Abstract
HTLV-1 proviral load (pvl) is an important risk marker for HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), but its value as prognostic marker is not well defined. Long-term prospective cohort studies are necessary to clarify this question. Here, we analyzed HTLV-1 pvl in the peripheral blood of 82 asymptomatic carriers (AC; 351 samples), 12 HAM/TSP patients (HAM; 46 samples), and six incident cases of HAM/TSP (iHAM), with serial samples collected before (n = 10) and after (n = 20) the disease onset. The mean interval of follow-up was 10 years in the AC group and 8 years in HAM and iHAM groups. pvl was not significantly different between the first and last measurements in the three groups, but there was a trend to decrease over time. Coefficient of variation of pvl was significantly lower in the AC group than in HAM (p = 0.015) and iHAM (p = 0.022) patients. AC and HAM individuals showed a significant and strong positive correlation between the first and last measurements of pvl, but not iHAM subjects. All individuals who developed HAM/TSP during the follow-up had high pvl level (>1 %) before the onset of disease, but a typical increase in pvl was not observed in that period. The data suggest that there is a trend to reach an equilibrium plateau of pvl over time, characteristic of each individual. A significant rate of AC keeps high pvl levels for a long time without developing clinical symptoms associated to HTLV-1 infection. Thus, serial quantification of pvl in the peripheral blood does not seem to be a good prognostic marker for HAM/TSP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The infection by human T cell lymphotropic virus 1 (HTLV-1) may lead to the development of adult T cell leukemia (ATL) (Hinuma et al. 1981; Yoshida et al. 1984) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (Gessain et al. 1985; Osame et al. 1986) in about 5–10 % of infected carriers. Other inflammatory clinical conditions, such as uveitis, inflammatory myositis, arthritis, and HTLV-1-associated pulmonary disease, as well as infective dermatitis and some types of skin lesions, are also associated with HTLV-1 (Martin et al. 2014; Gonçalves et al. 2003).
HAM/TSP is an inflammatory neurodegenerative disease, usually with a slow and insidious onset that progresses to motor and sensory disturbance, due to the atrophy of the thoracic spinal cord and involves perivascular demyelination and axonal degeneration (Izumo 2010; Yamano and Sato 2012). The pathogenesis of HAM/TSP supposes a strong inflammatory response in the central nervous system (CNS), with infiltration of HTLV-1-infected cells and specific anti-HTLV-1 T lymphocytes (Saito 2010).
Since only a relatively small percentage of the infected individuals develop HAM/TSP, which in general takes a long time for its installation, it is desirable to obtain good risk markers for monitoring HTLV-1-infected carriers. Cross-sectional studies comparing asymptomatic carriers to patients with HAM/TSP or other HTLV-1-associated diseases have shown that the median proviral load quantified in the peripheral blood is significantly higher in symptomatic patients (Ono et al. 1998; Hishizawa et al. 2004; Yakova et al. 2005; Olindo et al. 2005; Furtado et al. 2012), and therefore, a high proviral load is considered a risk marker for a poor clinical outcome throughout viral infection. The potential of the use of measurements of proviral load in the peripheral blood as a prognostic marker for HAM/TSP is hampered by the considerable frequency of asymptomatic carriers with high proviral load, leading to an important overlap of proviral load values among asymptomatic and symptomatic individuals (Furtado et al. 2012). Thus, it is not clear whether the high proviral load observed in patients with HAM/TSP is the cause or consequence of the disease.
Longitudinal studies monitoring proviral load may clarify the potential of the proviral load as a prognosis marker for HAM/TSP. The present study analyzed variation of proviral load in asymptomatic carriers and patients with HAM/TSP, during a long-term follow-up (5 to 16.6 years). It was also possible to analyze six incident cases of HAM/TSP, with quantification of proviral load in serial samples collected before and after the onset of illness.
Materials and methods
Study population
Individuals for whom serial HTLV-1 proviral load data in the peripheral blood were available were selected from all seropositive individuals followed up by the Interdisciplinary HTLV Research Group (GIPH). This open cohort study was started at Fundação Hemominas in March 1997 and follows up on blood donors with HTLV-1/2-positive serological results. HAM/TSP patients were from Rede Sarah, a rehabilitation hospital in Belo Horizonte, Brazil. Infection by HTLV-1 was detected by ELISA (different commercial kits were used during the enrollment of participants) and was confirmed by Western blot (WB HTLV 2.4, Genelabs Diagnostics, Singapore) or real-time PCR (Andrade et al. 2010). Subjects were submitted to neurological examinations and classified as asymptomatic carriers or HAM/TSP patients according to the WHO criteria (WHO 1989). All asymptomatic individuals enrolled in the present study were submitted to clinical examinations every time that blood sample was collected, and they did not show signs or symptoms. According to the GIPH protocol, when signs and symptoms suggestive of HAM/TSP are detected, an individual is referred to a specialized hospital (Hospital Sarah) for more specific tests in order to confirm or refute the diagnosis of HAM/TSP. Spinal cord injury was evaluated for HAM/TSP patients using the American Spinal Injury Association (ASIA) impairment scale that describes a person’s functional impairment as a result of their spinal cord injury. The ASIA scale (impairment calculation) was A (complete): no motor or sensory function in the lowest sacral segment (S4–S5); B (incomplete): sensory function below neurologic level and in S4–S5, no motor function below neurologic level; C (incomplete): motor function is preserved below neurologic level, and more than half of the key muscle groups below neurologic level have a muscle grade <3; D (incomplete): motor function is preserved below neurologic level, and at least half of the key muscle groups below neurologic level have a muscle grade >3; and E (normal): sensory and motor functions are normal.
In this study, HTLV-1 proviral load of 82 health carriers who remained asymptomatic until the end of the study (AC group) and 12 HAM/TSP patients (HAM group) was quantified in different periods of their follow-up ranging from 5 to 16.6 years, totaling 351 and 46 samples analyzed, respectively. Proviral load was also quantified in six incident cases of HAM/TSP (iHAM group), in serial samples collected before (n = 10) and after (n = 20) the onset of disease and during their follow-up (mean of 8 years, ranging from 3.8 to 16 years).
Quantification of proviral load
DNA was extracted from the peripheral blood by column extraction (QIAamp DNA Blood kit; Qiagen GmbH, Hilden, Germany). HTLV-1 proviral load was quantified by a real-time SYBR Green PCR method, using primers to pol region (SK110 [5′-CCCTACAATCCAACCAGCTCAG-3′] and SK111 [5′-GTGGTGAAGCTGCCATCGGGTTTT-3′) and to albumin gene (ALB-S [5′-GCTGTCATCTCTTGTGGGCTGT-3′] and ALB-AS [5′-AAACTCATGGGAGCTGCTGGTT-3′]) to determine the total cell number. For both HTLV-1 and the albumin gene, amplifications were performed in separate reactions using 240 ng DNA, 1× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), and 200 nM of each primer. Real-time PCR was performed using an ABI Prism 7300 Sequence Detector System (Applied Biosystems), with the following cycle conditions: 2 min at 50 °C and 10 min at 95 °C followed by 50 cycles of 15 s at 95 °C and 1 min at 65 °C. Melting curves were performed after the end of the amplification cycles to validate the specificity of the amplified products. Standard curves were generated using tenfold serial dilutions of DNA from MT2 cells (104–101) and normalized to three copies of the HTLV-1 pol gene and two copies of the cellular albumin gene per MT2 cell. All standard dilutions and individual samples were run in duplicate for both HTLV-1 and albumin DNA quantifications. Standard curves were accepted when slopes were between −3.10 and −3.74 and the r 2 was >0.99. The value for the HTLV-1 proviral load was reported as [(pol average copy number)/(albumin average copy number/2)] × 10,000 and expressed as the number of HTLV-1 proviral copies/10,000 cells. Mean coefficient of variation of Ct for pol in intraassays and interassays was 1.4 and 2 %, respectively. For albumin, mean coefficient of variation of Ct in intraassays and interassays was 0.4 and 1.5 %, respectively.
Statistical analysis
HTLV-1 proviral load among the groups was compared using the Mann–Whitney U test. Correlations between proviral load for the first and last samples of the same group were performed using Spearman’s rank correlation. Intraindividual proviral load variation was determined by the coefficient of variation (%), which was calculated as Std dev/mean × 100. A p value <0.05 was considered statistically significant.
Results
HTLV-1 proviral load was quantified in 351 peripheral blood samples from 82 health individuals who remained asymptomatic carriers (AC) during follow-up for a mean time of 10 years (5 to 16.6 years). Proviral load was also quantified in 46 samples from 12 HAM/TSP patients (HAM) collected during a mean time of 8 years (5.4 to 13.5 years). It was possible to measure proviral load in six HAM individuals before they develop HAM/TSP (10 samples) and after the disease onset (20 samples), during a mean time of 8 years (3.8 to 16 years) of follow-up.
AC group was composed by 50 females and 32 males, with mean age at the first sample collection of 39 years. During their follow-up, they remained without relevant signs, such as neurogenic bladder or intestine and walking disability until to the last proviral load analysis (last sample collection). The HAM group had eight females and four males, with mean age of 53 years. The iHAM group had five females and one male, with mean age of 44 years (Table 1). The difference of age was statistically significant between AC and HAM groups (p < 0.001), but not between AC and iHAM groups (p = 0.373) or between HAM and iHAM groups (p = 0.089; unpaired t test).
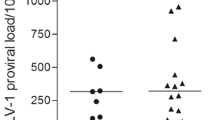
Mean proviral load of all samples analyzed during the follow-up was compared among the groups. The AC group showed significantly lower level of proviral load (median of 96 proviral copies/10,000 cells) when compared with the HAM group (median of 373 proviral copies/10,000 cells; p = 0.018) and with the iHAM group (median of 421 proviral copies/10,000 cells; p = 0.004), whereas the difference between HAM and iHAM groups was not significant (p = 0.349) (Fig. 1a).
HTLV-1 proviral load in asymptomatic carriers (AC), HAM/TSP patients (HAM), and individuals with incident HAM/TSP (iHAM). a Points represent mean proviral load value of each individual. The line indicates median proviral load of group, and the dotted line represents a threshold value (1 %) to define low (≤1 %) and high (>1 %) proviral load level. b Intragroup comparison of proviral load measured in the first (black symbols) and last (white symbols) samples. c Correlation between proviral load values for the first and last samples of AC, HAM, and iHAM groups. Mann–Whitney U test (a, b) and Spearman’s correlation (c)
The median of proviral load was 102 proviral copies/10,000 cells in the first samples and 65 proviral copies/10,000 cells in the last samples in the AC group, significantly lower than in the HAM group (556 proviral copies/10,000 cells in the first samples and 168 proviral copies/10,000 cells in the last samples) and in the iHAM group (235 proviral copies/10,000 cells in the first samples and 228 proviral copies/10,000 cells in the last samples) (Table 1 and Fig. 1b).
Although the difference of proviral load levels between the first and last samples from the same group was not significant, proviral load tended to decrease over time in the three groups analyzed. In addition, the groups tended to become more homogeneous regarding the level of proviral load, as shown by smaller standard deviation values observed in the analysis of the last samples (Table 1).
Analysis of correlation of proviral load quantified in the first and last samples showed a significant and strong positive correlation in AC (r = 0.78, p < 0.001) and HAM (r = 0.85, p < 0.001) groups, but not in individuals who showed incidence of HAM/TSP (r = −0.20, p = 0.714), suggesting that these individuals had not yet reached an equilibrium of proviral load level before the installation of disease (Fig. 1c).
Table 2 shows clinical characteristics and proviral load in the beginning and final of follow-up of HAM/TSP patients. Patients who remained able to walk without support (1 and 2) had low proviral load and showed only a slight increase of proviral load during the follow-up. Patients who showed worsening of walking disability (3 and 4) presented different ranges of proviral load. One (patient 3) had low proviral load that increased slightly during the follow-up; the other (patient 4) had high proviral load in his first sample, which decreased after 7 years. Patients with worse walking disability had high proviral load at the first sample (except patient 8) that decreased at the final of the follow-up (except patient 10). In general, considering all patients, proviral load tended to decrease or to remain stable during the period of follow-up (Fig. 2).
Table 3 shows clinical characteristics and proviral load in the incident cases of HAM/TSP, before and after the disease diagnosis. For all cases, proviral load was high before the onset of disease, which tended to decrease or to remain stable after HAM/TSP development (Fig. 2). Thus, the levels of proviral load observed in the period before the disease diagnosis did not show an increase that could predict the development of HAM/TSP.
We also analyzed the fluctuation of proviral load among all the intraindividual samples analyzed during the follow-up. Considering a variation of at least 0.5 log of proviral load between individual samples with the highest and lowest values of proviral load as a relevant fluctuation, variation was seen in 61 % (50/82) of AC, 75 % of HAM (9/12), and 67 % of iHAM (4/6) individuals. In spite of this variation, the majority of individuals in the three groups (AC 81 %; HAM 92 %; iHAM 83 %) remained at the same level of proviral load during the follow-up, which means that whoever had initial high proviral load (>1 %) remained with high levels and those who had low proviral load (≤1 %) remained with low values. Sixteen/82 (19 %) of AC individuals showed changes in the level of the proviral load at the final of the follow-up period. Of these, 12 changed from high to low proviral load levels.
The coefficient of variation of proviral load for individual samples was significantly higher in the HAM than in the AC group (p = 0.015) and in the iHAM group (p = 0.022) but was not statistically different between the AC and iHAM groups (p = 0.363) (Fig. 3). Comparing the coefficient of variation of proviral load for AC individual samples with low and high proviral loads, the mean was similar between them (63 and 57 %, respectively; p = 0.330).
Discussion
The level of HTLV-1-infected cells in the peripheral blood has been considered an important risk marker for several diseases associated with the virus, because of the significant differences of proviral load observed between groups of healthy carriers and symptomatic individuals, including HAM/TSP patients (Nagai et al. 1998; Furtado et al. 2012). Understanding how HTLV-1 proviral load ranges over time in infected individuals is necessary to define whether it could be used as a strong prognostic marker for HTLV-1-associated diseases. Only a few studies have measured HTLV-1 proviral load in samples from infected individuals collected in long-term follow-up. In this study, we quantified HTLV-1 proviral load in 94 Brazilian individuals, during a mean follow-up of 8 to 10 years, and including incident cases of HAM/TSP, whose serial samples were collected before and after the onset of disease. Although the Brazilian population is quite a mixed race, we do not consider the color of the skin to define ethnicity since, at the individual level, there is a significant dissociation of color and genomic ancestry (Parra et al. 2003). Thus, it is very difficult to classify the individuals as Caucasian, African-American, and others by self-reported skin color. For tri-hybrid Latin American populations, such as Brazilians, ancestry information is required for African, European, and Native American populations. Recently, we obtained data that estimated admixture in individuals from the GIPH cohort, using several ancestry informative markers (data not published). Among the individuals analyzed, we have 51 of 82 AC and 15 of 18 HAM patients who were enrolled in the current study. The ancestry frequencies of these AC and HAM were, respectively, 53.0 and 55.7 % European (p = 0.630), 37.7 and 36.0 % African (p = 0.765), and 9.3 and 8.2 % Amerindian (p = 0.592). The difference was not significant for any of the parental populations between AC and HAM. Therefore, we believe that the ethnic diversity in the individuals analyzed in the present study did not influence the results that were shown.
As expected, HAM patients in the present study showed significant higher proviral load than AC. Individuals who developed HAM/TSP during the cohort follow-up also showed significant higher proviral load than AC.
Although the proviral load was quite variable among individuals classified as AC and HAM groups, the difference of proviral load level quantified in the first and last samples from individuals belonging to the same group was not significant, indicating that the proviral load is rather stable over time, as reported elsewhere (Taylor et al. 1999; Takenouchi et al. 2003; Kwaan et al. 2006). Demontis et al. (2013) described a significant decrease of proviral load between the first and last samples in asymptomatic carriers, but not in HAM/TSP patients. We observed a decreasing trend of the proviral load measured from the first to the last sample for all groups, which suggests an equilibrium of the proviral load level in each individual, probably dependent on the ability to limit the expansion of infected cells. This decrease of proviral load over the course of infection tended to turn the groups more homogeneous, since individuals who initially had high levels of proviral load showed a reduction to levels that are closer to the group average.
It was interesting to observe a significant and strong correlation between the first and last measures of proviral load in AC and HAM groups, which means that individuals with low proviral load had a trend to remain with low levels, whereas those with high concentration will probably show high level (>1 %) of proviral load over time, in spite of its decreasing trend. In contrast, this correlation was not observed for the incident cases of HAM/TSP. This lack of correlation in this particular group (iHAM) may be due to the comparison of samples in different situations of clinical status (before and after HAM installation). This may suggest that after the onset of the neurologic disease the proviral load progressively achieves equilibrium.
Comparing the level of proviral load and clinical characteristics of HAM/TSP patients, those with worse walking disability showed higher proviral load than individuals who remained able to walk without support. An increase in proviral load from the beginning to the end of the follow-up was only observed in patients with low proviral load but did not necessarily correspond with clinical worsening. The range of proviral load in the incident cases of HAM/TSP also did not show a pattern in which clinical worsening was associated with increased proviral load.
The coefficient of variation showed that individual variation of proviral load was significantly higher in HAM than in the AC group. In addition, HAM patients showed more fluctuation (at least 0.5 log) of proviral load than AC. In spite of this fluctuation, the majority of AC and HAM individuals remained at the same proviral load level [low (≤1 %) or high (>1 %)]. Thus, slight change in proviral load seems to be common and not necessarily clinically relevant, since asymptomatic individuals did not show clinical worsening, such as neurogenic bladder or intestine and motor disability during the follow-up. Kwaan et al. (2006) described that subjects with urinary tract infections had a greater increase in proviral load over time than those without such infection. Comorbidities observed in the incident cases of HAM/TSP are very prevalent in the general population. However, we have observed in our cohort that major depression, hypertension, diabetes, hypothyroidism, dyslipidemia, and keratoconjunctivitis sicca seem to be more prevalent in individuals infected with HTLV-1, especially in HAM/TSP patients. Autoimmune mechanisms observed in chronic infections, which lead to increased inflammatory response, endovascular injury, and possible impairment of the microcirculation, may be involved.
We have observed, along with other authors (Demontis et al. 2013), a significant rate of asymptomatic carriers with high proviral load. In the GIPH cohort, these individuals have showed high proviral load level for a long time, which indicates that the levels of HTLV-1-infected cells in the peripheral blood are not enough to trigger HAM/TSP. The level of HTLV-1 proviral load seems to be regulated by the immune response of the host, which should be effective in detecting the infected cell and controlling its spread. For HAM/TSP onset, it seems to be essential that the infected cells cross the blood-brain barrier to reach the CNS, which leads to subsequent damage of neuronal cells by the chronic immune response. Studies have shown that HTLV-1 is able to disrupt the blood-brain barrier (Afonso et al. 2008), and Tax protein seems to play a major role in this process, enhancing the expression of cellular genes that promote proliferation and alter the blood-brain barrier permeability, enabling lymphocyte passage through it (Matsuura et al. 2010). This is corroborated by the evidence that proviral load in cerebrospinal fluid is always higher than in the peripheral blood in HAM/TSP patients, but not in asymptomatic carriers (Lezin et al. 2005; Demontis et al. 2013; Brunetto et al. 2014). Understanding how proviral load in the CSF varies in parallel with proviral load in the blood would help to understand the triggering process of HAM/TSP. However, collecting CSF is an invasive method, hindering its use in research of HTLV-1 pathogenesis and in the follow-up in viral carriers. In the present study, three patients (HAM, 1 and 8; iHAM, 5) had proviral load quantified in CSF and in blood. For all of them, proviral load in the CSF was higher than in the blood.
Why are there asymptomatic carriers with high proviral load in the peripheral blood who do not develop HAM/TSP is a question still unclear. However, we have previously described that high tax messenger RNA (mRNA) load instead of high HTLV-1 DNA proviral load seems to be a key factor associated to HAM/TSP development (Andrade et al. 2013). In addition, other studies described that both the level of Tax expression and lysis of infected cells were significant predictors of the proviral load and had been significantly and independently associated with HAM/TSP (Asquith et al. 2005a, b). Hence, proviral load may be rather a consequence of these processes than a factor that triggers HAM/TSP.
The measure of proviral load before and after the onset of HAM/TSP in six individuals showed that, in general, proviral load does not have a progressive increase before the neurologic disease is established. Each individual in our study had already high proviral load in the first measurement, before the onset of clinical disease, which tended to decrease over time. We did not observe a sharp progression of proviral load preceding the onset of disease; therefore, it was not possible to distinguish a profile that could differentiate them from asymptomatic carriers with high proviral load. Although only few incident cases were analyzed and few proviral load measurements were taken before the onset of disease, the data suggests that following up the levels of proviral load does not help to predict HAM/TSP development. These data is very rare in the literature, being possible only through prospective cohort studies, but additional studies with sample collection in regular and shorter intervals are necessary to confirm whether proviral load could be of use as a prognostic marker for neurologic disease.
In conjunction, the data presented here suggest that there is an individual threshold of proviral load typical to each carrier, with overall stability in time, and that it can be high in the peripheral blood for many carriers without necessarily implying in HTLV-1-associated disease progression. Therefore, we suggest that an expressive increase in proviral load in the peripheral blood, atypical of particular individual threshold, should be an alert to investigate other signs of HAM/TSP development, such as proviral load in the CSF and tax mRNA load. Unfortunately, they are much more difficult to assess. Thus, monitoring proviral load in the peripheral blood is important to assess the individual level to each carrier, being alert for atypical changes.
Conclusion
Our results suggest that serial quantification of proviral load in the peripheral blood does not seem to be a good prognostic marker for development of HAM/TSP. The data show that proviral load trends to reach an equilibrium plateau over time, characteristic of each individual, and an increase of the proviral load appears to be followed by stabilization and a slight decrease to lower levels. Individuals may show high proviral load for long periods of time in the peripheral blood, without developing HAM/TSP. Although proviral load is considered an important risk marker for the development of HAM/TSP, our study showed that change in clinical status does not coincide with significant changes in the peripheral blood proviral load.
References
Afonso PV, Ozden S, Cumont M-C, Seilhean D, Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, Couraud P-O, Pique C, Ceccaldi P-E, Romero IA (2008) Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog 4(11):e1000205
Andrade RG, Gonçalves PC, Ribeiro MA, Romanelli LCF, Ribas JG, Torres EB, Carneiro-Proietti ABF, Barbosa-Stancioli EF, Martins ML (2013) Strong correlation between tax and HBZ mRNA expression in HAM/TSP patients: distinct markers for the neurologic disease. J Clin Virol 56:135–140
Andrade RG, Ribeiro MA, Namen-Lopes MS, Silva SM, Basques FV, Ribas JG, Carneiro-Proietti AB, Martins ML (2010) Evaluation of the use of real-time PCR for human T cell lymphotropic virus 1 and 2 as a confirmatory test in screening for blood donors. Rev Soc Bras Med Trop 43:111–115
Asquith B, Mosley AJ, Barfield A, Marshall SE, Heaps A, Goon P, Hanon E, Tanaka Y, Taylor GP, Bangham CR (2005b) A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J Gen Virol 86:1515–1523
Asquith B, Mosley AJ, Heaps A, Tanaka Y, Taylor GP, McLean AR, Bangham CR (2005a) Quantification of the virus-host interaction in human T lymphotropic virus I infection. Retrovirology 2
Brunetto GS, Massoud R, Leibovitch EC, Caruso B, Johnson K, Ohayon J, Fenton K, Cortese I, Jacobson S (2014) Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations. J Neurovirol 20:341–351
Demontis MA, Hilburn S, Taylor GP (2013) Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retrovir 29:359–364
Furtado MSBS, Andrade RG, Romanelli LC, Ribeiro MA, Ribas JG, Torres EB, Barbosa-Stancioli EF, Proietti AB, Martins ML (2012) Monitoring the HTLV-1 proviral load in the peripheral blood of asymptomatic carriers and patients with HTLV-associated myelopathy/tropical spastic paraparesis from a Brazilian cohort: ROC curve analysis to establish the threshold for risk disease. J Med Virol 84:664–671
Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de Thé G (1985) Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 2:407–410
Gonçalves DU, Guedes AC, Proietti AB, Martins ML, Proietti FA, Lambertucci JR, Interdisciplinary HTLV-1/2 Research Group (2003) Dermatologic lesions in asymptomatic blood donors seropositive for human T cell lymphotropic virus type-1. Am J Trop Med Hyg 68:562–565
Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, Shirakawa S, Miyoshi I (1981) Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A 78:6476–6480
Hishizawa M, Imada K, Ishikawa T, Uchiyama T (2004) Kinetics of proviral DNA load, soluble interleukin-2 receptor level and tax expression in patients with adult T-cell leukemia receiving allogeneic stem cell transplantation. Leukemia 18:167–169
Izumo I (2010) Neuropathology of HTLV-1-associated myelopathy (HAM/TSP. Neuropathology 30:480–485
Kwaan N, Lee TH, Chafets DM, Nass C, Newman B, Smith J, Garratty G, Murphy EL, HTLV Outcomes Study (HOST) Investigators (2006) Long-term variations in human T lymphotropic virus (HTLV)-I and HTLV-II proviral loads and association with clinical data. J Infect Dis 194:1557–1564
Lezin A, Olindo S, Olière S, Varrin-Doyer M, Marlin R, Cabre P, Smadja D, Cesaire R (2005) Human T lymphotropic virus type I (HTLV-I) proviral load in cerebrospinal fluid: a new criterion for the diagnosis of HTLV-I-associated myelopathy/tropical spastic paraparesis? J Infect Dis 191:1830–1834
Martin F, Taylor GP, Jacobson S (2014) Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev Clin Immunol 10:1531–1546
Matsuura E, Yamano Y, Jacobson S (2010) Neuroimmunity of HTLV-I infection. J Neuroimmune Pharmacol 5:310–325
Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M (1998) Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol 4:586–593
Olindo S, Lézin A, Cabre P, Merle H, Saint-Vil M, Kaptue ME, Signate A, Césaire R, Smadja D (2005) HTLV-1 proviral load in peripheral blood mononuclear cells quantified in 100 HAM/TSP patients: a marker of disease progression. J Neurol Sciences 237:53–59
Ono A, Ikeda E, Mochizuki M, Matsuoka M, Yamaguchi K, Sawada T, Yamane S, Tokudome S, Watanabe T (1998) Provirus load in patients with human T-cell leukemia virus type 1 uveitis correlates with precedent Graves’ disease and disease activities. Jpn J Cancer Res 89:608–614
Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M (1986) HTLV-I associated myelopathy, a new clinical entity. Lancet 1:1031–1032
Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD (2003) Color and genomic ancestry in Brazilians. Proc Natl Acad Sci U S A 100:177–182
Saito M (2010) Immunogenetics and the pathological mechanisms of human T-cell leukemia virus type 1- (HTLV-1-) associated myelopathy/tropical spastic paraparesis (HAM/TSP). Interdiscip Perspect Infect Dis 2010:478461
Takenouchi N, Yamano Y, Usuku K, Osame M, Izumo S (2003) Usefulness of proviral load measurement for monitoring of disease activity in individual patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Neurovirol 9:29–35
Taylor GP, Tosswill JH, Matutes E, Daenke S, Hall S, Bain BJ, Davis R, Thomas D, Rossor M, Bangham CR, Weber JN (1999) Prospective study of HTLV-I infection in an initially asymptomatic cohort. J Acquir Immune Defic Syndr 22:92–100
WHO (1989) Report from the scientific group on HTLV-1 infection and its associated diseases, convened by the regional office for the Western Pacific of the World Health Organisation in Kagoshima, Japan, 10–15 December 1988. Wkly Epidemiol Rec 49:382–383
Yakova M, Lézin A, Dantin F, Lagathu G, Olindo S, Jean-Baptiste G, Arfi S, Césaire R (2005) Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology 2:4
Yamano Y, Sato T (2012) Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front Microbiol 3:389
Yoshida M, Seiki M, Yamaguchi K, Takatsuki K (1984) Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA 81:2534–2537
Acknowledgments
This study was funded by Fundação Hemominas, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). M.L. Martins, J.C. Guimarães, and A.B.F. Carneiro-Proietti received fellowships from FAPEMIG and CNPq. The authors would like to thank all the participants and all members of the Interdisciplinary HTLV Research Group for their ongoing collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were in accordance with the standards of the Hemominas Ethical Committee. Written consent for the study was obtained from all individuals.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Martins, M.L., Guimarães, J.C., Ribas, J.G. et al. Long-term follow-up of HTLV-1 proviral load in asymptomatic carriers and in incident cases of HAM/TSP: what is its relevance as a prognostic marker for neurologic disease?. J. Neurovirol. 23, 125–133 (2017). https://doi.org/10.1007/s13365-016-0484-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-016-0484-x