Abstract
The alternative prey hypothesis predicts that predators respond both functionally and numerically (with a time lag) to fluctuations in the main prey abundance, which affects the survival of alternative prey. This pattern was found in northern Europe in the community formed by voles (Microtidae), red foxes (Vulpes vulpes) and roe deer (Capreolus capreolus). We studied the same predator—prey community in a temperate latitude where, according to the predation hypothesis, only the functional response of predators to changes in main prey availability should occur. In the years 1997–2007, in western Poland, we estimated the index of common vole (Microtus arvalis) abundance (burrow counts), the density of foxes (spotlight counts), the young production in foxes (young/adult ratio), the index of fox predation on fawns (prey remains near dens) as well as the reproduction index (fawn/female ratio) and density of roe deer (total counts). The vole abundance fluctuated considerably, the young production in foxes did not correlate with the main prey availability, but the density of foxes showed direct numerical response. The index of fox predation on fawns decreased with the vole abundance and negatively affected the fawn/female ratio in roe deer. Thus, the relationships between voles and foxes were not fully consistent with the predation hypothesis. The direct numerical response of foxes should tend to stabilize this predator—prey community. It is suggested, however, that responses showed by vole-eating predators in temperate latitudes may sometimes affect their alternative prey, including animals with unfavourable conservation status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The population fluctuations of a number of species commonly occur in the boreal regions of Eurasia and North America (Krebs 1996). For example in Fennoscandia, small rodents undergo regular 3–4-year-long cycles featured by considerable amplitude and occurrence in large areas (Hanski et al. 1991; Hanski et al. 2001; Korpimäki et al. 2005). These rodent fluctuations result in synchronized population fluctuations of other species, e.g. hares and forest galliform birds, which are caused by numerical and functional responses of predators hunting primarily for small rodents (Angelstam et al. 1984, 1985; Lindström et al. 1987; Norrdahl and Korpimäki 2000). The abundance of those predators increases in the growth phase of small rodent populations and achieves the maximum in the next year after the rodent peak (as a result of high reproduction in the peak year). Simultaneously, with the decrease in the availability of main prey, predators switch to alternative prey. Consequently, predation on alternative prey species increases and the number of such prey decreases. That trend reverses when predator populations go down periodically as a result of insufficient food resources and the main prey starts again its growth phase. This mechanism, described as an alternative prey hypothesis (Angelstam et al. 1984; Norrdahl and Korpimäki 2000), indicates that the changes in alternative prey densities should follow the cycles of small rodent abundance with a time lag.
According to Kjellander and Nordström (2003), the relations compliant with the alternative prey hypothesis occurred in the community consisting of voles (Microtidae)—red foxes (Vulpes vulpes)—roe deer (Capreolus capreolus) in central Sweden. Fawn survival in roe deer turned out to be positively dependent on the current vole density and negatively dependent on the number of fox litters in the preceding year. Moreover, the number of litters produced by foxes in that study area increased with the abundance of voles. Thus, the population of adult foxes was apparently the largest 1 year after the peak of their main prey abundance.
Regular high-amplitude multiannual cycles of small rodents disappear in lower latitudes, and the reasons for this phenomenon include the increasing populations of generalist predators (which stabilize prey populations) and higher availability of alternative prey (Hanski et al. 1991; Turchin and Hanski 1997; Hanski et al. 2001). However, small rodent fluctuations and consecutive predator responses sometimes also occur in temperate latitudes (e.g. Jędrzejewska and Jędrzejewski 1998; Lambin et al. 2000; Salamolard et al. 2000; Dupuy et al. 2009). Nevertheless, these temperate fluctuations show an irregular course, low amplitude and synchronization on relatively limited areas (Korpimäki et al. 2005). According to a hypothesis formulated by Korpimäki et al. (2005), vole fluctuations in temperate latitudes do not result from interactions between voles and mainly specialist predators, as it takes place in the northern regions of Europe, but rather from a specific influence of generalist predators (Korpimäki et al. 2005). Still, the knowledge about relationships between predators and their prey in the aspect of population fluctuations in temperate climate regions seems to be limited.
The aim of this study was to describe the relationships occurring in a temperate latitude in the community formed by voles, red foxes and roe deer, i.e. the same already investigated in northern Europe by Kjellander and Nordström (2003). In accordance with the predation hypothesis (Hanski et al. 1991; Hanski et al. 2001), we expected that in the temperate latitude, foxes would not respond numerically but would functionally respond to the vole population fluctuations by increasing predation on roe deer fawns in years when the density of main prey is low. Due to the lack of delayed population response of foxes and numerous alternative prey species, vole fluctuations would only result in small changes in roe deer demography. Consequently, we tested the following detailed predictions resulting from the knowledge described above:
-
The abundance of voles affects positively the current density of foxes (direct numerical response) or the current productivity of foxes and in turn their density in the subsequent year (delayed numerical response);
-
Fox predation on roe deer fawns (i.e. their capture rate) is negatively correlated with vole abundance (functional response);
-
Changes in fawn predation by foxes or changes in the density of foxes affect current reproductive success in roe deer and their density in the subsequent year.
Materials and methods
Study area
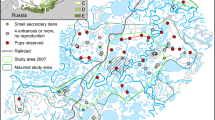
We carried out the study from 1997 to 2007 near Czempiń, south of Poznań, in western Poland (52°09′N, 16°45′E). The study area of 75 km2 contained mainly agricultural land (88%), with the dominance of arable fields (85%) and low proportion of grassland (3%). The size of individual crop fields varied in some regions from 10 to 100 ha (60% of agricultural land), but in other places, they usually did not exceed 5 ha (40%). The main crops were cereals (58%), and other cultivated plants included oilseed rape, maize, sugar beets, potatoes, and alfalfa. Only 3% of the study area constituted non-cultivated places in the form of belts with herbaceous vegetation, shrubs or trees (roadsides, drainage ditches, hedgerows) and small forest patches ranging from <1 to 10 ha. Human settlements and farms covered 9% of this area.
Considerable annual fluctuations in the abundance of common voles (Microtus arvalis) take place in the study area, and high vole densities occur especially in the alfalfa and oilseed rape plantations (Ryszkowski 1982). Red fox population increased at the end of the twentieth century after the initiation of vaccination against rabies and the spring density of foxes reached the level of about one individual per km2 (Panek and Bresiński 2002). The main food of foxes in that region previously constituted of small rodents, mainly common voles, and next brown hares Lepus europaeus (Goszczyński 1974). However, after a considerable decline in hare numbers in the 1980s and 1990s, foxes switched from hares to livestock carrion (Panek 2013). In the late 1990s, hunters intensified culling foxes in this region to limit their growing population in order to improve the situation of small game species (Panek et al. 2006). Annual hunting bag of foxes in the study period amounted to 0.41–1.96 individuals per km2. The hunts lasted from July to March, and used methods included lying in wait, flushing out with beaters and flushing out from dens with dogs. In this part of Poland, roe deer occur not only in forests but they also inhabit woodless agricultural landscape all year round, for example they group in open farmland during winter (Bresiński 1982; Kałuziński 1982a, b; Kamieniarz 2013). The exploitation of roe deer population by hunters depended on the results of total counts of this species carried out every year in early spring and adopted percentage quotas which decreased in the study period from 20 to 15% of spring numbers. Annual hunting bag of this species amounted to 0.68–1.42 individuals per km2 in the study period.
Vole abundance index
We used the number of active entrances to the burrows of common voles as an index of their abundance. It has been found in agricultural areas in Poland that the density of burrows used by common voles was positively correlated with the population density of these animals (Mackin-Rogalska et al. 1986). The counts of burrow entrances took place every year from 1997 to 2006 on two permanent polygonal-shaped transect routes with a length of about 15 km each (i.e. 30 km in total), evenly distributed throughout the agricultural land. The routes were traversed once a year, in March. We recorded only the entrances with clear signs of use (fresh digging, droppings, pieces of food), located within 3 m on both sides of the transect routes. Thus, the counts covered an area of 18 ha.
Fox abundance and productivity
We estimated the spring density of red foxes in the years 1997–2007 by night counts with the use of spotlight (Beltrám et al. 1991). The counts were carried out each year from the middle of March till the beginning of April at a permanent route (10 sections, 2–7 km each) running along secondary public roads and field roads, possibly evenly distributed throughout the study area. The length of this route amounted to 53 km from 1997 to 2004. In subsequent years, we gradually shortened the route, and finally, in 2007, it had a length of 32 km. The same team carried out the counts during the first night hours from a slowly moving car within a 200 m wide strip of land illuminated by a halogen spotlight. Days with adverse weather (fog, rain, strong wind) were avoided. The counts were performed on the whole route during the same night and repeated 3–5 times a year. The density of foxes was a quotient of the average number of spotted individuals and the area of the counting strip.
To assess the productivity of red fox population, we used the ratio of young to adults among foxes shot by hunters in the study area from July to September. At this time of year, young foxes differ from adult ones by the appearance of their teeth, i.e. by the lack of incisor wear (Goszczyński 1995). All shot foxes came from the same group of three gamekeepers using rifles and hunting every year in accordance with the same rules. They collected the sufficient annual samples of 26–67 foxes in the years 1998–2006.
Fox dens and remains of roe deer fawns
The average number of fawns found among prey remains in the vicinity of fox breeding dens was used as an index of fox predation on roe deer fawns (i.e. their capture rate). Each year from 1997 to 2006, dens and other shelters of foxes were located in early spring. All places with clear signs of use were subsequently visited in the first half of June, when cubs start leaving their birth places. Traces left by cubs (treaded patches, scats, tracks, food remains) were the basis for distinguishing breeding dens from shelters used only as by adult foxes. If we found remains of fawns (usually limbs, fragments of spine and skin) near such breeding dens, we determined the number of individuals from which these remains originated by counting the same body parts. As during cub rearing by foxes only newly born fawns occurred, which vary clearly in size and coloration from adults (Pielowski 1999), it was easy to distinguish between the remains of fawns and adult individuals (sometimes also found near dens, probably carrion). The annual number of checked breeding dens varied from 11 to 20.
Roe deer abundance and fawn/female ratio
We estimated the spring density of roe deer each year from 1997 to 2007 by total counts in the whole study area (Kałuziński 1982a; Kamieniarz 2013). We carried out the counts at the end of February or in March, choosing the period after the withdrawal of winter conditions but before agricultural works started, during sunny and windless weather favourable to spotting roe deer groups in open space. The counts lasted three consecutive days and breaks were taken in places constituted barriers to animal movements (e.g. villages). The counting team (3–4 persons) moved in a car viewing successive parts of agricultural area. They observed located roe deer groups from a distance (without scaring them away) through a telescope with magnification of 20–40× to determine their number and sex. If some tree or shrub clumps occurred in searched area where roe deer could hide, some members of counting team noisily penetrated such places to drive the animals out of these shelters, trying to direct them towards open fields checked previously. The density of the whole roe deer population and, separately, the density of females were quotients of the total number of animals observed during this single count and the acreage of study area.
The ratio of fawns to female adults was a measure of the roe deer reproduction success in 1997–2006. We collected these data each year in October and November when the majority of high crops disappear from fields and roe deer more frequently stay in open places. At the same time, fawns still differ by size from adults (Kałuziński 1982a; Kamieniarz 2013). We recorded all encountered females and fawns during single and cursory searching the whole study area. The annual sample of observed females ranged from 50 to 128. Some females seen in autumn were individuals born in the preceding year which did not reproduce in the past season (Pielowski 1999). We subtracted them from the total amount of recorded females on the basis of fawn female to old female proportion assessed from the fawn/female ratio found in the autumn of the preceding year (assuming equal sex ratio among fawns). Thus, the annual reproduction index was a quotient of the number of observed fawns and the number of at least 2-year-old females.
Data analysis
We analysed relationships between the index of vole abundances, the fox abundance and productivity parameters, the index of fox predation on fawns and roe deer demographic parameters with the use of correlation and multiple regression (GLM, STATISTICA software).
Due to a curvilinear relationship between the density of burrow entrances and the population density of voles (Mackin-Rogalska et al. 1986), we transformed logarithmically the observed numbers of vole burrow entrances per 1 km of a transect. In order to check whether foxes showed a direct numerical response or a response with a 1-year-long delay, we compared the vole abundance index with the spring density of foxes in the same year and 1 year later. In the study period, the intense culling of foxes started and the change in hunting exploitation of the roe deer took place which could affect their numbers. Therefore, we inserted the year (as a continuous variable) into multiple regression models testing factors responsible for changes in density. Next, during the analysis of factors affecting productivity of both species, we used their densities in regression models because previous studies indicated density dependence of young production in these species (Goszczyński 1995; Vincent et al. 1995). Additionally, we took into account the average depth of snow from January to March in the case of fawn/female ratio as in that period the development of embryos starts in roe deer, so winter weather conditions may affect reproduction in this species (Pielowski 1999). The data on the depth of snow come from a nearby meteorological station. We created two regression models to analyse relationships between the fawn/female ratio and explanatory variables, i.e. with or without the vole abundance index and the index of fox predation on fawns, as the second factor potentially depended on the first one.
Results
Vole abundance
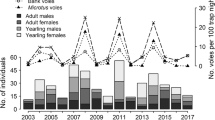
The annual number of active burrow entrances of common voles ranged from 0.4 to 36.9 per km of transects, and after logarithmic transformation, the vole abundance index obtained ranged from −0.92 to 3.61. Two peaks and two low phases of vole abundance occurred in the study period (Fig. 1a).
The vole abundance index (a the number of common vole burrow entrances per km of transects, transformed logarithmically), the spring density of red foxes (b individuals per km2), the index of fox predation on fawns (c the average number of fawns found among prey remains near fox breeding dens) and the fawn/female ratio in roe deer (d the average number of fawns per an adult female) in western Poland in 1997–2006
Fox numerical response
The spring density of foxes in 1997–2007 ranged from 0.6 to 1.1 individuals per km2 (Fig. 1b for the years 1997–2006) and did not correlate with the vole abundance index (r 8 = 0.264, p = 0.5). However, in the multiple regression (R 2 = 0.908), after controlling for the year effect (partial regression coefficient rp 7 = −0.949, p < 0.001), the density of foxes positively depended on the vole abundance index (Fig. 2a; rp 7 = 0.867, p = 0.002).
The relationship between the spring density of red foxes (a) or the index of red fox predation on fawns of roe deer (b the average number of fawns found among prey remains near fox breeding dens) and the vole abundance index in the same spring (the number of common vole burrow entrances per km of transects, transformed logarithmically) in western Poland in 1997–2006. In the case of a, the y-axis shows values corrected due to the significant trend in the density of foxes during the study, i.e. the residuals from the regression between the real fox density (individuals per km2) and year
The spring density of foxes in the subsequent year did not correlate with the vole abundance index (r 8 = −0.206, p = 0.6). The absence of statistically significant relationship between the subsequent year density and the vole abundance index also occurred in the multiple regression taking into account the year effect (R 2 = 0.810; voles: rp 7 = 0.154, p = 0.7; year: rp 7 = −0.896, p = 0.001).
The young/adult ratio among foxes culled in summer, i.e. the production index in this predator, ranged from 1.0 to 3.1 in 1998–2006. The ratio did not correlate with the vole abundance index (r 7 = −0.359, p = 0.3). The multiple regression analysis also did not reveal any impact of the vole abundance index and the fox density on the young production index in foxes (R 2 = 0.129; voles: rp 6 = −0.333, p = 0.4; density: rp 6 = 0.021, p = 0.96).
Fox predation on fawns
The average number of fawns found among prey remains near fox breeding dens, i.e. the index of fox predation on fawns, ranged from 0.08 to 0.86 (Fig. 1c) and decreased with the vole abundance index (Fig. 2b; r 8 = −0.777, p = 0.01). Similar results showed the multiple regression (R 2 = 0.763) including both the density of females (range from 2.9 to 4.3 per km2; rp 7 = 0.205, p = 0.6) and the index of vole abundance (rp 7 = −0.862, p = 0.003).
Fawn/female ratio and roe deer abundance
The average number of fawns per female with reproduction capacity ranged from 0.83 to 1.22 (Fig. 1d). No correlations were found between the fawn/female ratio and the vole abundance index (r 8 = 0.407, p = 0.2) or the index of fox predation on fawns (r 8 = −0.511, p = 0.1). We carried out the analyses of multiple regression for the fawn/female ratio in relation to explanatory variables in two variants—with the exclusion of the index of fox predation on fawns and with the exclusion of vole abundance index. When the index of vole abundance entered the regression model, none of the variables gave statistically significant effect. However, when the fox predation index supplemented the equation, this variable as well as the average depth of snow cover (range from 0.3 to 5.0 cm) gave a negative effect, and the impact of the female density was near significance, whereas the effect of fox density was not significant (Table 1). The fawn/female ratio in roe deer decreased with the fox predation on fawns also when only the changes in winter severity entered the multiple regression model (Fig. 3; R 2 = 0.695; predation: rp 7 = −0.787, p = 0.01; snow: rp 7 = −0.767, p = 0.02).
The relationship between the fawn/female ratio in roe deer and the index of red fox predation on fawns (the average number of fawns found among prey remains near fox breeding dens) in western Poland in 1997–2006. The y-axis shows values corrected for the effect of winter severity, i.e. the residuals from the regression between the real fawn/female ratio and the average depth of snow cover from January to March
The spring density of roe deer in the study area ranged from 5.3 to 7.2 individuals per km2 in 1997–2007. The subsequent year density (1998–2007) did not correlate with the index of fox predation on fawns (r 8 = −0.359, p = 0.3) and fawn/female ratio (r 8 = −0.222, p = 0.5). When the year effect was controlled in multiple regression models, the subsequent year density of roe deer did not show any significant relationship with the index of fox predation on fawns (R 2 = 0.423; predation: rp 7 = −0.195, p = 0.6; year: rp 7 = 0.581, p = 0.1) or with the fawn/female ratio (R 2 = 0.401; ratio: rp 7 = −0.032, p = 0.9; year: rp 7 = 0.608, p = 0.08).
Discussion
Contrary to our predictions, red foxes in western Poland responded numerically to the changes in vole abundance. However, the response was not compliant with the alternative prey hypothesis as in Fennoscandia, where the availability of small rodents affected the cub productivity of foxes in the same year and as a result fox population increase occurred 1 year later (Angelstam et al. 1984, 1985; Kjellander and Nordström 2003). We did not find any relationship between the spring abundance of voles and productivity of foxes in western Poland, which suggests that foxes were able to reproduce successfully also in years with limited availability of voles by switching into alternative food which satisfied their needs in such periods. On the other hand, our index of fox productivity is rather rough, so it seems possible that some moderate changes in the litter size of foxes or their cub survival with the fluctuations of vole abundance occurred in our study area, but without a substantial impact on the numbers of these animals in the next years. The reports from other regions of central Europe gave inconsistent findings, i.e. about the lack of relationships between the fluctuations in the main prey abundance and the reproduction of foxes, i.e. the size of their litters (Weber et al. 1999) or about the increase in the number of cubs per a litter with the increasing share of small mammals in fox diet, which indicated their better availability (Goszczyński 1989).
In our study area, foxes responded numerically without time lag. It may suggest that vole abundance affected fox survival rate in previous winter. During this season, the availability of alternative prey seems to be more limited than in spring for instance due to lack of young of medium-sized mammals (hares, roe deer) or migrating birds and bird broods. It is probable that the results of our spring counts of vole burrows also described well winter fluctuations in the availability of that prey. Potential changes in the survival rate of foxes in winter may have resulted in the observed relationship between the abundance of voles and the spring density of foxes. On the other hand, the numerical response without the time lag is especially typical for nomadic predators, i.e. birds of prey which move to regions with abundant food resources (Korpimäki and Norrdahl 1989, 1991; Salamolard et al. 2000; Millon and Bretagnolle 2008). In that case, the fluctuations in local populations of predators are not a result of changes in their reproduction or survival rates, but in immigration and emigration. In the populations of red foxes, especially those with high densities, there are individuals which do not participate in reproduction—usually young animals which enter social groups or wander; and moreover, the tendency to stay in the region of birth or to migrate seems to depend on the availability of food resources (Schantz 1981, 1984a; Cavallini 1996). The dispersion distance of red foxes ranges in various populations from 1 to 2 km to several dozens of kilometres (Trewhella et al. 1988). The formation of social groups in the red fox is linked with the availability of resources. According to this point of view (the resource dispersion hypothesis), the size of fox territories is delimited according to the period of lowest resource availability; so, the average food abundance is greater than the requirements of breeding pair, and therefore, these dominants may tolerate subordinate animals on their territory (Macdonald 1983; Schantz 1984a; Baker et al. 2004). The level of this tolerance seems to depend on the food availability, which may affect the number of such additional individuals living in fox territories (Lindström 1989; Schantz 1984b). Furthermore, territoriality in the red fox seems to be not very strict, and it promotes the presence of floating individuals in populations (Cavallini 1996). The research carried out in our study area at the end of 1990s indicated that the local population of foxes included individuals which did not participate in reproduction (Panek and Bresiński 2002). Thus, it is probable that in the years of high vole abundance in the study area, the emigration of foxes was lower than their immigration from neighbouring areas where the availability of the main prey may have been limited. Consequently, relatively large numbers of non-breeding individuals could stay then in our area. Conversely, foxes could mainly emigrate from the study area in the years of minimal vole availability as they searched for better feeding grounds. The small size of our area was undoubtedly a factor favouring this pattern. Such relatively short-distance movements of predatory mammals, similar to migrations of birds of prey following their prey, seem probable in temperate latitudes also because synchronization in fluctuations of small rodent populations frequently occur there only over small areas (Korpimäki et al. 2005). As a result, even not very mobile species may probably move from places with limited food resources into neighbouring areas where more food is available.
In accordance with the alternative prey hypothesis, fox predation on fawns increased in the years with limited availability of small rodents. The research method applied to measure the rate of this predation did not allow us to assess its real magnitude because during the inspections of breeding dens, only a part of animals killed by fox families was undoubtedly detected (Sargeant et al. 1984). According to the studies carried out in other regions of Europe, red foxes may kill from 13 to 25% to about half the fawns born, so the fox predation was an important factor influencing fawn survival and recruitment in roe deer, and moreover, acting more strongly in open agricultural landscapes than in forests (Lindström et al. 1994; Aanes and Andersen 1996; Jarnemo and Liberg 2005; Panzacchi et al. 2008, 2009). Another important cause of roe deer fawn mortality in rural areas is agricultural activity, mainly mowing (Kałuziński 1982b; Jarnemo 2002). In our study area, hay cutting took partially place in the final part of cub rearing by foxes, so a part of fawn remains found near fox dens might be individuals killed by mowing machines. However, grass and alfalfa fields are also places preferred by common voles (Ryszkowski 1982). Therefore, it seems unlikely that foxes visited such fields less often and thus had less opportunity to find dead fawns in the years of high vole abundance. This indicates that the use of dead fawns by foxes is not able to create the observed changes in the frequency of fawn remains near fox dens, and such potential scavenging does not affect considerably our findings concerning the relationship between the availability of voles and fawn predation.
Fox predation on fawns turned out to be so intense in our agricultural study area that it was one of the factors affecting the reproductive index in roe deer. But we did not find a significant relationship between the abundance of voles and fawn presence, probably because the availability of voles affected positively the density of foxes, and in this way presumably increased the probability of killing fawns by these predators (which compensated the influence of fox functional response on fawn capture rate). The fox numerical response was probably also the cause for which we have not observed relationships between the index of fox predation on fawns and the density of roe deer in the subsequent year. Thus, the direct response of fox numbers to the abundance of voles stabilized this fox—roe deer system in compliance with predictions of the predation hypothesis (Hanski et al. 2001).
Nevertheless, the fact that we have not detected any relationship between fox predation on fawns or fawn/female ratio and roe deer density might result from a specific pattern of exploitation of the local roe deer population by hunters. That exploitation based primarily on the results of spring counts of those animals used to calculate shooting quotas for the nearest hunting season. However, according to regulations existing in game management in Poland, the hunting quotas of big game animals should be reduced if numerous cases of their losses caused by predation, diseases or starvation are observed before or during a given hunting season. Furthermore, local hunters took into account the results of annual autumn estimations of the fawn/female ratio and if this index was low then they limited the autumn and early winter hunting for females and yearlings in order to counteract the decrease in the population size (R. Kamieniarz, unpubl. data). Hunting is an important factor affecting roe deer survival rates, especially in the absence of lynxes and wolves (Strandgaard 1972; Pielowski 1999). The strategy of local hunters resulted undoubtedly in stabilizing the roe deer numbers in winter and early spring and could mask a potential impact of fluctuations in the number of fawns killed by foxes on the roe deer population. An additional reason for the lack of numerical response in this species was probably the relatively small size of our study area, resulting in the possible impact of migration and dispersal.
Our research has revealed that the fluctuations of vole abundance in a temperate latitude led to changes in the rate of fox predation on roe deer fawns. However, we did not find significant relationships between these fluctuations and the population density of roe deer probably because of the described pattern of functional and numerical response of foxes and the specific behaviour of hunters. It seems, however, that if predators react to the availability of small rodents in temperate latitudes, it is possible that at least local and minor synchronized fluctuations in the abundance of alternative prey species, similar to those described in Fennoscandia (Angelstam et al. 1984), may occur in temperate conditions. For instance, in central Poland, red foxes switched into hares and game birds in years with limited availability of small rodents, at least before the long-term decrease in the number of these species (Goszczyński 1986). In the case of roe deer fawns, the red fox seems to be the only successful predator in agricultural areas in many regions of Europe, but the list of predator species hunting field-living small-game animals is definitely more numerous (Potts 1986; Hill and Robertson 1988; Pielowski et al. 1993). Therefore, the effects of total responses showed by all vole-eating predator species (even if these responses are functional only) may have a marked impact on the populations of some alternative prey. The status of a part of such potential prey species, for instance brown hares and grey partridges, has recently been unfavourable in Europe, as considerable declines in their numbers have occurred in the last decades (Smith et al. 2005; Kuijper et al. 2009). If population densities of such species become low, potential fluctuations in their numbers resulting from fluctuations in small rodent abundance would constitute an additional threat for their existence. Thus, it seems vital to carry out further studies on the effects of predator responses to fluctuations in availability of small rodents in agricultural landscapes of temperate Europe.
References
Aanes R, Andersen R (1996) The effects of sex, time of birth, and habitat on the vulnerability of roe deer fawns to red fox predation. Can J Zool 74:1857–1865. doi:10.1139/z96-209
Angelstam P, Lindström E, Widén P (1984) Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 62:199–208. doi:10.1007/BF00379014
Angelstam P, Lindström E, Widén P (1985) Synchronous short-term population fluctuations of some birds and mammals in Fennoscandia – occurrence and distribution. Holarct Ecol 8:285–298. doi:10.1111/j.1600-0587.1985.tb01181.x
Baker PJ, Funk SM, Bruford MW, Harris S (2004) Polygynandry in a red fox population: implications for the evolution of group living in canids? Behav Ecol 15:766–778. doi:10.1093/beheco/arh077
Beltrám JF, Delibes M, Rau JR (1991) Methods of censusing red fox (Vuples vulpes) populations. Hystrix 3:199–214. doi:10.4404/hystrix-3.1-3957
Bresiński W (1982) Grouping tendencies in roe deer under agrocenosis conditions. Acta Theriol 27:427–447. doi:10.4098/AT.arch.82-38
Cavallini P (1996) Variation in the social systems of the red fox. Ethol Ecol Evol 8:323–342. doi:10.1080/08927014.1996.9522906
Dupuy G, Giraudoux P, Delattre P (2009) Numerical and dietary responses of a predator in a temperate zone of Europe. Ecography 32:227–290. doi:10.1111/j.1600-0587.2008.04930.x
Goszczyński J (1974) Studies on the food of foxes. Acta Theriol 19:1–18. doi:10.4098/AT.arch.74-1
Goszczyński J (1986) Diet of foxes and martens in central Poland. Acta Theriol 31:491–506. doi:10.4098/AT.arch.86-44
Goszczyński J (1989) Population dynamics of the red fox in central Poland. Acta Theriol 34:141–154. doi:10.4098/AT.arch.89-10
Goszczyński J (1995) Lis. OIKOS, Warszawa
Hanski I, Hansson L, Henttonen H (1991) Specialist predators, generalist predators, and the microtine rodent cycle. J Anim Ecol 60:353–367. doi:10.2307/5465
Hanski I, Henttonen H, Korpimäki E, Oksanen L, Turchin P (2001) Small-rodent dynamics and predation. Ecology 82:1505–1520. doi:10.1890/0012-9658(2001)082[1505:SRDAP]2.0.CO;2
Hill DA, Robertson P (1988) The pheasant: ecology, management and conservation. Blackwell Sci. Publ, Ltd, Oxford
Jarnemo A (2002) Roe deer Capreolus capreolus fawns and mowing – mortality rates and countermeasures. Wildl Biol 8:211–218
Jarnemo A, Liberg O (2005) Red fox removal and roe deer fawn survival – a 14 year study. J Wildl Manag 69:1090–1098. doi:10.2193/0022-541X(2005)069[1090:RFRARD]2.0.CO;2
Jędrzejewska B, Jędrzejewski W (1998) Predation in vertebrate communities. The Białowieża Forest as a case study. Springer, Berlin
Kałuziński J (1982a) Dynamics and structure of a field roe deer population. Acta Theriol 27:385–408. doi:10.4098/AT.arch.82-35
Kałuziński J (1982b) Roe deer mortality due to mechanization of work in agrocenoses. Acta Theriol 27:449–455. doi:10.4098/AT.arch.82-39
Kamieniarz R (2013) The structure of agricultural landscape and the functioning of the field roe deer population. Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu, Rozprawy Naukowe 463
Kjellander P, Nordström J (2003) Cyclic voles, prey switching in red fox, and roe deer dynamics – a test of the alternative prey hypothesis. Oikos 101:338–344. doi:10.1034/j.1600-0706.2003.11986.x
Korpimäki E, Norrdahl K (1989) Predation of Tengmalm’s owls: numerical responses, functional responses and dampening impact on population fluctuations of microtines. Oikos 54:154–164. doi:10.2307/3565261
Korpimäki E, Norrdahl K (1991) Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology 72:814–826. doi:10.2307/1940584
Korpimäki E, Oksanen L, Oksanen T, Klemola T, Norrdahl K, Banks PB (2005) Vole cycles and predation in temperate and boreal zones of Europe. J Anim Ecol 74:1150–1159. doi:10.1111/j.1365-2656.2005.01015.x
Krebs CJ (1996) Population cycles revisited. J Mammal 77:8–24. doi:10.2307/1382705
Kuijper DPJ, Oosterveld E, Wymenga E (2009) Decline and potential recovery of the European grey partridge (Perdix perdix) population – a review. Eur J Wildl Res 55:455–463. doi:10.1007/s10344-009-0311-2
Lambin X, Petty SJ, Mackinnon JL (2000) Cyclic dynamics in field vole populations and generalist predation. J Anim Ecol 69:106–118. doi:10.1046/j.1365-2656.2000.00380.x
Lindström E (1989) Food limitation and social regulation in a red fox population. Holarct Ecol 12:70–79. doi:10.1111/j.1600-0587.1989.tb00824.x
Lindström E, Angelstam P, Widén P, Andrén H (1987) Do predators synchronize vole and grouse fluctuations? – an experiment. Oikos 48:121–124. doi:10.2307/3565847
Lindström ER, Andrén H, Angelstam P, Cederlund G, Hörnfeldt B, Jäderberg L, Lemnell P-A, Martinsson B, Sköld K, Swenson JE (1994) Disease reveals the predator: sarcoptic mange, red fox predation, and prey populations. Ecology 75:1042–1049. doi:10.2307/1939428
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301:379–384. doi:10.1038/301379a0
Mackin-Rogalska R, Adamczewska-Andrzejewska K, Nabagło L (1986) Common vole numbers in relation to the utilization of burrow systems. Acta Theriol 31:17–44. doi:10.4098/AT.arch.86-2
Millon A, Bretagnolle V (2008) Predator population dynamics under a cyclic prey regime: numerical responses, demographic parameters and growth rates. Oikos 117:1500–1510. doi:10.1111/j.0030-1299.2008.16458.x
Norrdahl K, Korpimäki E (2000) Do predators limit the abundance of alternative prey? Experiments with vole-eating avian and mammalian predators. Oikos 91:528–540. doi:10.1034/j.1600-0706.2000.910315.x
Panek M (2013) Long-term changes in the feeding pattern of red foxes Vulpes vulpes and their predation on brown hares Lepus europaeus in western Poland. Eur J Wildl Res 59:581–586. doi:10.1007/s10344-013-0709-8
Panek M, Bresiński W (2002) Red fox Vulpes vulpes density and habitat use in a rural area of western Poland in the end of 1990s, compared with the turn of 1970s. Acta Theriol 47:433–442. doi:10.1007/BF03192468
Panek M, Kamieniarz R, Bresiński W (2006) The effect of experimental removal of red foxes Vulpes vulpes on spring density of brown hares Lepus europaeus in western Poland. Acta Theriol 51:187–193. doi:10.1007/BF03192670
Panzacchi M, Linnell JDC, Odden J, Odden M, Andersen R (2008) When a generalist becomes a specialist: patterns of red fox predation on roe deer fawns under contrasting conditions. Can J Zool 86:116–126. doi:10.1139/Z07-120
Panzacchi M, Linnell JDC, Odden M, Odden J, Andersen R (2009) Habitat and roe deer fawn vulnerability to red fox predation. J Anim Ecol 78:1124–1133. doi:10.1111/j.1365-2656.2009.01584.x
Pielowski Z (1999) Sarna. Oficyna Wydawnicza „Wydawnictwo Świat”, Warszawa
Pielowski Z, Kamieniarz R, Panek M (1993) Report about game animals in Poland. Biblioteka Monitoringu Środowiska, PIOŚ, Warszawa
Potts GR (1986) The partridge. Pesticides, predation and conservation. Collins, London
Ryszkowski L (1982) Structure and function of the mammal community in an agricultural landscape. Acta Zool Fennica 169:45–59
Salamolard M, Butet A, Leroux A, Bretagnolle V (2000) Responses of an avian predator to variations in prey density at a temperate latitude. Ecology 819:2428–2441. doi:10.1890/0012-9658(2000)081[2428:ROAAPT]2.0.CO;2
Sargeant AB, Allen SH, Eberhardt RT (1984) Red fox predation on breeding ducks in midcontinent North America. Wildlife Monograph, series number 89. The Wildlife Society, Canada, United States, pp. 1–41
von Schantz T (1981) Female cooperation, male competition, and dispersal in the red fox Vulpes vulpes. Oikos 37:63–68. doi:10.2307/3544073
von Schantz T (1984a) ‘Non-breeders’ in the red fox Vulpes vulpes: a case of resource surplus. Oikos 42:59–65. doi:10.2307/3544609
von Schantz T (1984b) Spacing strategies, kin selection, and population regulation in altricial vertebrates. Oikos 42:48–58. doi:10.2307/3544608
Smith RK, Jennings NV, Harris S (2005) A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mamm Rev 35:1–24. doi:10.1111/j.1365-2907.2005.00057.x
Strandgaard H (1972) The roe deer (Capreolus capreolus) population at Kalø and the factors regulating its size. Danish Review of Game Biology 7
Trewhella WJ, Harris S, McAllister FE (1988) Dispersal distance, home-range size and population density in the red fox (Vulpes vulpes): a quantitative analysis. J Appl Ecol 25:423–434. doi:10.2307/2403834
Turchin P, Hanski I (1997) An empirically based model for latitudinal gradient in vole population dynamics. Am Nat 149:842–874. doi:10.1086/286027
Vincent JP, Bideau E, Hewison AJM, Angibault JM (1995) The influence of increasing density on body weight, kid production, home range and winter grouping in roe deer (Capreolus capreolus). J Zool 236:371–382. doi:10.1111/j.1469-7998.1995.tb02719.x
Weber J-M, Meia J-S, Meyer S (1999) Breeding success of the red fox Vulpes vulpes in relation to fluctuating prey in central Europe. Wildl Biol 5:241–244
Acknowledgments
We are grateful to all colleagues from the Research Station PHA in Czempiń for their help in field data collection, and to Krzysztof Kujawa, Maciej Skorupski and anonymous referees for their valuable comments to the manuscript. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Rafał Kowalczyk
Rights and permissions
About this article
Cite this article
Panek, M., Kamieniarz, R. Vole fluctuations, red fox responses, predation on fawns, and roe deer dynamics in a temperate latitude. Mamm Res 62, 341–349 (2017). https://doi.org/10.1007/s13364-017-0327-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-017-0327-z