Abstract
A classic question in community ecology is how species coexist within a community. Studies have sought to understand how species occurrence vary according to habitat structure, space, food, predators, and competitors. Small mammals are widely used as a model system in community ecology, since they represent the most diverse group of mammals in the neotropical forests. Hence, we investigated whether microhabitat features, food resource (fruits), and presence of medium and large mammals can explain fine-spatial scale richness, abundances, and habitat use of small mammals in a forest in Brazil. Three species represented 83% of all captured individuals (Didelphis albiventris, Oligoryzomys nigripes, Akodon montensis). Species richness, abundance, and habitat use of small mammals were affected positively by the distance of bamboo (Chusquea sp.) thickets. The occurrence of predators (carnivores and omnivores) and potential competitors (large herbivores), however, did not affect richness, abundance, and habitat use of small mammals at small spatial scales. Our findings suggest that the bamboo patches can influence spatial distribution and shape small mammal communities in tropical forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A classic question in community ecology is how species manage to coexist (Hutchinson and MacArthur 1959; Macarthur and Levins 1964). Neotropical forest mammals (small, mid, and large) that occur in the same space and time must have different characteristics in at least one dimension of the niche (Chesson and Kuang 2008; Galetti et al. 2016; Hutchinson 1957; Pinotti et al. 2011). Species can partition space and time according to (1) habitat structure: forest cover, bamboo, leaf litter, and fallen logs on forest (e.g., Delciellos et al. (2018); Lima et al. (2010); Melo et al. (2011); Naxara et al. (2009); Pinotti et al. (2011)); (2) vertical stratification: some species, for example, can use the canopy whereas others forage in the understory (Melo et al. 2013; Melo et al. 2011; Vieira and Monteiro Filho 2003); (3) food: a certain species can feed more on fruits, leaves, grasses, roots, other species more on insects, or seeds with different sizes (Ben-Moshe et al. 2001; Galetti et al. 2015a; Galetti et al. 2016; Leite et al. 1996), (4) predators: limiting the population size by predation, affecting reproduction, population growth, and behavior (Magioli and Ferraz 2021; Melo et al. 2013; Moura et al. 2009), and (5) competitors: by limiting space, resource availability, and displacement, affecting abundance, composition, and behavior (Akkawi et al. 2020; Pedó et al. 2010). Despite the number of recent studies, the factors that allow coexistence of mammals in Neotropical communities are still poorly understood (DeMattia et al. 2004; HilleRisLambers et al. 2012; Santos et al. 2019).
Neotropical small non-flying mammals (rodents and marsupials) are the most diverse group of mammals inhabiting Neotropical forests, with approximately 289 species described for Brazil (Paglia et al. 2012), with 124 species in the Atlantic Forest, 94 species of rodents, and 30 species of marsupials (Bovendorp et al. 2017b). Rodents, according to classic dietary studies, can be classified as omnivorous, granivorous, frugivorous, insectivorous, and herbivorous (Carvalho et al. 1999; Galetti et al. 2016; Pinotti et al. 2011; Vieira et al. 2003; Vieira et al. 2006; Vieira et al. 2011), with the majority being seed predators (Galetti et al. 2015a; Galetti et al. 2015b). Marsupials have a diet consisting of fruits, small invertebrates, nectar, and flowers (Vieira and De Moraes 2003). Small mammals are of great importance for the functioning of ecosystem processes, as they can act as pollinating agents (Amorim et al. 2020; Vieira and de Carvalho-Okano 1996), seed dispersers, and seed predators (Iob and Vieira 2008). In addition, they contribute to the maintenance of trophic connections, being a source of food for carnivorous mammals (Facure and Giaretta 1996), birds (Cabral et al. 2006), and snakes (Henderson et al. 1987). Some species can also be considered biological indicators, as they are sensitive to changes in habitat (Banks-Leite et al. 2014).
Given the rapid response to changes in the environment, these animals constitute an ideal model group to assess the effect of resource availability, spatial distribution, and ecological interactions on species coexistence and composition (Naxara et al. 2009; Pinotti et al. 2011; Püttker et al. 2019; Püttker et al. 2008). Studies focusing in microhabitat preference show that the amount of canopy cover, density of vegetation, presence of ferns, presence of fallen logs, leaf litter structure, and rocky outcrops used by different species of small mammals can influence reproduction and coexistence between species (Bovendorp 2013; Lima et al. 2010; Melo et al. 2013; Püttker et al. 2013). Small mammal species with morphological and behavioral similarity, for example, may show spatial segregation to avoid direct competition for resources (Dalmagro and Vieira 2005; Naxara et al. 2009; Pinotti et al. 2011). In this way, species tend to present spatial segregation in the environment using different microhabitats and resources (e.g., fallen logs, litter, arboreal strata, availability of insects, and fruits) (Bergallo and Magnusson 2004; Bergallo and Magnusson 1999; Dueser and Hallett 1980; Melo et al. 2013); however, if resources are grouped or scarce in the environment, species can aggregate around resources (Pinotti et al. 2011). The spatial structure of the forest surface (i.e., spatial variation in quality and quantity of litter) creates distinct microhabitats that can allow the coexistence of multiple species and influence the richness, diversity, and abundance of small mammal communities (Melo et al. 2013; Naxara et al. 2009; Pinotti et al. 2011).
However, changes in population dynamics and composition of the small mammal community may occur with local or functional extinction of large- and medium-sized mammals that act as potential competitors (e.g., Tayassu pecari) and predators (e.g., Puma concolor) (Akkawi et al. 2020; Bovendorp et al. 2018; Fonseca and Robinson 1990; Galetti et al. 2017; Galetti et al. 2015b). Indeed, small mammals tend to be overabundant in defaunated forest fragments and/or predator-free patches with very low density or extinct large- and medium-sized mammals (see Dirzo et al. 2007; Galetti et al. 2017; Lopez and Terborgh 2007; Terborgh et al. 2001). This may be more significant for generalist species (Bovendorp et al. 2018; Galetti et al. 2021), since the increase in the abundance of generalist species can relate to changes in the structure of the small mammal community through interspecific competition (Galetti et al. 2015b; Püttker et al. 2019).
Here we focus on four dimensions that may affect coexistence: microhabitat feature, resource availability, and occurrence of predators and potential competitors. We evaluate how these factors affect richness, abundance, and habitat use of small mammals at a fine-spatial scale in the largest remnant of semideciduous Atlantic Forest (Caetetus Ecological Station) in Brazil. We hypothesize that richness, abundance, and habitat use of small mammals are affected positively by microhabitat feature and resources (Bergallo and Magnusson 1999; Fonseca and Robinson 1990; Lima et al. 2010; Melo et al. 2013) and negatively by the occurrence of predators and potential competitors (Bovendorp et al. 2018; Fonseca and Robinson 1990; Galetti et al. 2015b).
Methods
Study area
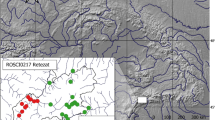
The study was conducted at the Caetetus Ecological Station, Gália, São Paulo, Brazil (hereafter ESEC, 22° 24′ 11″ S, 49° 42′ 08″ W), a 2178-ha remnant of semideciduous forest of Atlantic Forest (Fig. 1). ESEC holds a complete community of large- and medium-sized forest mammals like the endangered black-lion tamarin Leontopithecus chrysopygus (Tabanez et al. 2005), white-lipped peccary Tayassu pecari, collared peccary Pecari tajacu, agouti Dasyprocta azarae, tapir Tapirus terrestris, paca Cuniculus paca, red brocket deer Mazama americana (Akkawi et al. 2020), cougar Puma concolor, ocelot Leopardus pardalis, andoncilla Leopardus tigrinus (Tabanez et al. 2005). The ESEC presents a semideciduous seasonal vegetation (Veloso et al. 1991) and holds threatened plants species such as the peroba rosa Aspidosperma polyneuron, juçara Euterpe edulis, and jequitibá branco Cariniana estrellensis (Durigan et al. 2000). The Köppen climate classification is Cwa, mesothermic with dry winter, rains from October to March ranging from 120 to 200 mm with temperatures between 22 and 26°C, and periods of drought from April to September with rainfall ranging from 35 to 75 mm and lower monthly and temperatures from 16 to 25°C.
Capture of small mammals
We sampled the small mammal community using the capture-mark-recapture method for 10 days every 2 months from June 2017 to April 2018 comprising six campaigns during this period. Small mammals were captured in a 3-ha trapping grid located in the north-east of ESEC. The grid consisted of 11 parallel 150-m lines, 15 m from each other, with trapping stations located every 15 m containing one Sherman trap (37.5×10.0×12.0 cm or 23.0×7.5×8.5 cm; H. B. Sherman Traps, Inc., Tallahassee, Florida) or Tomahawk (42.0 × 12 × 15 cm) placed on the ground. Additionally, pitfall traps (60-L buckets, 53.0 cm in depth, and 40.0 cm in diameter) connected by a 50-cm-high plastic fence were placed at each trap station in 5 of the 11 lines (Appendix Fig. 3). Three different types of traps were used to maximize both capture and recapture rates because pitfall traps result in higher capture rates and a higher proportion of young individuals (Barros et al. 2015; Bovendorp et al. 2017a; Umetsu et al. 2006). All traps were baited with a mixture of sardines, peanut butter, banana, and cornmeal. Captured animals were marked with a numbered ear tag (Small Animal Tags OLT; A. Hartenstein GmbH, Würzburg-Versbach, Germany), recording capture station, trap type, and species of each individual prior to releasing in the location of capture. Capture and recapture data at the station level were used to estimate richness, abundance, and habitat use of small mammals. The habitat use metric was calculated summing captures and recaptures. This metric is closely related to the habitat use once animals use the area for foraging, having positive (mating, food resources, shelter) and negative (fear from predators, possible competitors, etc.) interactions in the habitat (Fonseca and Robinson 1990; Melo et al. 2013; Oliveira et al. 2007; Puttker et al. 2006). Nonetheless, species differing in home range sizes, occupying and using different parts of the habitat. Ten individuals who accidentally died during the fieldwork were deposited at Escola Superior de Agricultura “Luiz de Queiroz” - ESALQ (University of São Paulo, Piracicaba - SP) under supervision of Dr. Alexandre R. Percequillo as testimony material, for taxidermy and species identification. All capture, handling, and tagging protocols followed the guidelines of the American Society of Mammalogists (Sikes et al. 2016).
Sampling of medium and large mammals
In the first moment, we placed 40 camera traps (Bushnell) at the end of each grid line during 30 consecutive days in December 2017 and January 2018. After removing the 40 camera traps, we installed 24 camera traps in 24 randomly selected grid intersections for 15 consecutive days in January and February 2018 (Appendix Fig. 3). From a total of 64 camera traps, 16 did not work in the first sampled period and 4 in the second sampled; therefore, were excluded from the analysis. The camera traps were set to record videos with 10 s to identify the medium and large mammals that used the grid area. Individuals of the same species that were recorded more than once within a 30-min period and that were not possible to identify based on natural marks (stripes, spots, moles, etc.) were considered a single record. For further analyses, we classified medium and large mammals into two categories: predators (carnivores) and potential competitors (herbivores) of small mammals. (Appendix Table 3). We estimated their abundances as the average number of predators and competitors individuals recorded in each sampling station per day. The small mammals (Rodentia and Didelphimorphia) captured in the videos were not considered.
Microhabitat features and resource availability
To characterize the microhabitat features in the grid, we delimited a 5-m radius plot around each trapping station and we measured (I) number of trees with a diameter at breast height (DBH) greater than 20 cm; (II) number of fruits and seeds on the ground; (III) number of fallen logs with a diameter greater than 20 cm; (V) litter height (cm); (VI) distance to water bodies, and (VII) distance to bamboo Chusquea sp. thickets. We measured fruits and seeds on the ground every field campaign. Due to the semideciduous nature of the vegetation in the ESEC, we measured the litter height every two campaigns.
The litter height was measured at 10 random points within the 5-m radius plots with a metallic skewer introduced perpendicularly to the ground until it reached the surface of the soil. The height of the litter in the metallic skewer was then measured with a ruler with millimeter precision. The average litter height per plot was calculated using the 10 measurements. We measured all points in the first campaign and then measured again only in the third campaign. Because the average litter height did not vary between the two campaigns, we replaced the litter missing values from the non-measured campaigns with the overall average for each plot.
Knowing that presence of water bodies can influence the occurrence of small mammals, we calculated the distance of each trap station in the grid to the nearest water body. Similarly, we calculated the distance to the nearest bamboo thickets.
Data analysis
Diversity and abundance of mammals
We recorded the richness (number of species), abundance (number of captures), and habitat use (all captures and recaptures by sampling station—see Appendix Fig. 4) for small mammals using the capture-mark-recapture methodology. Also we record all explanatory variables by sampling station (see Appendix Fig. 5). To evaluate if our sampling effort was sufficient to estimate small mammal diversity, we generated the collector curve using vegan package (Oksanen et al. 2016) in the software R (R Core Team 2019).
Factors that influence small mammal spatial patterns
Because large mammals were sampled in two fieldwork campaigns (December 2017–January 2018 and January–February 2018) and camera traps recorded only a fraction of the small mammal traps, we analyzed two sets of data: one including all six fieldwork campaigns and sampling points (full sample hereafter), but without the predator and competitor variables (N = 121 sampling points) and another including only the two campaigns and the sampling points at which predators and competitors were sampled (subset sample hereafter, N = 35 sampling points, December 2017 and January 2018), thus containing information on all explanatory variables (except for the number of fruits that almost did not vary across sampling points of the subset dataset). We centered and standardized all continuous variables using z-scores and then checked for collinearity (r > |0.60|) between variables to avoid biases in coefficient estimates. We found two collinear variables: distance to bamboo thickets and distance to water bodies (r = −0.74, Appendix Fig. 6) and we removed distance to water bodies because it had higher correlation with all other variables.
Using the full sample dataset, we tested which microhabitat feature covariates affected small mammals in ESEC using generalized linear mixed-effects models (GLMMs) using the package lme4 (Bates et al. 2014). We considered trap type and fieldwork campaigns as random effects in all models, coding them as (1|Campaing) and (1|Trap). We built three sets of models to test the effects of variables on small mammals. The response variables were richness (number of species), abundance (number of individuals captured), and habitat use (all captured and recaptured individuals), while microhabitat features were represented by distance to the nearest bamboo thicket (m), number of fruits, number of fallen logs, number of trees, and litter thickness (cm). Because we used three trap types to maximize the captures of small mammals (Barros et al. 2015; Bovendorp et al. 2017a) and we conducted six campaigns during the entire period of sampling (totalizing 10 months) we considered trap type and the fieldwork campaigns as random effects in the models.
Using the subset sample dataset, we tested which variables affected small mammals in ESEC. Unfortunately, due to the low number of observations, we were not able to fit models for richness of small rodents. Therefore, we built two sets of models (one for abundance and another for habitat use) to test the effects of variables on small mammals. We included the same microhabitat feature covariates used to analyze the full dataset, except for the number of fruits. There were too few observations with fruits to estimate the coefficients. We also included the recorded number of potential competitors and predators as additional covariates. Because the GLMMs fitted using this dataset were zero inflated and/or suffered from “singularity,” we fitted the models using zero-inflated Poisson mixed models (ZIPMM) using R package glmmTMB (Brooks et al. 2017). We considered trap type and fieldwork campaigns as random effects in all models, coding them as (1|Campaing) and (1|Trap). ZIPMM are constituted of two components: the fixed effects that include all covariates of GLMMs and the zero-inflated fixed effects (ZI hereafter), which include the variables that account for the excess of zeroes in the data. Variables included in the ZI component were specified in the same way as variables of the fixed effects component.
Model selection
For model selection, we built sets of models containing all possible combinations of explanatory variables, compared models using second-order AIC (AICc) weights (w), and built the averaged model for each model set (Anderson 2008; Burnham and Anderson 2002). Because of the smaller sample size of the subset dataset and because of the additional ZI component of ZIPMM that doubles the number of covariates, we restricted the number of candidate models (N = 37) in the model set. This was performed by creating models with all combinations of two variables, one in the fixed effect component and another in the ZI component, plus the null model. We also assessed the statistical evidence of the variables (variable importance) by summing the AICc weights (w) of the model in which the explanatory variables were present (Burnham and Anderson 2002). Some combinations of variables did not converge for the ZIPMM model selection and the log likelihood of these models was not calculated. These models were excluded from the calculations of variable importance and the number of times each variable appeared across the candidate set was not fully balanced. The 85% confidence interval of each variable in the averaged models was used to check for uninformative parameters (Arnold 2010). For model building, comparison, and averaging, we used the R package “MuMIn” (Barton 2019). We assessed the quality (overdispersion, zero-inflation, and singular model fits) of global models using the R package “performance” (Lüdecke et al. 2020). All analyses were conducted in program R (R Core Team 2019).
Results
Diversity of small mammal species
Our total trapping effort was 7260 trap nights (represented by 3300 pitfall traps, 1320 small Sherman, 1320 large Sherman and 1320 Tomahawk trap nights). We obtained a low capture rate (0.99%) and captured 72 individuals from 11 species of small mammal (Appendix Table 4). The most captured species were Didelphis albiventris (n=31 individuals), Oligoryzomys nigripes (n=20), and Akodon montensis (n=9). The species richness, as well abundance and habitat use curves, indicates slow increment of species, individuals, and captures, respectively, with increasing sampling effort (Appendix Fig. 7). We did not record any species considered threatened with extinction by the IUCN red list (Appendix Table 4).
Occurrence of medium and large mammals
The total trapping effort was 1080 camera-trap-days and we had 283 records from 14 species of medium and large mammals (Appendix Table 3). In total, we had 49 records of predators (n=6 species) and 234 records of competitors (n=8 species). The species with higher number of records belonging to the competitors’ group were Dasypus novemcinctus (n=93), Tayassu pecari (n=85), and Sylvilagus brasiliensis (n=28), and the most frequent predators were Nasua nasua (n=35), Leopardus pardalis (n=4), and Leopardus wiedii (n=3). Four species are considered threatened with risk of extinction based on the IUCN red list and these are Tayassu pecari, Sylvilagus brasiliensis, Leopardus wiedii, and Tapirus terrestris (Appendix Table 3).
Factors affecting small mammals
Exploring only the microhabitat features in the full sample dataset, from all 37 models in the model set, 11 were better than the null model for richness (Appendix Table 5), 16 for abundance (Appendix Table 6), and 19 for habitat use (Appendix Table 7). Distance to bamboo thickets was the most important variable, positively affecting all small mammal responses (Fig. 2; Appendix Fig. 8). The relative importance was 0.87 for richness, 0.97 for abundance, and 0.94 for habitat use (Table 1). Distance to bamboo thickets was the only informative variable (i.e. confidence intervals did not overlap with 0) for richness and abundance, and was informative together with number of fallen logs for habitat use, both positively related to the respective response variables.
None of the 37 models of the subset sample dataset was considered more plausible than the null model to explain abundance (Appendix Table 8) or habitat use of small mammals (Appendix Table 9). For abundance, 18 models did not converge while one model did not converge for habitat use. These models were excluded from the candidate set. Predators and competitors had greater importance than most covariates in the abundance and habitat use models (Table 2); however, our analyses using the subset dataset did not have enough statistical power to outrun the null model.
Discussion
Our hypotheses were partially corroborated. Species richness, abundance, and habitat use of small mammals were affected only by a single microhabitat feature, more specifically by the distance to the nearest bamboo patch). In the other hand, litter and number of fallen logs were not significant in the models, contrasting with other studies that highlight the importance of theses microhabitat features for small mammals (Melo et al. 2013; Pinotti et al. 2012). Also, divergent to our expectation, the occurrence of potential competitors and predators did not significantly affect any small mammals’ dimension.
Many species of small mammals captured in ESEC are common and have a wide distribution, like D. aurita, O. nigripes, A. montensis, M. americana, N. lasiurus, C. subflavus, and C. tener (Patton et al. 2015). It is important to note that we have no capture of arboreal rodent or marsupials like Caluromys philander, but we found one specimen of C. philander killed in a road nearby (C. André personal comm.). Probably the use of canopy platform traps in the grid could have captured arboreal species (Graipel et al. 2003), like C. philander. Nevertheless, the abundant species D. albiventris and O. nigripes are common in the ecotone of Atlantic Forest and Cerrado and these species occurred in more than 50% of the sites in the Atlantic Forest (Bovendorp et al. 2017b).
The small mammal assemblage in ESEC is dominated by very few species. It is suggested that large mammals can shape small mammals communities (Bovendorp et al. 2018), so that in defaunated sites, Oligoryzomys sp. and Akodon sp. are highly abundant compared to sites with peccaries (Galetti et al. 2017; Galetti et al. 2015b). Indeed, the abundance or diversity of large mammals helps maintaining the diversity of small mammals, decreasing the abundance of dominant species (Bovendorp et al. 2018; Galetti et al. 2017). However, our best models, when using the subset dataset, indicate that potential competitors and predators did not significantly affect the abundance or habitat use of small mammals even with high abundance of large mammals (Appendix Table 5). It is important to note that both predators and competitors ranked as the most important covariates among all variables in the model’s subset, but the null model was top-ranked. Therefore, it is possible that the extremely low capture rate of small mammals during the camera traps survey or the very fine-spatial scale sampled in this study was not sufficient to detect the effect of larger mammals.
Bamboo emerged as the most important microhabitat feature in our best models, affecting richness, abundance, and habitat use by small mammals. The positive correlation was found between the distance of bamboo thickets and all three response variables. We did not expect such an influence from bamboo, especially because its shoots and seeds can be used as food resources by some small mammal species (Bovendorp et al. 2020; Silva 2005), but bamboos were not reproductive during our study. Also, small mammals usually use dense bush and more complex structures for foraging and hiding (Fonseca and Robinson 1990; Lima et al. 2010; Melo et al. 2013). Bamboo thickets generate open areas inside the forest and this happens because the bamboo leaves cover the soil and prevent seedlings to grow, creating a less complex and open structure in the area (Campanello et al. 2007; Lima et al. 2012; Rother et al. 2016). It is important to mention that the distance to bamboos was negatively and significantly correlated with distance to water in our study, pointing out that may the capture of small mammals have been some influence of proximity of water bodies (Fonseca and Kierulff 1989; Honorato et al. 2015).
Litter and fallen logs were marginally important explanatory variables in the models. Because terrestrial species comprised most of our captures, these elements in the forest floor may be important to shape the small mammal community in ESEC (Grelle 2003; Pardini et al. 2005; Pinotti et al. 2015). Our results show that open areas inside the forest, such as the ones created by bamboo thickets, may influence more the small mammal behavior than other microhabitat features (liana, simple tree, forked tree, ferns) (Grelle 2003; Melo et al. 2013; Pardini et al. 2005). Indeed forest specialists tend to avoid open areas and sometimes those areas act as an barrier for displacement and population dispersion (Olifiers et al. 2005; Pardini et al. 2005).
Conversely, an unexpected result was that the presence of fruits was not a significant explanatory variable in the models. The ESEC presents a semideciduous seasonal vegetation (Veloso et al. 1991) and holds 76 plants species (Durigan et al. 2000), among them important palm trees, like Euterpe edulis, that are source of fruits for fauna (Akkawi et al. 2020). Our findings contrast with other studies that found influence of variables such as fruit, litter, and trees in small mammal communities (Bergallo and Magnusson 1999; Bovendorp 2013; Melo et al. 2013; Naxara et al. 2009; Pinotti et al. 2011). One plausible explanation for these results is that during the campaigns, the availability of fruits in the trees and on the ground was overall very low in the grid.
As a conclusion, we found that only bamboo is shaping community of small mammals in the fine scale in the ESEC. In this way, the lack of fruits during our campaigns and the strong influence of water (strongly negatively correlated with bamboo) might associate with the finding result. On the other hand, the low captured rate of small mammals in this study may was not capable to capture all nuances of the factors explored here. However, to solve this puzzle, we encourage future studies about small mammals in the ESEC once the low capture rate may be associated with other factors that we have not quantified and/or because there is no replication. In addition, we encourage to collect other variables that may influence the community of small mammal in the ESEC, such as the presence and abundance of fungi and/or invertebrates once mostly of small mammals are omnivorous and/or insectivorous.
References
Akkawi P, Galetti M, Mendes CP, Villar N (2020) Dominance hierarchy on palm resource partitioning among Neotropical frugivorous mammals. J Mammal 101:697–709. https://doi.org/10.1093/jmammal/gyaa052
Amorim FW et al (2020) Good heavens what animal can pollinate it? A fungus-like holoparasitic plant potentially pollinated by opossums. Ecology 101:e03001. https://doi.org/10.1002/ecy.3001
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer. https://doi.org/10.1007/978-0-387-74075-1
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s Information Criterion. J Wildl Manag 74:1175–1178
Banks-Leite C et al (2014) Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 345:1041–1045. https://doi.org/10.1126/science.1255768
Barros CS, Püttker T, Pinotti BT, Pardini R (2015) Determinants of capture-recapture success: an evaluation of trapping methods to estimate population and community parameters for Atlantic forest small mammals. Zoologia (Curitiba) 32:334–344. https://doi.org/10.1590/s1984-46702015000500002
Barton K (2019) MuMIn: multi-model inference. R package version 1.43.6. https://CRAN.R-project.org/package=MuMIn. Accessed 10 Feb 2021
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7. Retrived from http://CRAN.R-project.org/package=lme4. Accessed 10 Feb 2021
Ben-Moshe A, Dayan T, Simberloff D (2001) Convergence in morphological patterns and community organization between Old and New World rodent guilds. Am Nat 158:484–495
Bergallo HG, Magnusson WE (1999) Effects of climate and food availability on four rodent species in southeastern Brazil. J Mammal 80:472–486. https://doi.org/10.2307/1383294
Bergallo HdG, Magnusson WE (2004) Factors affecting the use of space by two rodent species in Brazilian Atlantic forest 68
Bovendorp RS (2013) História natural e ecologia de duas espécies de roedores simpátricas da tribo Oryzomyini (Cricetidae: Sigmodontinae) na Floresta Atlântica. Escola Superior de Agricultura “Luiz de Queiroz”. Universidade de São Paulo
Bovendorp RS, McCleery RA, Galetti M (2017a) Optimising sampling methods for small mammal communities in Neotropical rainforests. Mammal Rev 47:148–158. https://doi.org/10.1111/mam.12088
Bovendorp RS, Villar N, de Abreu-Junior EF, Bello C, Regolin AL, Percequillo AR, Galetti M (2017b) Atlantic small-mammal: a dataset of communities of rodents and marsupials of the Atlantic forests of South America. Ecology 98:2226. https://doi.org/10.1002/ecy.1893
Bovendorp RS, Brum FT, McCleery RA, Baiser B, Loyola R, Cianciaruso MV, Galetti M (2018) Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography 42:23–35. https://doi.org/10.1111/ecog.03504
Bovendorp RS, Heming NM, Percequillo AR (2020) Bottom-up effect: a rodent outbreak following the bamboo blooming in a Neotropical rainforest. Mamm Res. https://doi.org/10.1007/s13364-020-00505-y
Brooks M et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.3929/ethz-b-000240890
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information - theoretical approach. Spinger, New York
Cabral JC, Granzinolli MAM, Motta-Junior JC (2006) Dieta do quiriquiri, Falco sparverius (Aves: Falconiformes), na Estação Ecológica de Itirapina, SP. Rev Bras Ornitol 14:393–399
Campanello PI, Gatti MG, Ares A, Montti L, Goldstein G (2007) Tree regeneration and microclimate in a liana and bamboo-dominated semideciduous Atlantic Forest. For Ecol Manag 252:108–117
Carvalho FMV, Pinheiro PS, Fernandez FAS, Nessimian JL (1999) Diet of small mammals in Atlantic forest fragments in southeastern Brazil. Rev Bras Zoociencias 1:91–101
Chesson P, Kuang JJ (2008) The interaction between predation and competition. Nature 456:235–238
Dalmagro A, Vieira E (2005) Patterns of habitat utilization of small rodents in an area of Araucaria forest in Southern Brazil. Austral Ecol 30:353–362
Delciellos AC, Barros CS, Prevedello JA, Ferreira MS, Cerqueira R, Vieira MV (2018) Habitat fragmentation affects individual condition: evidence from small mammals of the Brazilian Atlantic Forest. J Mammal 99:936–945. https://doi.org/10.1093/jmammal/gyy078
DeMattia EA, Curran LM, Rathcke BJ (2004) Effects of small rodents and large mammals on Neotropical seeds. Ecology 85:2161–2170
Dirzo R, Mendoza E, Ortiz P (2007) Size-related differential seed predation in a heavily defaunated neotropical rain forest. Biotropica 39:355–362
Dueser RD, Hallett JG (1980) Competition and habitat selection in a forest-floor small mammal fauna vol 35. Oikos
Durigan G, Franco GADC, Saito M, Baitello JB (2000) Estrutura e diversidade do componente arbóreo da floresta na Estação Ecológica dos Caetetus, Gália, SP. Rev Bras Bot 23(371):383
Facure KG, Giaretta AA (1996) Food habits of carnivores in a coastal Atlantic forest of Brazil. Mammalia 60:499–502
Fonseca G, Kierulff M (1989) Biology and natural history of Brazilian Atlantic forest small mammals Bulletin of the Florida State Museum. Biological Sciences 34:99–152
Fonseca G, Robinson J (1990) Forest size and stucture: competitive and predator effects on small mammal communities. Biol Conserv 53:265–294
Galetti M, Bovendorp RS, Guevara R (2015a) Defaunation of large mammals leads to an increase in seed predation in the Atlantic forests. Glob Ecol Conserv 3:824–830. https://doi.org/10.1016/j.gecco.2015.04.008
Galetti M et al (2015b) Defaunation affects the populations and diets of rodents in Neotropical rainforests. Biol Conserv 190:2–7. https://doi.org/10.1016/j.biocon.2015.04.032
Galetti M, Rodarte RR, Neves CL, Moreira M, Costa-Pereira R (2016) Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS One 11:e0152494. https://doi.org/10.1371/journal.pone.0152494
Galetti M et al (2017) Defaunation and biomass collapse of mammals in the largest Atlantic forest remnant. Anim Conserv 20:270–281. https://doi.org/10.1111/acv.12311
Galetti M et al (2021) Causes and consequences of large-scale defaunation in the Atlantic forest. In: The Atlantic forest, pp 297–324. https://doi.org/10.1007/978-3-030-55322-7_14
Graipel ME, Cherem JJ, Miller PRM, Glock L (2003) Trapping small mammals in the forest understory: a comparison of three methods. Mammalia 67:551–558
Grelle CEV (2003) Forest structure and vertical stratification of small mammals in a secondary Atlantic forest, southeastern Brazil. Stud Neotropical Fauna Environ 38:81–85
Henderson RW, Dixon JR, Soini P (1987) On the seasonal incidence of tropical snakes. Milwaukee Public Mus Contrib Biol Geol 17:1–15
HilleRisLambers J, Adler PB, Harpole W, Levine JM, Mayfield MM (2012) Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst 43:227–248
Honorato R, Crouzeilles R, Ferreira MS, Grelle CEV (2015) The effects of habitat availability and quality on small mammals abundance in the Brazilian Atlantic Forest. Natureza & Conservação 13:133–138. https://doi.org/10.1016/j.ncon.2015.11.010
Hutchinson GE (1957) Population studies - animal ecology and demography - concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427
Hutchinson GE, MacArthur RH (1959) A theoretical ecological model of size distributions among species of animals. Am Nat 93:117–125
Iob G, Vieira EM (2008) Seed predation of Araucaria angustifolia (Araucariaceae) in the Brazilian Araucaria Forest: influence of deposition site and comparative role of small and ‘large’ mammals. Plant Ecol 198:185–196. https://doi.org/10.1007/s11258-007-9394-6
Leite Y, Costa L, Stallings J (1996) Diet and vertical space use of three sympatric opossums in a Brazilian Atlantic forest reserve. J Trop Ecol 12:435–440
Lima DO, Azambuja BO, Camilotti VL, Caceres NC (2010) Small mammal community structure and microhabitat use in the austral boundary of the Atlantic forest. Brazil Zool 27:99–105
Lima RAF, Rother DC, Muler AE, Lepsch IF, Rodrigues RR (2012) Bamboo overabundance alters forest structure and dynamics in the Atlantic Forest hotspot. Biol Conserv 147:32–39. https://doi.org/10.1016/j.biocon.2012.01.015
Lopez L, Terborgh J (2007) Seed predation and seedling herbivory as factors in tree recruitment failure on predator-free forested islands. J Trop Ecol 23:129. https://doi.org/10.1017/s0266467406003828
Lüdecke D, Makowski D, Waggoner P, Patil I (2020) Package ‘performance’.
Macarthur R, Levins R (1964) Competition, habitat selection, and character displacement in a patchy environment. Proc Natl Acad Sci U S A 51:1207–1210. https://doi.org/10.1073/pnas.51.6.1207
Magioli M, Ferraz KMPM (2021) Deforestation leads to prey shrinkage for an apex predator in a biodiversity hotspot. Mamm Res 66:245–255. https://doi.org/10.1007/s13364-021-00556-9
Melo GL, Sponchiado J, Machado AF, Caceres NC (2011) Small-mammal community structure in a South American deciduous Atlantic forest. Community Ecol 12:58–66. https://doi.org/10.1556/ComEc.12.2011.1.8
Melo GL, Miotto B, Peres B, Cáceres NC (2013) Microhabitat of small mammals at ground and understorey levels in a deciduous, southern Atlantic Forest. An Acad Bras Cienc 85:727–736
Moura MC, Vieira MV, Cerqueira R (2009) Occasional intraguild predation structuring small mammal assemblages: the marsupial Didelphis aurita in the Atlantic Forest of Brazil. Austral Ecol 34:481–489. https://doi.org/10.1111/j.1442-9993.2009.01948.x
Naxara L, Pinotti BT, Pardini R (2009) Seasonal microhabitat selection by terrestrial rodents in an old-growth Atlantic Forest. J Mammal 90:404–415. https://doi.org/10.1644/08-mamm-a-100.1
Oksanen J et al (2016) Vegan: community ecology package. R package version 2.3-5. https://CRAN.R-project.org/package=vegan. Accessed 27 Jan 2021
Olifiers N, Gentile R, Fiszon JT (2005) Relation between small-mammal species composition and anthropic variables in the Brazilian Atlantic forest. Braz J Biol 65:495–501
Oliveira FFR, Nessim R, Costa LP, Leite YLR (2007) Small mammal ecology in an urban Atlantic forest fragment in southeastern Brazil. Lundiana 8:27–34. https://doi.org/10.35699/2675-5327.2007.23171
Paglia AP et al (2012) Lista anotada dos mamiferos do Brasil / Annotated checklist of Brazilian mammals. Occas Pap Conserv Int 6:1–76
Pardini R, de Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv 124:253–266. https://doi.org/10.1016/j.biocon.2005.01.033
Patton JL, Pardiñas UFJ, D’Elía G (2015) Mammals of South America. Volume 2: rodents, vol 2. University of Chicago Press, Chicago
Pedó E, Freitas TRO, Hartz SM (2010) The influence of fire and livestock grazing on the assemblage of non-flying small mammals in grassland-Araucaria forest ecotones, southern Brazil. Zoologia (Curitiba Impr) 27:533–540
Pinotti BT, Naxara L, Pardini R (2011) Diet and food selection by small mammals in an old-growth Atlantic forest of south-eastern Brazil. Stud Neotropical Fauna Environ 46:1–9
Pinotti BT, Pagotto CP, Pardini R (2012) Habitat structure and food resources for wildlife across successional stages in a tropical forest. For Ecol Manag 283:119–127. https://doi.org/10.1016/j.foreco.2012.07.020
Pinotti BT, Pagotto CP, Pardini R (2015) Wildlife recovery during tropical forest succession: assessing ecological drivers of community change. Biotropica 47:765–774
Puttker T, Meyer-Lucht Y, Sommer S (2006) Movement distances of five rodent and two marsupial species in forest fragments of the coastal Atlantic rainforest, Brazil. Ecotropica 12:131–139
Püttker T, Pardini R, Meyer-Lucht Y, Sommer S (2008) Responses of five small mammal species to micro-scale variations in vegetation structure in secondary Atlantic Forest remnants. Brazil BMC Ecol 8:9
Püttker T, Bueno AA, dos Santos de Barros C, Sommer S, Pardini R (2013) Habitat specialization interacts with habitat amount to determine dispersal success of rodents in fragmented landscapes. J Mammal 94:714–726. https://doi.org/10.1644/12-mamm-a-119.1
Püttker T, Barros CS, Pinotti BT, Bueno AA, Pardini R (2019) Co-occurrence patterns of rodents at multiple spatial scales: competitive release of generalists following habitat loss? J Mammal 100:1229–1242
R Core Team (2019) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrived from http://www.R-project.org/. Accessed 15 Dec 2021
Rother DC, Rodrigues RR, Pizo MA (2016) Bamboo thickets alter the demographic structure of Euterpe edulis population: a keystone, threatened palm species of the Atlantic forest. Acta Oecol 70:96–102
Santos F et al (2019) Prey availability and temporal partitioning modulate felid coexistence in Neotropical forests. PLoS One 14:e0213671
Sikes RS, Animal C, Use Committee of the American Society of M (2016) 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Silva RB (2005) Ecologia do rato-da-taquara (Kannabateomys amblyonyx) no Parque Estadual de Itapuã, São Leopoldo, RS. Universidade do Vale do Rio dos Sinos
Tabanez MF et al (2005) Plano de manejo da Estação Ecológica dos Caetetus Instituto Florestal. Série Registros 29:1–104
Terborgh J et al (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926. https://doi.org/10.1126/science.1064397
Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. J Mammal 87:757–765. https://doi.org/10.1644/05-mamm-a-285r2.1
Veloso HP, Filho ALRR, Lima JCA (1991) Classificação da vegetação brasileira, adaptada a sistema universal. vol 1. IBGE
Vieira MF, de Carvalho-Okano RM (1996) Pollination biology of Mabea fistulifera (Euphorbiaceae) in southeastern Brazil Biotropica:61-68
Vieira EM, De Moraes DA (2003) Carnivory and insectivory in Neotropical marsupials Predators with pouches: the biology of carnivorous marsupials:271-284
Vieira EM, Monteiro Filho ELA (2003) Vertical stratfication of small mammals in the Atlantic rain forest of south-eastern Brazil. J Trop Ecol 19:501–507
Vieira E, Pizo M, Izar P (2003) Fruit and seed exploitation by small rodents of the Brazilian Atlantic forest. Mammalia 67:533–539
Vieira EM, Paise G, Machado PHD (2006) Feeding of small rodents on seeds and fruits: a comparative analysis of three species of rodents of theAraucaria forest, southern Brazil. Acta Theriol 51:311–318. https://doi.org/10.1007/bf03192683
Vieira EM, Ribeiro JF, Iob G (2011) Seed predation of Araucaria angustifolia (Araucariaceae) by small rodents in two areas with contrasting seed densities in the Brazilian Araucaria forest. J Nat Hist 45:843–854. https://doi.org/10.1080/00222933.2010.536265
Acknowledgements
We thank the Fundação Florestal (COTEC Proc. SMA no. 002.169/2017) for allowing us to work at ESEC. We also thank Sérgio Nazareth, Sean Keuroghlian, and other colleagues for their assistance with the fieldwork. We are deeply in debt with two anonymous reviewers who made their suggestions in the earlier versions to improve the quality of this manuscript.
Funding
This project was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, Programa Biota (FAPESP, Proc 2014/01986-0). CLA received a Master’s fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Financial code 001, Proc PPG – Unesp - RC: 2017), MG a Senior Fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and RSB a Postdoctoral Fellowship from FAPESP (Proc 2013/25441-0).
Author information
Authors and Affiliations
Contributions
CLA, MCC, MG, and RSB contributed to the study conception and design. Data collection was performed by CLA and RSCA. Data analysis was performed by MCC and NMH. The first draft of the manuscript was written by CLA, NMH, and RSB, and MCC and MG commented and contributed on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Thales Renato Ochotorena de Freitas
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Sample grid design for capturing small mammals and medium and large mammals in the Caetetus Ecological Station, Brazil. Large Sherman trap (23 × 7.5 × 8.5 cm) are represented by dark rectangle, small Sherman trap (23 × 7.5 × 8.5 cm) are represented by light gray rectangle, and Tomahawk (42.0 × 12 × 15 cm) are represented by open rectangle. The open circles connected by continuous line represent the pitfall traps connected by plastic fence. The cameras trap is represented by the dark blue triangle for the period to December 2017 to January 2018 and dark green from January to February 2018 in the grid
Rights and permissions
About this article
Cite this article
André, C.L., Côrtes, M.C., Heming, N.M. et al. Bamboo shapes the fine-scale richness, abundance, and habitat use of small mammals in a forest fragment. Mamm Res 67, 199–218 (2022). https://doi.org/10.1007/s13364-021-00616-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-021-00616-0