Abstract
Tropical rainforest canopies harbor nearly half of the world’s biodiversity. Previous research on rainforest ecosystems has primarily focused on the terrestrial stratum, leading to a limited understanding of forest canopies. Camera traps have seen a wide application in the studies of terrestrial mammals, but their utility for documenting arboreal mammal communities has been far more limited. Financial resources, field training, and access to equipment and logistical constraints may have precluded researchers from undertaking systematic arboreal camera surveys, especially in the Global South countries. We deployed arboreal and terrestrial cameras to document the mammal assemblage in Kadumane estate, Western Ghats, India. During April–May 2022, we documented 3 exclusively arboreal, 11 semi-arboreal and 14 terrestrial species, using 16 cameras in the canopies and 13 cameras on the ground. Using rarefaction curves, we find that 648 trap-nights were sufficient to document all the arboreal species, while > 1350 trap-nights of additional effort would have been required to document all the terrestrial species in our study site. For each species, we generated an arboreality index (calculated from proportional capture rates) to gauge its propensity for arboreal habits. We also compared the efficacy of using different baits; species responses to shrimp–dry fish baits indicated a reduction in rodent captures when carnivore captures were higher. Our study deliberates on the resources, logistical considerations, and advantages of arboreal camera surveys to study mammal assemblages in forest canopies. Importantly, we highlight the utility of such surveys for understanding the ecology of rare, elusive, and hitherto under-studied species that may be threatened with extinction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical rainforests are among the most biodiverse terrestrial ecosystems (Ashton 1977; Benton et al. 2022). Rainforests are structurally complex habitats that include not only plants at ground level, but also multiple layers of vegetation extending up to their towering canopies (Moffett 2000). Most research in rainforests has been restricted to the terrestrial stratum owing to difficulties in accessing the canopies that can sometimes extend up to 40 m above the forest floor. Aptly termed “the last biotic frontier” (Godoy-Güinao et al. 2018), research forays into and exploration of the high canopies continue to be hampered by inadequate resources or other logistical challenges, particularly in the Global South countries (Nadkarni et al. 2011; Wardhaugh 2023). Given the gaps in the current knowledge of arboreal habitats within tropical forest systems, several recent studies have called for further exploration and a thorough understanding of the composition and functioning of rainforest ecosystems focusing on these canopies (Basham et al. 2023; Cudney-Valenzuela et al. 2023; Lowman 2009).
Tropical forest canopies are thought to support nearly half of the world’s biodiversity (Lowman et al. 2012), containing three quarters of forest vertebrates and a large proportion of mammals. Collectively, these species play important ecological roles such as predation, seed dispersal, pollination, and herbivory (Kays and Allison 2001; Whitworth et al. 2019). With the exception of large, conspicuous, and vocal species such as primates, the ecology of arboreal mammals in the tropics remains severely under-studied (Haysom et al. 2021; Kays and Allison 2001). The conservation and management of these canopies and the species network they support require, at the very least, baseline inventories/checklists, distribution assessments, and population estimates. Such information is typically difficult to obtain, given the cryptic nature and nocturnal habits of most canopy species, in addition to the inaccessibility of their habitats using most commonly applied survey methods.
The use of motion-triggered cameras for studying terrestrial mammals has become widespread due to advancements in technology (O’Connell et al. 2011) but has seen limited application for arboreal animals (Moore et al. 2021). Previous camera-trap studies in the canopy have documented the presence, behavior, and activity of animals (Bezerra et al. 2014; Oliveira-Santos et al. 2008; Suzuki and Ando 2019) in relation to specific habitat features, such as fruiting trees (Ganesh and Devy 2006; Jayasekara et al. 2007; Otani 2001) or canopy bridges (Gregory et al. 2017). Studies that used camera-trap data to survey arboreal mammal communities are limited to six sites in the Neotropics, Africa, and South East Asia (Bowler et al. 2017; Haysom et al. 2021; Hongo et al. 2020; Moore et al. 2021; Whitworth et al. 2019; Whitworth et al. 2016); these studies were primarily focused on medium- to large-bodied mammals. Additionally, many canopy-based camera-trap studies place the traps at heights of < 10 m, which does not a fully optimize data acquisition across the vertical dimension. While camera-trapping in high canopies holds certain promise, it is yet to be widely adopted as a standard tool for monitoring arboreal mammal communities.
Here, we test and compare the results from terrestrial-only versus terrestrial-plus-arboreal sampling to inventory the mammal community in a tropical rainforest in India’s Western Ghats. We also explore the influence of different baits on the trap-encounter rates of various mammal groups. Our overall goal is to establish the utility of arboreal camera-trapping as an effective approach for detecting species that are rare, elusive, and cryptic, and thereby making a case for its application in future studies of such species.
Methods
Study area
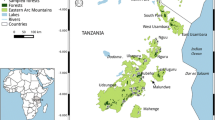
The Western Ghats is a mountain range in South West India (8–25° N 73–76° E). The range receives more than 6000 mm of rainfall annually, most of which falls between June and September (Venkatesh et al. 2021) covered by a mix of tropical moist evergreen forest, tropical semi-evergreen forest, and tropical moist deciduous forests. The location is a global biodiversity hotspot, supporting many mammal species that are arboreal or semi-arboreal, including endemic rodents, squirrels, small carnivores, and primates (Norman 2003). In the recent past, the region has undergone extensive deforestation, and degraded forests have increased in area. (Jha et al. 2000). Our study was conducted within the privately-owned Kadumane tea estate, in the Hassan district of Karnataka state, Western Ghats. With an elevation range of 900–1100 m, the estate has a mosaic of tea, abandoned cardamom and coffee plantations, and old growth forests. Some of the forest fragments lie in close proximity to protected areas (Ramachandra et al. 2015). There are 36 species of non-volant mammals documented in the estate (Supplementary Table 1). Our study was conducted within an area of approximately 72 ha in the northernmost part of the estate (Fig. 1).
Top-left Map inset showing the location of the study site in Western Ghats, in Southwest India. Bottom-left The study site, Kadumane tea estate, which is part of an important forest corridor between Kudremukh National Park to the north and Pushpagiri Wildlife Sanctuary in the south. Right Location of cameras from block 1 (red squares) and block 2 (blue squares) in the forested area within Kadumane
Data collection
We undertook camera-trap surveys during the pre-monsoon period between April and June 2022. Considering the logistical challenges involved in setting up cameras atop tree canopies, and that we had a limited number of camera-trap units, we adopted a block-shifting sampling design following protocols detailed in O’Connell et al. (2011). Trapping effort in block 1 was used for standardizing the protocols, and 6 sites were selected within this block. Once the protocols were standardized, we selected 10 sites in block 2. We first surveyed the area for potential sites with canopy contiguity as the main criteria. Trees for the arboreal cameras were selected based on field observations of animal movement and logistics of access and safety. As Kadumane is embedded in an agro-forestry landscape, we were cautious of overhead electric lines. To capture the arboreal assemblage across the vertical strata in the canopy, cameras were deployed in the mid- (n = 16) or upper-canopy (n = 9; a subset of the 16 sites) at a height of 4–15 m (mean height = 6 m). In cases where the site had cameras in mid- as well as upper-canopy, the cameras were placed 2–5 m apart (vertically). The average distance between adjacent arboreal camera-trap locations was 115 m (range: 29 to 491 m). Terrestrial cameras were placed in 13 sites within a radius of ~20 m, from the corresponding tree with arboreal cameras (with one exception, where the site only had a terrestrial camera but no arboreal cameras). The average distance between adjacent terrestrial camera-trap locations was 134 m (range: 39 to 494 m). Terrestrial cameras were positioned along forest roads, foot trails, dead logs, and nallas to maximize the probability of detecting all species found in the area.
We used the Rapid Ascent Descent System (RADS; Maher 2006; Fig. 2), a single-rope technique to access the canopy and deploy the arboreal camera units. We deployed Browning Strike Force Pro XD (Model: BTC5PXD, Browning Arms Company Ltd, USA) camera-traps equipped with infrared flash (IR) and motion sensors. The cameras were mounted onto the trunk or the branches using camera mounts (AlexVyan 5 mm ¼″ 7cm metal camera bracket) which help position and lock the camera in a suitable angle and direction; straps were used to secure the camera and prevent accidental fall due to animal movement. The zone of detection was targeted along branches connected to the surrounding trees. To reduce false triggers, some leaves within the view-shed area were removed (Gregory et al. 2014), while ensuring minimal alteration to the vegetation. Cameras were set to record videos, initially with a 10 s delay between consecutive encounter events, which was later changed to 1 s since the number of false triggers were fewer than anticipated. Flash intensity setting was set to “Fast Motion” (as recommended by the manufacturer) to obtain clear captures of fast-moving species, and sensor “Motion Detect” was set to “Long” to maximize the detections of species that were not necessarily within close proximity of the camera. All sites were revisited regularly to check the cameras and replace memory cards/batteries. To further improve the detection frequency, we tested five types of baits (n = number of camera-trap sites, trap nights = total effort across camera-trap sites): (1) banana–papaya mashed, mixed and fermented overnight (n = 12, trap nights = 79); (2) banana–papaya mashed, mixed and fermented overnight, with honey added while baiting (n = 19, trap nights = 80); (3) dry fish (n = 19, trap nights = 101); (4) dry fish and shrimp paste (n = 18, trap nights = 139); and (5) dry fish, aniseed oil, and fish sauce (n = 19, trap nights = 138). The baits were used one at a time across locations, i.e., at any given time all locations had a single bait type. Baits were changed or replenished when cameras were revisited. There were no un-baited sites as control. In the absence of a scientifically robust protocol, we refrained from conducting any comparative statistical tests on bait efficacy and restrict our results to observations of capture rates per 100 trap nights across bait types.
a Schematic of each sampling site showing location of arboreal cameras with respect to the tree; b picture demonstrating Rapid Ascent Descent System (RADS); c equipment used for RADS, which include (i) helmet, (ii) descender (Petzl I’D), (iii) ascender, (iv) harness, (v) pulley, (vi) carabiners, (vii) foot loop, and (viii) climbing rope
Analysis
We used digiKam software (version 7.8.0; http://www.digikam.org) to catalogue and tag the videos. Species were identified using Menon (2014); in cases where we could not identify the species, especially small rodents and shrews, we assigned the identity up to the family level. Videos where the animal was only partially visible and not identifiable were tagged as “unidentified”. False triggers from vegetation and canopy movement were separated out, and video captures of team members, local people, domestic animals, birds, reptiles, invertebrates, and vehicles were tagged separately. The data were processed and synthesized using R package camtrapR (Niedballa et al. 2016).
We examined mammal species richness based on video captures from terrestrial and arboreal cameras. Species accumulation curves were generated for (i) arboreal, (ii) terrestrial, and (iii) arboreal–terrestrial combined, using the number of trap-nights as effort, with R package iNEXT (Hsieh et al. 2013). Following this, we used the accumulation curve to extrapolate species detections to 2000 trap-nights, which is approximately thrice the minimum observed sample size, as recommended by Hsieh et al. (2013). We did so to evaluate the adequacy of our effort, while also obtaining an estimate of the effort required to document the complete species assemblage. We used 84% confidence intervals to evaluate the significance of the difference between the richness (MacGregor-Fors and Payton 2013).
We parsed our data and grouped the species based on (1) the stratum where they were detected, i.e., arboreal (only captured in canopy), semi-arboreal (captured in canopy and on the ground), or terrestrial (only captured on the ground); (2) IUCN threat status—threatened (CR, EN, and VU) or non-threatened (LC, NT, and DD); (3) body size—small (< 1 kg), medium (1–5 kg), or large (> 5 kg); (4) time of detection (nocturnal or diurnal); (5) taxonomy—Rodentia, Primates, Carnivora, Artiodactyla, Proboscidea, and Pholidota. Information on body size and taxonomic classification was sourced from Menon (2014), and the IUCN Red List (accessed December 2022). For each of the categories listed above, we examined species richness across strata, based on rarefied species accumulation curves at the maximum sample size with 84% confidence intervals.
For each species, we computed “capture rate” as the number of captures per 100 trap-nights. Encounters that were at least 30 min apart were treated as independent captures (Laughlin et al. 2020). First, we compared taxonomic group-wise capture rates for each bait type across all cameras (stratum-agnostic). Second, using the same capture rates but calculated separately for arboreal cameras and terrestrial cameras, we generated species-wise “Arboreality Index”, calculated as:
The index values (modified from Hongo et al. 2020) range between 0 and 1. Species whose values are closer to 0 are more terrestrial; values closer to 0.5 indicate semi-arboreal habits, and values closer to 1 indicate a greater propensity for arboreal habitats.
Results
Survey effort summary
We recorded 28 species of mammals during 1196 independent capture events across 648 and 392 trap-nights from arboreal cameras and terrestrial cameras, respectively. We recorded a total of 2112 videos of reptiles, birds, and mammals across arboreal and terrestrial cameras. We excluded 74 videos from our analysis which were of species that could not be identified; 55 of these were of small rodents and shrews. There were 1512 videos from false triggers, of which 1299 were from arboreal cameras. From the arboreal cameras, we detected 14 species of which 3 were exclusively in the canopy. Terrestrial cameras detected a total of 25 species (see Fig. 3).
Species captured during the survey; top (left to right) Nilgiri marten (Martes gwatkinsii), grey slender loris (Loris lydekkerianus), Indian giant flying squirrel (Petaurista philippensis), and the Malabar giant squirrel (Ratufa indica); bottom (left to right) Malabar spiny dormouse (Platacanthomys lasiurus), Asian small-clawed otter (Aonyx cinereus), leopard cat (Prionailurus bengalensis), and the Indian pangolin (Manis crassicaudata)
Sampling effectiveness across strata
The rarefaction curve for arboreal species appeared to reach an asymptote between 500 to 1000 trap-nights, suggesting that the arboreal inventory was near complete (Fig. 4). In contrast, the terrestrial curve continued to rise much beyond the maximum observed sample size, indicating that our sampling effort was inadequate to document all mammals in the terrestrial stratum. This was corroborated by the extrapolated curves, based on which we predict that terrestrial communities may require 1500–2000 trap-nights for a near-complete inventory.
Species accumulation curves for arboreal, terrestrial, and arboreal–terrestrial (combined) captures. Shaded areas around the curves represent the 84% confidence intervals. No overlap in the adjacent confidence intervals indicates a significant difference in the number of species with respect to trap-nights
Mammal communities across strata
The number of species detected by arboreal and terrestrial cameras was split into groups according to the stratum in which they were detected, IUCN threat status, body size, and taxonomic group are presented in Fig. 5. We had similar success in detecting semi-arboreal species in both arboreal and terrestrial cameras. On the other hand, terrestrial camera-traps detected more viverrids, herpestids, and felids (Fig. 6).
Number of species in arboreal and terrestrial cameras based on the stratum where they were detected (top-left), IUCN threat status (top-right), body size (bottom-left), and the time of detection (bottom-right). Error bars are standard error values calculated from species accumulation curves at the maximum sample size using the iNEXT package (Hsieh et al. 2013)
Efficacy of bait type
Species within the Rodentia group did not appear to show a clear preference for bait type, though the number of rodent captures reduced where dried fish and shrimp paste were used. For Carnivora, captures were higher in locations with dried fish and dried fish and shrimp paste and extremely low when only banana–papaya paste was used. We did not find any discernible patterns for the other taxonomic groups with relation to bait type (Supplementary Figure 1).
Arboreality of the mammal community
The Malabar spiny dormouse (Platacanthomys lasiurus) and the Sahyadri forest rat (Rattus satarae) had the highest capture rate per 100 trap-nights in arboreal and terrestrial cameras, respectively. Six of the 11 species had an arboreality index > 0.7, while the remaining had an arboreality index below 0.25 (Table 1). The Nilgiri marten (Martes gwatkinsii) was the only semi-arboreal carnivore with a high arboreality index (0.71); the brown palm civet (Paradoxurus jerdoni) was the only other semi-arboreal carnivore (Table 1). The endangered Nilgiri long-tailed tree mouse (Vandeleuria nilagirica) had a marginally higher capture rate in the canopies. We had one detection each of the black-footed grey langur (Semnopithecus hypoleucos), jungle cat (Felis chaus), leopard (Panthera pardus), Indian pangolin (Manis crassicaudata), and greater bandicoot rat (Bandicota indica) during the sampling period (Supplementary Table 1); only the langur was detected in the canopy, while the rest were in the terrestrial stratum.
Discussion
We present the first comparative account of terrestrial-only versus terrestrial-plus-arboreal sampling for mammals in India. Our arboreal cameras recorded the giant flying squirrel and the grey slender loris—known to be obligate arboreal species—which would have been missed in terrestrial surveys. Sampling techniques for such species have typically relied on direct observations, spot-lighting (Kumara and Radhakrishna 2013), or by way of tracking vocal responses to predator call playbacks (Babu and Jayson 2009). Such conventional sampling approaches can be sub-optimal; for example, line transect and fixed-width strip transect based studies have generally resulted in the underestimation of the population size for the grey slender loris (Kumara and Radhakrishna 2013). We highlight the significance of camera trapping in the canopies as an effective method to detect arboreal species in the tropics.
Our study was primarily aimed at gauging the feasibility and efficacy of camera trapping in tropical forest canopies to detect rare and elusive arboreal species in India’s Western Ghats. This exploratory survey was therefore limited to ~50 days, with a sampling effort of < 1000 trap-nights. Given this premise, we were unable to detect all the mammal species in the community. The projected species accumulation curve for data from terrestrial cameras predicted the presence of more species that may have been detected with additional effort. The missed species likely include gaur (Bos gaurus), tiger (Panthera tigris), dhole (Cuon alpinus), black-naped hare (Lepus nigricollis), crested porcupine (Hystrix indica), Asian house shrew (Suncus murinus), house mouse (Mus musculus), and the Indian field mouse (Mus booduga) known to be present in the landscape but not recorded in our survey (Supplementary Table 1). In comparison, our cameras in the canopies detected all arboreal species known from the landscape. This may be attributed to the relatively low diversity of arboreal species in the Western Ghats (Menon 2014) as compared to elsewhere in the tropics (Haysom et al. 2021; Moore et al. 2021; Whitworth et al. 2019).
Understanding rodent communities in tropical forests is crucial for making inferences on seed dispersal, seed predation, examining predator assemblages (whose primary prey consist of rodents), and disease ecology in the context of zoonotic spillovers (Ansil et al. 2021; Blackwell et al. 2001; Gopal et al. 2021). Such studies typically rely on live-trapping or terrestrial camera-trapping surveys. Our results highlight the importance of documenting rodents in the canopies (alongside terrestrial surveys), since a substantial proportion of species may go undetected when other methods are employed (Mills et al. 2016). Our surveys yielded a total of 1500 captures of 10 species that belong to the family Rodentia. Rodent captures may have been higher because of our choice of baits (Woodman et al. 1996). We note that the relative proportion of carnivore captures was high when baited using dry fish and shrimp paste. Importantly, rodent captures—which were consistently high across all bait types used in the study—showed lower rates when shrimp was used, possibly because these sites had higher carnivore visitation (Randler et al. 2020).
We use the arboreality index as a simple yet useful metric since it relies on proportional capture rates to determine a species’ propensity for arboreal habits while also accounting for unequal sampling effort across strata. Of particular importance is the value of this index with respect to the Nilgiri marten, a charismatic and elusive carnivore endemic to the Western Ghats that remains extremely under-studied (Anil et al. 2018; Krishna and Karnad 2010; Vijay et al. 2022). Almost all previous papers on the Nilgiri marten include opportunistic encounters; camera-trap studies have consistently reported low capture rates of the species, underscoring the logistical difficulties in documenting this threatened mustelid and understanding its ecology (Srivathsa et al. 2022). In comparison, we had 15 independent captures of the species within a short duration of ~50 days. Given the arboreality index of the marten was 0.7 (high arboreality), we propose that future studies on the species should be tailored to include arboreal surveys targeted at forest canopies. The same recommendation applies to studies of the endangered Nilgiri long-tailed tree mouse (arboreality index = 0.6), another information-poor endemic species in the landscape.
Studies undertaking spatio-temporal assessments of arboreal biodiversity using motion-triggered cameras generally cite increased financial costs and time effort as the main challenges (Haysom et al. 2021). Arboreal camera trapping does entail more effort as compared to terrestrial camera trapping—(i) safety and equipment training of survey personnel and (ii) finding ideal trap locations within the canopy that are conducive for deployment, optimizing target species detections and reducing false triggers. Selecting memory cards with sufficient storage is crucial as regular visits to check or service the arboreal traps may not be feasible. Various methods have been developed and tested for accessing tree canopies, and the use of the single-rope technique (SRT) over other methods has been well discussed by Anderson et al. (2015), Maher (2006), and Perry (1978). SRTs do not require the installation of permanent structures, do not injure the tree, but provide easy access to the entire tree. We highly recommend the use of RADS as compared to other single-rope or double-rope techniques, given that the costs of the climbing equipment employed were reasonable for our camera trapping exercise (see Supplementary Table 2).
To the best of our knowledge, this is the first study from South Asia to employ arboreal cameras in conjunction with terrestrial sampling to create a faunal inventory. While we advocate for the implementation of arboreal camera-trapping, we also suggest that researchers and practitioners planning to conduct arboreal surveys remain cognizant of all the above logistical and practical considerations. Many regions such as Northeast India, Northern Myanmar, and Southeast China have extensive tropical wet forests that are relatively unexplored. We recommend the systematic use of canopy cameras for its tremendous potential in the discovery, study, and monitoring of elusive arboreal wildlife.
Data availability
The datasets generated and analyzed as part of this study are available from the corresponding author on reasonable request.
References
Anderson DL, Koomjian W, French B, Altenhoff SR, Luce J (2015) Review of rope-based access methods for the forest canopy: safe and unsafe practices in published information sources and a summary of current methods. Methods Ecol Evol 6:865–872. https://doi.org/10.1111/2041-210X.12393
Anil G, Kishor N, Gafoor N, Ommer N, Nameer PO (2018) Observations on the Nilgiri marten Martes gwatkinsii (Mammalia: Carnivora: Mustelidae) from Pampadum Shola National Park, the southern Western Ghats, India. J Threat Taxa 10:11226–11230. https://doi.org/10.11609/jott.3446.10.1.11226-11230
Ansil BR, Mendenhall IH, Ramakrishnan U (2021) High prevalence and diversity of Bartonella in small mammals from the biodiverse Western Ghats. PLoS Negl Trop Dis 15:e0009178. https://doi.org/10.1371/journal.pntd.0009178
Ashton PS (1977) A contribution of rain forest research to evolutionary theory. Ann Missouri Bot Gard 64:694–705. https://doi.org/10.2307/2395295
Babu S, Jayson EA (2009) Anti-predator behaviour of large brown flying squirrel (Petaurista philippensis): is this an effective census method to survey the species? Curr Sci 96:772–773 http://www.jstor.org/stable/24104512. Accessed 14 Jan 2023
Basham EW, Baecher JA, Klinges DH, Scheffers BR (2023) Vertical stratification patterns of tropical forest vertebrates: a meta-analysis. Biol Rev 98:99–114. https://doi.org/10.1111/brv.12896
Benton MJ, Wilf P, Sauquet H (2022) The angiosperm terrestrial revolution and the origins of modern biodiversity. New Phytol 233:2017–2035. https://doi.org/10.1111/nph.17822
Bezerra BM, Bastos M, Souto A, Keasey MP, Eason P, Schiel N, Jones G (2014) Camera trap observations of nonhabituated critically endangered wild blonde capuchins, Sapajus flavius (Formerly Cebus flavius). Int J Primatol 35:895–907. https://doi.org/10.1007/s10764-014-9782-4
Blackwell GL, Potter MA, Minot EO (2001) Rodent and predator population dynamics in an eruptive system. Ecol Modell 142:227–245. https://doi.org/10.1016/S0304-3800(01)00327-1
Bowler MT, Tobler MW, Endress BA, Gilmore MP, Anderson MJ (2017) Estimating mammalian species richness and occupancy in tropical forest canopies with arboreal camera traps. Remote Sens Ecol Conserv 3:146–157. https://doi.org/10.1002/rse2.35
Cudney-Valenzuela SJ, Arroyo-Rodríguez V, Morante-Filho JC, Toledo-Aceves T, Andresen E (2023) Tropical forest loss impoverishes arboreal mammal assemblages by increasing tree canopy openness. Ecol Appl 33:e2744. https://doi.org/10.1002/eap.2744
Ganesh T, Devy MS (2006) Interactions between non-flying mammals and flowers of Cullenia exarillata Robyns (Bombacaceae), a canopy tree from the wet forests of Western Ghats, India. Curr Sci 90:1674–1679. http://www.jstor.org/stable/24091918. Accessed 14 Apr 2022
Godoy-Güinao J, Díaz IA, Celis-Diez JL (2018) Confirmation of arboreal habits in Dromiciops gliroides: a key role in Chilean temperate rainforests. Ecosphere 9:e02424. https://doi.org/10.1002/ecs2.2424
Gopal A, Mudappa D, Raman TRS, Naniwadekar R (2021) Seed fates of four rainforest tree species in the fragmented forests of Anamalais in the southern Western Ghats, India. Acta Oecol 110:103698. https://doi.org/10.1016/j.actao.2020.103698
Gregory T, Carrasco Rueda F, Deichmann J, Kolowski J, Alonso A (2014) Arboreal camera trapping: taking a proven method to new heights. Methods Ecol Evol 5:443–451. https://doi.org/10.1111/2041-210X.12177
Gregory T, Carrasco-Rueda F, Alonso A, Kolowski J, Deichmann JL (2017) Natural canopy bridges effectively mitigate tropical forest fragmentation for arboreal mammals. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-04112-x
Haysom JK, Deere NJ, Wearn OR, Mahyudin A, Jami J, Reynolds G, Struebig MJ (2021) Life in the canopy: using camera-traps to inventory arboreal rainforest mammals in Borneo. Front For Glob 4:673071. https://doi.org/10.3389/ffgc.2021.673071
Hongo S, Dzefack Z, Vernyuy L, Minami S, Nakashima Y, Djiéto-Lordon C, Yasuoka H (2020) Use of multi-layer camera trapping to inventory mammals in rainforests in southeast Cameroon. Afr Study Monogr Suppl 60:21–37. https://doi.org/10.14989/250126
Hsieh TC, Ma KH, Chao A (2013) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Jayasekara P, Weerasinghe U, Wijesundara D, Takatsuki S (2007) Identifying diurnal and nocturnal frugivores in the terrestrial and arboreal layers of a tropical rain forest in Sri Lanka. Ecotropica 13:7–15
Jha CS, Dutt CBS, Bawa KS (2000) Deforestation and land use changes in Western Ghats, India. Curr Sci 79:231–238. http://www.jstor.org/stable/24103455. Accessed 1 Jan 2023
Kays R, Allison A (2001) Arboreal tropical forest vertebrates: current knowledge and research trends. Plant Ecol 153:109–120. https://doi.org/10.1007/978-94-017-3606-0_9
Krishna CY, Karnad D (2010) New records of the Nilgiri marten Martes gwatkinsii in Western Ghats, India. Small Carniv Conserv 43:23–27
Kumara HN, Radhakrishna S (2013) Evaluation of census techniques to estimate the density of slender loris (Loris lydekkerianus) in Southern India. Curr Sci 104:1083–1086. http://www.jstor.org/stable/24092199. Accessed 25 Dec 2022
Laughlin MM, Martin JG, Olson ER (2020) Arboreal camera trapping reveals seasonal behaviors of Peromyscus spp. in Pinus strobus canopies. Am Midl Nat 183:210–222. https://doi.org/10.1637/0003-0031-183.2.210
Lowman M(M)D, Schowalter TD, Franklin JF (2012) Methods in forest canopy research. University of California Press. https://doi.org/10.1525/9780520953925
Lowman MD (2009) Canopy research in the twenty-first century: a review of arboreal ecology. Trop Ecol 50:125
MacGregor-Fors I, Payton ME (2013) Contrasting diversity values: statistical inferences based on overlapping confidence intervals. PLoS One 8:e56794. https://doi.org/10.1371/journal.pone.0056794
Maher J (2006) Canopy access: beyond basic single rope technique. Inst Trop Ecol Conserv pp 1–11. https://verticalsection.caves.org/nh/55/CanopyAccess.pdf. Accessed 18 Apr 2022
Menon V (2014) Indian mammals a field guide. Hachette India
Mills CA, Godley BJ, Hodgson DJ (2016) Take only photographs, leave only footprints: novel applications of non-invasive survey methods for rapid detection of small, arboreal animals. PLoS One 11:e0146142. https://doi.org/10.1371/journal.pone.0146142
Moffett MW (2000) What’s “Up”? A critical look at the basic terms of canopy biology. Biotropica 32:569–596. https://doi.org/10.1111/j.1744-7429.2000.tb00506.x
Moore JF, Soanes K, Balbuena D, Beirne C, Bowler M, Carrasco-Rueda F, Cheyne SM, Coutant O, Forget P-M, Haysom JK, Houlihan PR, Olson ER, Lindshield S, Martin J, Tobler M, Whitworth A, Gregory T (2021) The potential and practice of arboreal camera trapping. Methods Ecol Evol 12:1768–1779. https://doi.org/10.1111/2041-210X.13666
Nadkarni NM, Parker GG, Lowman MD (2011) Forest canopy studies as an emerging field of science. Ann For Sci 68:217–224. https://doi.org/10.1007/s13595-011-0046-6
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR: an R package for efficient camera trap data management. Methods Ecol Evol 7:1457–1462. https://doi.org/10.1111/2041-210X.12600
Norman M (2003) Biodiversity hotspots revisiteD. Bioscience 53:916–917. https://doi.org/10.1641/0006-3568(2003)053[0916:BHR]2.0.CO;2
O’Connell AF, Nichols JD, Karanth KU (2011) Camera traps in animal ecology: methods and analyses. Springer
Oliveira-Santos LGR, Tortato MA, Graipel ME (2008) Activity pattern of Atlantic forest small arboreal mammals as revealed by camera traps. J Trop Ecol 24:563–567. https://doi.org/10.1017/S0266467408005324
Otani T (2001) Measuring fig foraging frequency of the Yakushima macaque by using automatic cameras. Ecol Res 16:49–54. https://doi.org/10.1046/j.1440-1703.2001.00370.x
Perry DR (1978) A method of access into the crowns of emergent and canopy trees. Biotropica 10:155–157. https://doi.org/10.2307/2388019
Ramachandra TV, Shivamurthy V, Aithal DB (2015) Environmental flow assessment in Yettinaholé: Where is 24 TMC to divert? Sahyadri Conservation Series 48. ENVIS Tech Rep 91. https://doi.org/10.13140/RG.2.1.5136.4323
Randler C, Katzmaier T, Kalb J, Kalb N, Gottschalk TK (2020) Baiting/luring improves detection probability and species identification—a case study of mustelids with camera traps. Animals 10:2178. https://doi.org/10.3390/ani10112178
Srivathsa A, Banerjee A, Banerjee S, Chawla MM, Das A, Ganguly D, Rodrigues RG, Adhya T, Bhatia S, Kshettry A, Majgaonkar I (2022) Chasms in charismatic species research: seventy years of carnivore science and its implications for conservation and policy in India. Biol Conserv 273:109694. https://doi.org/10.1016/j.biocon.2022.109694
Suzuki KK, Ando M (2019) Early and efficient detection of an endangered flying squirrel by arboreal camera trapping. 83:372–378. https://doi.org/10.1515/mammalia-2018-0055
Venkatesh B, Nayak PC, Thomas T, Jain SK, Tyagi JV (2021) Spatio-temporal analysis of rainfall pattern in the Western Ghats region of India. Meteorol Atmos Phys 133:1089–1109. https://doi.org/10.1007/s00703-021-00796-z
Vijay A, Chandran K, Hameed H (2022) First sighting record of the elusive Nilgiri marten Martes gwatkinsii in Wayanad, Southern Western Ghats, India. Mammalia 8:439–443. https://doi.org/10.1515/mammalia-2021-0119
Wardhaugh CW (2023) Forest canopy ecology: what do we know about canopy species and their interactions? CABI Rev. https://doi.org/10.1079/PAVSNNR201510039
Whitworth A, Beirne C, Pillco Huarcaya R, Whittaker L, Serrano Rojas SJ, Tobler MW, MacLeod R (2019) Human disturbance impacts on rainforest mammals are most notable in the canopy, especially for larger-bodied species. Divers Distrib 25:1166–1178. https://doi.org/10.1111/ddi.12930
Whitworth A, Braunholtz LD, Huarcaya RP, MacLeod R, Beirne C (2016) Out on a limb: arboreal camera traps as an emerging methodology for inventorying elusive rainforest mammals. Trop Conserv Sci 9:675–698. https://doi.org/10.1177/194008291600900208
Woodman N, Timm RM, Slade NA, Doonan TJ (1996) Comparison of traps and baits for censusing small mammals in neotropical lowlands. J Mammal 77:274–281. https://doi.org/10.2307/1382728
Acknowledgements
We thank the Nature Conservation Foundation, Mysore, for offering logistical support and the Kadumane Tea Estate, Karnataka, for granting us permission to carry out the study.
Funding
This study was funded by On the Edge Conservation, UK, through a grant to the Wildlife Biology and Conservation Program, National Centre for Biological Sciences–TIFR. A.S. was supported by the Department of Science and Technology–Government of India’s Innovation in Science Pursuit for Inspired Research Faculty Award.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design; material preparation and data collection: ON; analysis: ON and VR; first draft of the manuscript was written by ON, AS, and VR; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was not conducted in any protected areas and did not require research permits. Since the methods used were non-invasive in nature, animal care and use committee approval was not required.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Dries Kuijper
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 872 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazareth, O., Srivathsa, A. & Ramachandran, V. Trunks and treetops: integrating terrestrial and arboreal camera-trap surveys to document elusive mammal communities in India. Mamm Res 69, 43–52 (2024). https://doi.org/10.1007/s13364-023-00714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-023-00714-1