-C-l-methionine positron emission tomography was normal. In January 2016, the patient stopped valproate and started lacosamide 200 mg bid and clobazam 10 mg bid.
One year later, a progressive focal atrophy involving the mesial polar, uncus, and amygdala regions on the left side appeared at MRI, with definite signs of hippocampal sclerosis.
In February 2017, the patient underwent tailored left antero-mesial temporal lobectomy for his epilepsy: polar portions of T1, T2, T3, and T4 and anterior portion of T4 temporal lobe were resected and a complete amygdalo-hippocampectomy was performed. Histology showed an inflammatory process characterized by neuronal loss, presence of microglial activation with microglial nodules, astrogliosis, and infiltrating perivascular immune cells together with focal atrophy of the temporo-mesial cortical structures. The patient was dismissed without neurological deficit after an uneventful postoperative course.
Eight days after surgery, the patient came to the emergency room complaining of headache, fever, and confusion. Hematological tests showed leucocytosis and high levels of C-reactive protein.
The neurological examination revealed global aphasia, normal strength, sensibility, and coordination. CSF examination was performed urgently and revealed 48 cells/mm3 (all mononuclear), with normal protein and glucose levels; PCR examination demonstrated 12,216 copies/ml of HSV-1 DNA. Brain MRI findings were consistent with herpetic encephalitis involving residual temporal lobe areas (Fig. 1c, d). Intravenous acyclovir (10 mg/kg 3 times a day) was started and continued for 3 weeks.
Severe aphasia persisted for 2 weeks, with progressive recovery within 2–3 months. At 6-month follow-up, the patient was seizure-free and antiepileptic medication has been progressively reduced.
Discussion
The pathogenesis of HSE is not completely understood, with 30% of cases related to primary infection and 70% attributed to HSV reactivation (Gnann Jr and Whitley 2017). HSE relapses are uncommon and reported in more than 10% of patients treated with acyclovir, most cases occurring within 4 months (Skoldenberg et al. 2006).
Occurrence of HSE after surgical procedures, especially neurosurgical interventions, has been reported: postoperative HSE is usually caused by a primary infection, with only few cases considered as relapse of a previously diagnosed HSE (Bourgeois et al. 1999; Lellouch-Tubiana et al. 2000; Gong et al. 2010; Lund 2011; Kim et al. 2013; Uda et al. 2013; Lo Presti et al. 2015; Monteiro de Almeida et al. 2015; Jaques et al. 2016; Alonso-Vanegas et al. 2016). In neurosurgical patients, an atypical presentation of HSE with extratemporal involvement is possible (Gnann Jr and Whitley 2017).
Previous studies have suggested that late relapsing HSE may be caused either by reactivation of the virus or by an immune-mediated encephalopathy (De Tiège et al. 2003; Joos et al. 2003). In our case, surgical manipulation of brain parenchyma in the site of the previous infection may probably have facilitated viral reactivation.
Different pathophysiological mechanisms have been advocated, including reactivation of the HSV in the trigeminal ganglion, with subsequent retrograde axonal transport into the CNS and in situ reactivation (Jaques et al. 2016).
A lumbar puncture must be performed as soon as possible in patients with suspected HSE: CSF pleocytosis with predominance of lymphocytes is frequently present, but the diagnostic test of choice is the demonstration of HSV DNA PCR, with a sensitivity of 98% and a specificity of 94–99% (Gnann Jr and Whitley 2017).
Early start of antiviral therapy with intravenous acyclovir without waiting for laboratory confirmation is crucial (Tunkel et al. 2008). Treatment must be maintained for at least 21 days, even with a negative PCR, if the clinical picture strongly suggests a diagnosis of HSE. In fact, as reported by Jaques et al. (2016), early antiviral treatment seems to be a strong predictor of clinical outcome, with death or neurological sequelae having been observed in all untreated patients.
No studies on preoperative prophylactic treatment with acyclovir in patients with a previous history of HSE are available. Moreover, there are no data regarding the usefulness of presurgical CSF testing for persistence of HSV genome as a tool in the decision of using prophylactic therapy.
Considering the good tolerability of the antiviral medications and the risk of relapse, we recommend prophylactic treatment for patients with a previous HSE undergoing neurosurgery, as suggested in other cases (Lo Presti et al. 2015; Monteiro de Almeida et al. 2015; Jaques et al. 2016; Alonso-Vanegas et al. 2016).
References
Aldea S, Joly L-M, Roujeau T, Oswald A-M, Devaux B (2003) Postoperative herpes simplex virus encephalitis after neurosurgery: case report and review of the literature. Clin Infect Dis 36:e96–e99
Alonso-Vanegas MA, Quintero-López E, Martínez-Albarrán AA, Moreira-Holguín JC (2016) Recurrent herpes simplex virus encephalitis after neurologic surgery. World Neurosurg 89:731
Bourgeois M, Vinikoff L, Lellouch-Tubiana A, Sainte-Rose C (1999) Reactivation of herpes virus after surgery for epilepsy in a pediatric patient with mesial temporal sclerosis: case report. Neurosurgery 44:633–635
De Tiège X, Rozenberg F, Des Portes V et al (2003) Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 61:241–243
Fearnside MR, Grant JM (1972) Acute necrotizing encephalitis complicating bifrontal craniotomy and pituitary curettage. Report of two cases. J Neurosurg 36:499–502
Filipo R, Attanasio G, De Seta E, Viccaro M (2005) Post-operative herpes simplex virus encephalitis after surgical resection of acoustic neuroma: a case report. J Laryngol Otol 119:558–560
Gnann JW Jr, Whitley RJ (2017) Herpes simplex encephalitis: an update. Curr Infect Dis Rep 19:13
Gong T, Bingaman W, Danziger-Isakov L, Tuxhorn I, Goldfarb J (2010) Herpes simplex virus reactivation after subtotal hemispherectomy in a pediatric patient. Pediatr Infect Dis J 29:1148–1150
Ihekwaba UK, Battersby RD (2009) Type 2 herpes simplex reactivation after craniocervical decompression for hind brain hernia and associated syrinx. Br J Neurosurg 23:326–328
Jalloh I, Guilfoyle MR, Lloyd SKW, Macfarlane R, Smith C (2009) Reactivation and centripetal spread of herpes simplex virus complicating acoustic neuroma resection. Surg Neurol 72:502–504
Jaques DA, Bagetakou S, L'Huillier AG, Bartoli A, Vargas M, Fluss J, Kaiser L (2016) Herpes simplex encephalitis as a complication of neurosurgical procedures: report of 3 cases and review of the literature. Virol J 13:83
Joos AA, Ziyeh S, Rauer S, Keller E, Huzly D, Lücking CH (2003) Postinfectious autoimmune-mediated encephalitis eight months after herpes simplex encephalitis. Eur Neurol 50:54–56
Kennedy PGE, Chaudhuri A (2002) Herpes simplex encephalitis. J Neurol Neurosurg Psychiatry 73:237–238
Kennedy PGE, Steiner I (2013) Recent issues in herpes simplex encephalitis. J Neuro-Oncol 19:346–350
Kim SH, Lee SG, Kim SH, Kim DS, Kim HD (2013) Relapsed herpes simplex virus encephalitis after epilepsy surgery. J Epilepsy Res 3:28–31
Kwon J-W, Cho B-K, Kim EC, Wang K-C, Kim S-K (2008) Herpes simplex encephalitis after craniopharyngioma surgery. J Neurosurg Pediatr 2:355–358
Lellouch-Tubiana A, Fohlen M, Robain O, Rozenberg F (2000) Immunocytochemical characterization of long-term persistent immune activation in human brain after herpes simplex encephalitis. Neuropathol Appl Neurobiol 26:285–294
Lo Presti A, Weil AG, Niazi TN, Bhatia S (2015) Herpes simplex reactivation or postinfectious inflammatory response after epilepsy surgery: case report and review of the literature. Surg Neurol Int 6:47
Lund M (2011) Herpes simplex virus reactivation and encephalitis after topectomy. J Pediatr Health Care 25:323–327
Mallory GW, Wilson JW, Castner ML, Driscoll CLW, Link MJ (2012) Herpes simplex meningitis after removal of a vestibular schwannoma: case report and review of the literature. Otol Neurotol 33:1422–1425
Molloy S, Allcutt D, Brennan P, Farrell MA, Perryman R, Brett FM (2000) Chemotherapy, surgery, and stereotactic radiotherapy for medulloblastoma. Arch Pathol Lab Med 124:1809–1812
Monteiro de Almeida S, Crippa A, Cruz C, de Paola L, de Souza LP, Noronha L, Torres LF, Koneski JA, Pessa LF, Nogueira MB, Raboni SM, Silvado CE, Vidal LR (2015) Reactivation of herpes simplex virus-1 following epilepsy surgery. Epilepsy Behav Case Rep 4:76–78
Ochsner F (1981) Contamination of a glioma by herpes simplex virus. Schweiz Arch Neurol Neurochir Psychiatr 129:19–30
Perry JD, Girkin CA, Miller NR, Kerr DA (1998) Herpes simplex encephalitis and bilateral acute retinal necrosis syndrome after craniotomy. Am J Ophthalmol 126:456–460
Ploner M, Turowski B, Gabriele W (2005) Herpes encephalitis after meningioma resection. Neurology 65:1674–1675
Prim N, Benito N, Montes G, Pomar V, Molet J, Rabella N (2013) Human herpesvirus 1 meningoencephalitis after trigeminal neuralgia surgery. J Inf Secur 67:79–81
Raper DMS, Wong A, McCormick PC, Lewis LD (2011) Herpes simplex encephalitis following spinal ependymoma resection: case report and literature review. J Neuro-Oncol 103:771–776
Rigamonti A, Lauria G, Mantero V, Salmaggi A (2013) A case of late herpes simplex encephalitis relapse. J Clin Virol 58:269–270
Sheleg S, Nedzved M, Nedzved A, Kulichkovskaya I (2001) Contamination of glioblastoma multiforme with type 1 herpes simplex virus. J Neurosurg 95:721
Skoldenberg B, Aurelius E, Hjalmarsson A et al (2006) Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 253:163–170
Spuler A, Blaszyk H, Parisi JE, Davis DH (1999) Herpes simplex encephalitis after brain surgery: case report and review of the literature. J Neurol Neurosurg Psychiatry 67:239–242
Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ, Infectious Diseases Society of America (2008) The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 47:303–327
Uda T, Koide R, Ito H, Hosono A, Sunaga S, Morino M (2013) Relapse of herpes simplex virus encephalitis after surgical treatment for temporal lobe epilepsy: rare complication of epilepsy surgery. J Neurol 260:318–320
Vik-Mo EO, Krossnes BK, Stanisic M, Egge A, Holter E, Taubøll E, Heuser K, Lund CG (2014) Reactivation of occult herpes simplex meningoencephalitis after temporal lobe resection for refractory epilepsy–a case report. Seizure 23:321–323
Author information
Authors and Affiliations
Contributions
All authors approved the contents of the manuscript and validated the accuracy of the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mantero, V., Cossu, M., Rigamonti, A. et al. HSV-1 encephalitis relapse after epilepsy surgery: a case report and review of the literature. J. Neurovirol. 26, 138–141 (2020). https://doi.org/10.1007/s13365-019-00796-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-019-00796-1