Abstract
Flying lemurs are able to glide using a thin membrane of skin (patagium) connected to their limbs, body, and part of tails. The Malayan flying lemur (Galeopterus variegatus) is endemic to Southeast Asia. Previous information on food habits of this species has been anecdotal, and few studies on their dietary composition have been conducted. From the information of sibling species, we predicted that the Malayan flying lemurs to be folivorous. We firstly tried a quantitative analysis of the dietary compositions of the Malayan flying lemurs together with food availability in West Java, Indonesia, throughout the year. The Malayan flying lemurs seasonally changed foods: leaves from December to July largely overlapping with the rainy season (October–June) and fruits from August to November largely overlapping with the dry season (July–September). When fruits were abundant, the proportions of fruits in the feces increased while proportions of leaves in the feces decreased, or the flying lemur shifted their foods from leaves to fruits. In this period, tree leaves were abundant, which did not explain the decrease in the feces. This implies that the flying lemurs fed on tree leaves in the majority of the year but they shifted to fruits when they were abundant though leaves were also abundant. The abrupt changes in the dietary composition of the flying lemurs were different from those of the sympatric folivorous primate, Javan lutung (Trachypithecus auratus), for which dietary composition showed a gradual change from leaves to fruits. Such inter-specific differences would be caused by differences in body sizes and/or digestive physiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are seasonal variations in the dietary characteristics of mammals, including dietary composition, food diversity, and its seasonality (Sovada et al. 2001; Ciucci et al. 2018; Sharma et al. 2014). To fulfil the dietary requirements and maximize the nutrition, mammals have adapted to temporal and spatial variations in food availability (e.g., Bojarska and Selva 2011; Diaz-Ruiz et al. 2013). Such adaptations include behavioral traits known as feeding strategy (Schoener 1971; Weise et al. 2010; Bakaloudis et al. 2012; Hocking et al. 2017). In some cases, dietary compositions are affected by food availability, resulting in nutritional condition and consequent reproduction. A good example is known in a wild boar population in Mediterranean area (Massei et al. 1996). Such variations of dietary compositions affected by food availability sometimes result in population dynamics as was shown in the Taihangshan macaques Macaca mulatta tcheliensis in Henan Province, China (Cui et al. 2020). Therefore, studies on food habits can provide useful insights to understand mammals what and how much proportion the mammal feed on, and if we can know the food availability, we may understand how the diets relate to the habitat (Riney 1992; Pyke 2010; Sinclair 2003). For endangered species, studying their food habits would help to quantify their suitable habitats because we can know which foods are important for them. Such information would be important for their conservation including habitat management, supplementary feeding, or reintroduction to new habitats (e.g., Hejcmanová et al. 2010; Lovari et al. 2013; Castle et al. 2020).
The Malayan flying lemur (or colugo, Galeopterus variegatus = Cynocephalus variegatus), the Order Dermoptera, is able to glide using a thin membrane of skin, called a patagium, which is connected to their limbs, their body, and part of their tails. It is endemic to Southeast Asia, ranging in southern parts of Thailand, Indochina, Malay Peninsula, Sumatra, Java, and Borneo (Lim 2007). It inhabits forests, including primary and secondary forests (Lim 2007). It is arboreal and nocturnal, and therefore studies on its behavioral aspects have been limited (Lim 2007; Byrnes et al. 2011). Although recent studies have shown its home range size, nocturnal activity, and habitat preference (Baba 2008; Lim et al. 2013; Tsuji et al. 2015a, 2019), majority of information on its dietary composition is anecdotal (Agoramoorthy et al. 2006; Lim 2007; Dzulhelmi and Abdullah 2009), and only one systematic study has been conducted for a sibling species, Philippine flying lemurs (Cynocephalus volan) (Wischusen and Richmond 1998). It showed that the Philippine flying lemurs were folivorous.
In this study, we tried to reveal the dietary compositions of the flying lemur by fecal analysis, a noninvasive method, with supplemental behavioral observation. In addition, to address their foraging strategy, we conducted phenological survey of diet trees to find relationship between food availability and dietary compositions. If the flying lemur is strictly folivorous, the diet would be composed of only leaves regardless plant phenology. However, if it is adaptively folivorous, it would feed on some more tasteful or nutritious foods depending on plant phenology. In order to address this, quantitative analysis is needed. However, no quantitative analysis of dietary compositions of flying lemurs has not been done. Therefore, we studied both dietary compositions of the Malayan flying lemur and food availability. Folivorous food habits of the Philippine flying lemur (Wischusen and Richmond 1998) are partly interpreted by its well-developed caecum (48 cm in length) which can ferment ingested leaves (Lim 2007). Therefore, we predicted that the Malayan flying lemur is also folivorous. Our particular concern is whether their dietary compositions are constant through the year or they vary seasonally relating to food availability.
Materials and methods
Study area
This study was conducted at Pangandaran Nature Reserve (hereafter PNR), located at the southern coast of western Java, Indonesia (Fig. 1). It is composed of a forest park (38 ha) and a strictly protected nature reserve (370 ha) (Mitani et al. 2009; Tsuji et al. 2015a, b). PNR has an average elevation of < 100 m above sea level. The average annual rainfall (2005 to 2013) is 3272 mm (AccuWeather.com 2015, http://www.accuweather.com [accessed: January 14, 2015]), and the average annual air temperature and humidity are 25–30 °C and 85–95%, respectively (Brotoisworo 1991; Kool 1993). The dry season ranges from July to September and the rainy season ranges from October to June. The main vegetation of the forest park is secondary forest; however, several areas of planted Tectona grandis L. and Swietenia macrophylla King forest stands exist. There are also several grassland patches (Sumardja and Kartawinata 1977; Tsuji et al. 2015a, b). Although PNR is small and isolated, the reserve has a diverse fauna. Forest rangers listed 23 mammalian species, including two diurnal primates, and 62 avian species (Tsuji et al. 2016).
Collection and analysis of fecal samples
A fecal pellet of the flying lemurs was sampled from ten aggregated pellets (Fig. 2) collected beneath tall trees where the flying lemurs were resting. Collections were conducted inside the forest park every month from August 2017 to July 2018 (n = 120). Immediately after collection, the fecal pellets were preserved in plastic bags with 70% alcohol, and brought back the IPB University, Bogor, West Java, Indonesia, until analyses.
The fecal samples were washed over a 0.5 mm-meshed sieve, and the retained materials were preserved in 70% ethanol. The fragments were microscopically analyzed by the point-frame method (Stewart 1967; Uresk and Dietz 2018). Fragments were spread over a slide-glass with 1 mm aperture grids, and points of grids covered by the fragments were counted. More than 100 points were counted, and proportions in the fecal compositions (%) of the food categories were calculated. Foods were classified into six categories: fruit (including fruit coat and pulp), flower, leave (including veins, and grasses), fiber (including culms), animal material (insects and mammal hairs), and others (unidentified materials).
Observation of feeding behavior
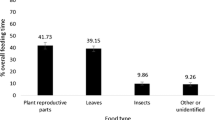
As a supplemental investigation, we conducted intermittent field surveys at the forest park between 2014 and 2019 (228 days in total, 3–4 days a month in average). Besides this study, we recorded ranging behavior of flying lemurs with radio telemetry (Tsuji et al. 2019a). We traced them every 30 min during the night (18:00–06:00 of the next day) and four times during the day (07:00, 10:00, 13:00, and 16:00). Whenever we observed a flying lemur feeding, we recorded plant species and part eaten. Our methodology adhered to Indonesian/Japanese legal requirements.
Plant phenology
We had established 685 monitoring trees along the forest path (ca. 2.5 km in length and ca. 5 m in width) inside the forest park for a phenological survey (Tsuji et al. 2019b). Diameters at breast height (DBH) of trees were measured and the basal areas (BA) or the cross-sectional areas were calculated from the DBHs. To evaluate the availability of the target food items, phenology index (PI) of (1) mature leaves, (2) young leaves, (3) flowers, (4) ripen fruits, and (5) young fruits were checked twice a month and averaged for month. The PI for each tree/part was evaluated by three grades: absence (0), small amount (1), and abundant (2).
In order to evaluate food availability in the PNR, we calculated the food availability index (hereafter FAI), defined by the following formula:
When we calculate FAI of ripen fruits, for example, we summed PI scores of ripen fruits of each monitoring tree and summed up the scores. For analyses, we used two different FAI data sets: one across all the plant species (685 trees) and another one for Ficus spp. (16 trees), on which the flying lemurs in the PNR stayed for foraging frequently (Tsuji et al. 2015a).
Statistical analyses
We employed the generalized linear models (GLMs) to evaluate monthly differences in the target food parts. In the GLM, we set month as an explanatory variable. An error structure of a dependent variable (number of counted points for target food part) was assumed to follow the Poisson distribution (linked function: log), and the total number of counted points was set as an offset-term. We conducted the Spearman’s rank correlation tests between the FAI and proportion (%) of food parts (young leaves, ripen fruits, young fruits, flowers, fiber, animals, and others). As we mentioned above, we separately conducted corresponding correlation analyses for FAI of Ficus spp. All analyses were performed in R 3.2.3 (R Development Core Team 2015), with the statistical standard (α) set at 0.05.
Results
During the study period (2014–2019), we recorded 14 items (13 species of 10 families) as foods of the flying lemurs by direct observations. Total number of food items in the PNR is 24 (19 species of 12 families) (Supplementary Data SD1) according to the results of the present study and our previous study (Tsuji et al. 2015a).
Figure 3 shows major food categories whose proportions showed more than 20% at least in a month. Proportions of leaves occupied more than 40% from December to July, which largely corresponded with the rainy season (October–June, Fig. 3) while they accounted for less than 20% in the dry season (July–September). Particularly, it reached more than 60% in December, January, April, and June. The monthly change in proportions of leaves also differed significantly (Supplementary Data SD2). These results imply that they are the staple foods for the flying lemurs.
Proportions of fruits occupied 40–80% from August to November, which largely corresponded with in the dry season (July–September), after which decreased to less than 30% except March (32%). Monthly changes in the proportions of fruits differed significantly (Supplementary Data SD2).
Out of these major food categories, flowers and animal materials (insects and mammal hair) appeared in limited months (flowers: December, January, and February, animal materials: July, October, and November) and the proportions were small (proportions were less than 3%). Others occupied less than 20% in all months.
Food availability indices (FAIs) of young leaves peaked in October and those of ripen fruits had a bottom in February and March. The FAI of young fruits and flowers showed no clear seasonal changes (Fig. 4). Young leaves of Ficus spp. had lower values from May to July. Finally, the FAIs of Ficus fruits (both ripen and young) peaked in October–December.
Correlation analyses showed that the FAI of young fruits positively correlated with proportions of fruits, and negatively correlated proportions of leaves. In addition, the FAI of young leaves of Ficus spp. positively correlated to proportion of fiber in the fecal composition (Supplementary Data SD3).
Discussion
Due to their nocturnal and arboreal nature, the information of the food of the Malayan flying lemur has been fragmental (Agoramoorthy et al. 2006; Lim 2007; Dzulhelmi and Abdullah 2009; Tsuji et al. 2015a). Our study is therefore first quantitative information on their diets.
It was described that the Philippine flying lemur, a sibling species, is folivorous (Wischusen and Richmond 1998). The food habits seem to relate to its well-developed caecum (48 cm in length) which enables it to ferment ingested leaves (Lim 2007). We thus predicted that the Malayan flying lemur would be also folivorous. In fact, the fecal compositions were greatly occupied by leaves for long period from December to July (Fig. 3). Besides, correlation analyses showed that the flying lemur fed on leaves regardless of its availability and fed more leaves in fruit-scarce season. In addition, dependence on young leaves of Ficus spp. was positively correlated with its availability. From these, leaves seem to be staple foods for the Malayan flying lemur. This supported our prediction. However, contrary to our prediction, our fecal analysis showed that he Malayan flying lemur fed on fruits in a short period from August to November (Fig. 3), reflecting increase of ripen fruits (Fig. 4). This frugivorous nature was not found for the Philippine flying lemur (Wischusen and Richmond 1998). During this period, the proportion of leaves decreased in the fecal composition, that is, fruits replaced leaves in the diet. It is noteworthy that leaves were abundant in this period (Fig. 4). Therefore, the flying lemur shifted from leaves to fruits not because of leaf supply but because of increase of ripen fruits. In fact, proportion of fruits in the feces positively correlated with availability of ripen fruits (rs = 0.545, p = 0.071, SD3) and young fruits (rs = 0.713, p = 0.012, SD3). In general, fleshy fruits contain much sugar, a carbohydrate-rich food source (Robbins 1993). Short-term use seems similar to be a “fallback” food” (Harrison and Marshall 2011; Smith 2013), but since a fallback food is less nutritious and less favored, fruits are not fallback foods for the flying lemur. The shift from leaves to fruits occurred at a habitat of enough foods (leaves). The flying lemur chose better foods (fruits) according to additional more favorable foods, which may be called as a “bonus” food.
Our new finding of fruit use by a flying lemur was attained by continuous sampling of feces as well as by recording plant phenology. Findings of other mammals suggest that we need to pay attention to availability of food resources and animals’ responses, such as ranging (Norscia et al. 2006) and daily activity (Karasov 1992) to address their feeding strategy.
In PNR, another frugi-folivorous mammal species, Javan lutungs (Trachypithecus auratus) inhabit. Tsuji et al. (2019b) studied the food habits of the lutungs during the same period (August 2017–July 2018) and showed that their diet is composed of leaves occupying 80–90% from February to July and accounting for around 50% from August to January. The lutung fed on fruits when the availability of leaves decreased (Tsuji et al. 2019b). The seasonal change of the lutungs’ diet was gradual while those of the flying lemur was abrupt. The inter-specific difference in the monthly change in dietary composition would be originated to difference in digestive physiology: the lutungs moved for several hundred meters a day (Kool 1993) within home range (5–10 ha) and visit five to six trees to feed on leaves and sometimes pick up fruits and flowers (Browoisworo 1991). The passage rate of foods through digestive tract of a captive Javan lutungs was around 100 h (Tsuji et al. 2017). This means that their foods were mixed in their digestive tracts. This explains their gradual change in their diet. Contrary, the Malayan flying lemur stays several hours on a tree and use a few patches within small home ranges (0.5–1.5 ha) during a night (Tsuji et al. 2019a). It is likely that their food passage is rapid: Wischusen et al. (1994) reported the passage rate of the Philippine flying lemurs to be 14–22 h. The rapid passage rate is partly explained by the smaller body of the Malayan flying lemur (adult body weight: 1.0–1.1 kg) than the lutung (adult body weight: 5–7 kg) (Tsuji et al. 2016). This suggests that fecal compositions of the flying lemur directly reflect their past feeding both spatially and temporally. The abrupt seasonal change in their fecal compositions seems to be explained by this. It may be possible that such an inter-specific difference in dietary shift would contribute to weaken the inter-specific competition over leaves and/or fruits. In future, community-based research focusing on the inter-specific competition would confirm this.
In summary, feeding strategy of the Malayan flying lemurs is characterized by the following: (1) to feed on leaves regularly as “staple foods,” (2) to shift to fruits if available even leaves are abundant, which may be called as “bonus foods,” and (3) to other parts optionally.
Data availability
Data are available upon request.
References
Agoramoorthy G et al (2006) Population, diet and conservation of Malayan flying Lemurs in altered and fragmented habitats in Singapore. Biodiv Cons 15:2177–2185. https://doi.org/10.1007/s10531-004-6900-1
Baba M (2008) Ecology of the Malayan flying lemur. In: Katayama T (ed) Colugo: are they flying monkeys? Yasaka Shobo, Tokyo Japan
Bakaloudis DE et al (2012) Diet composition and feeding strategies of the stone marten (Martes foina) in a typical Mediterranean ecosystem. The Sci World J 2012:ID163920. https://doi.org/10.1100/2012/163920
Bojarska K, Selva N (2011) Spatial patterns in brown bear Ursus arctos diet: the role of geographical and environmental factors. Mammal Rev 42:120–143. https://doi.org/10.1111/j.1365-2907.2011.00192.x
Brotoisworo E (1991) The lutung (Presbytis cristata) in Pananjung- Pangandaran Nature Reserve. Comp Primatol Monogr 3:45–148
Byrnes G et al (2011) Sex differences in the locomotor ecology of a gliding mammal, the Malayan colugo (Galeopterus variegatus). J Mammal 92:444–451
Castle ST et al (2020) Diet composition analysis provides new management insights for a highly specialized endangered small mammal. PloS ONE 15(10):e0240136. https://doi.org/10.1371/journal.pone.0240136
Ciucci P et al (2018) Inter-pack, seasonal and annual variation in prey consumed by wolves in Pollino National Park, southern Italy. Eur J Wildl Res 64:1–16. https://doi.org/10.1644/13-MAMM-A-218
Cui Z et al (2020) Living near the limits: Effects of interannual variation in food availability on diet and reproduction in a temperate primate, the Taihangshan macaque (Macaca mulatta tcheliensis). Am J Primatol 82:e23080. https://doi.org/10.1002/ajp.23080
Diaz-Ruiz F et al (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian Peninsula. Mamm Rev 43:59–70. https://doi.org/10.1111/j.1365-2907.2011.00206.x
Dzulhelmi MN, Abdullah MT (2009) Foraging ecology of the Sunda colugo (Galeopterus variegatus) in Bako National Park, Sarawak, Malaysia. Malay Nat J 61:285–294
Harrison ME, Marshall AJ (2011) Strategies for the use of fallback foods in apes. Int J Primatol 32:531–565. https://doi.org/10.1007/s10764-010-9487-2
Hejcmanová P et al (2010) Exclusion of livestock grazing and wood collection in dryland savannah: an effect on long-term vegetation succession. Afr J Ecol 48:408–417. https://doi.org/10.3957/056.040.0105
Hocking DP et al (2017) A behavioural framework for the evolution of feeding in predatory aquatic mammals. Proc Roy Soc b: Biol Sci 284:20162750. https://doi.org/10.1098/rspb.2016.2750
Karasov WH (1992) Daily energy expenditure and the cost of activity in mammals. Am Zool 32:238–248. https://doi.org/10.1093/icb/32.2.238
Kool KM (1993) The diet and feeding behavior of the silver leaf monkey (Trachypithecus auratus sondaicus) in Indonesia. Int J Primatol 14:667–700. https://doi.org/10.1007/BF02192186
Lim NTL (2007) Colugo: the flying lemur of South-East Asia. Draco Publishing and Distribution Pte Ltd, Singapore
Lim TL et al (2013) Occurrence of the Sunda colugo (Galeopterus variegatus) in the tropical forests of Singapore: a Bayesian approach. Mamm Biol 78:63–67. https://doi.org/10.1016/j.mambio.2012.06.008
Lovari S et al (2013) Food habits of two leopard species competition climate change and upper treeline: a way to the decrease of an endangered species? Ethol Ecol Evol 25:305–318. https://doi.org/10.1080/03949370.2013.806362
Massei G et al (1996) Diet, food availability and reproduction of wild boar in a Mediterranean coastal area. Acta Theriol 41:307–320
Mitani M et al (2009) Plant species list from the Pananjung Pangandaran Nature Reserve, west Java, Indonesia, sampled in the El Niño-Southern Oscillation year of 1997. Humans Nat 20:113–120
Norscia I et al (2006) Influence of dry season and food quality and quantity on behavior and feeding strategy of Propithecus verreauxi in Kirindy, Madagascar. Int J Primatol 27:1001–1022
Pyke GH (2010) Optimal foraging theory: introduction. Encycl Anim Behav 2:601–603
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Riney T (1992) Study and management of large mammals. Wiley, Hoboken, NJ, USA
Robbins CT (1993) Wildlife feeding and nutrition, 2nd edn. Academic Press, San Francisco
Schoener TW (1971) Theory of feeding strategies. Ann Rev Ecol Syst 2:369–404. https://doi.org/10.1146/annurev.es.02.110171.002101
Sharma HP et al (2014) Seasonal food habits of the red panda (Ailurus fulgens) in Rara National Park. Nepal J Mammal 25:47–50. https://doi.org/10.4404/hystrix-25.1-9033
Sinclair ARE (2003) Mammal population regulation, keystone processes and ecosystem dynamics. Philos Trans Roy Soc B 358:1729–1740. https://doi.org/10.1098/rstb.2003.1359
Smith DAE (2013) Feeding ecology of red langurs in Sabangau tropical peat-swamp forest, Indonesian Borneo: extreme granivory in a non-masting forest. Am J Primatol 75:848–859
Sovada MA et al (2001) Seasonal food habits of swift fox (Vulpes velox) in cropland and rangeland landscapes in western Kansas. Am Midl Nat 145:101–111. https://doi.org/10.1674/0003-0031(2001)145[0101:SFHOSF]2.0.CO;2
Stewart DRM (1967) Analysis of plant epidermis in faeces: a technique for studying the food preferences of grazing herbivores. J Appl Ecol 4:83–111
Sumardja EA, Kartawinata K (1977) Vegetation analysis of the habitat of banteng (Bos javanicus) at the Pananjung Pangandaran Nature Reserve, West Java. Biotrop Bull 18:1–49
Tsuji Y et al (2015a) Diurnal resting site selection and daytime feeding behaviour of wild Malayan flying lemur Galeopterus variegatus in Western Java, Indonesia. Mamm Stud 40:35–45. https://doi.org/10.3106/041.040.0107
Tsuji Y et al (2015b) “Deer” friends: feeding associations between colobine monkeys and deer. J Mammal 96:1152–1161. https://doi.org/10.1093/jmammal/gyv123
Tsuji Y et al (2016) The notes on mammal carcasses collected in Pangandaran Nature Reserve West Java Indonesia. HAYATI 23:35–38. https://doi.org/10.1016/j.hjb.2016.01.001
Tsuji Y et al (2017) Neglected seed dispersers: endozoochory by Javan lutungs (Trachypithecus auratus) in Indonesia. Biotropica 49:539–545. https://doi.org/10.1111/btp.12439
Tsuji Y et al (2019a) A report on ranging behavior of Malayan flying lemurs, Galeopterus variegatus, in West Indonesia: Relationships with habitat characteristics. Biodiv 20:430–435. https://doi.org/10.13057/biodiv/d200218
Tsuji Y et al (2019b) Dietary habits of wild Javan lutungs (Trachypithecus auratus) in a secondary-plantation mixed forest: effects of vegetation composition and phenology. Mamm Biol 98:80–90. https://doi.org/10.1016/j.mambio.2019.08.001
Uresk DW, Dietz DR (2018) Fecal vs. rumen contents to determine white-tailed deer diets. Int J Sci 24:118–122
Weise MJ et al (2010) The role of body size in individual-based foraging strategies of a top marine predator. Feeding ecology of red langurs in Sabangau tropical peat-swamp forest, Indonesian Borneo: extreme granivory in a non-masting forest. Ecol 91:1004–1015. https://doi.org/10.1890/08-1554.1
Wischusen EW, Richmond ME (1998) Foraging ecology of the Philippine flying lemur (Cynocephalus volans). J Mamm 79:1288–1295. https://doi.org/10.2307/1383020
Acknowledgements
The study was conducted according to the permission from Ministry for High Education, Research and Technology (Kemdikti Ristek), the Republic of Indonesia.
Funding
This study was funded by a grant from JSPSAS-HOPE, ITP-HOPE, and KAKENHI (Nos. 23780160, 24405018, 15H05242, 16K18619, and 21KK0130), JSPS Bilateral Open Partnership Joint Research, Future Development Funding Program of Kyoto University Research Coordination Alliance, and JSPS Core-to-Core Program (Advanced Research Networks).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Our research was carried out following the legal standards of universities of Indonesia and Japan.
Consent for publication
The manuscript has been approved by all co- authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Yayoi Kaneko.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takatsuki, S., Tsuji, Y., Prayitno, B. et al. Seasonal changes in dietary compositions of the Malayan flying lemur (Galeopterus variegatus) with reference to food availability. Mamm Res 68, 77–83 (2023). https://doi.org/10.1007/s13364-022-00658-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00658-y