Abstract
We applied molecular analysis methods to faecal samples to determine both the overall level of occupancy for pine marten (Martes martes) and current stone marten (Martes foina) distribution in the western Po plain. Surveys were carried out in a 10 × 10-km grid, applying a hybrid sampling design. The specific identification of faecal samples was accomplished either by a polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) method or by amplifying and sequencing a 330-bp mtDNA fragment of the control region (D-loop). Identification success was 93.7 % by the PCR-RFLP and 71.7 % by DNA sequencing. Overall, we collected 47 pine marten records and 24 stone marten records. Thirty-six squares (81.8 %) were found to be positive for at least one marten species, the distribution range of the two species scarcely overlapping. The pine marten was shown to be widespread in lowland areas on the north bank of the River Po, which is probably acting as a barrier to its expansion. In this area, stone marten records were few, while it is was widespread on the south bank of the river. Pine marten expansion may have forced the stone marten to restrict itself to less suitable agricultural and urban areas. Nonetheless, we cannot exclude that stone marten range and/or numbers may being declining as a consequence of pine marten expansion. Six pine marten samples belonged to the Central-Northern European (CNE) phylogroup. The relatively high percentage of CNE martens is consistent with the hypothesis of an ongoing expansion of Alpine and trans-Alpine pine marten populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species ranges can be highly dynamic, continuously changing in size and shifting in space as a consequence of variation in both abiotic and biotic conditions over time (Davis and Shaw 2001; Gaston 2003; Wisz et al. 2013). Research on species’ distribution ranges has recently gained a renewed interest, due to the need to address large-scale, human-induced environmental changes (reviewed by Sexton et al. 2009). Several studies have focused on the expansion of invasive alien species (Andow et al. 1990; Hastings 1996; Von Holle and Simberloff 2005), which allows the investigation of spatio-temporal dynamics in the absence of historical complexities (Svenning et al. 2014); the impact of global climate change (Parmesan 2006; Moritz et al. 2008), which makes it urgent to assess the capability of species to track shifting environments (Cianfrani et al. 2011) and the effects on ecosystems of the extinction of large carnivores (e.g. “mesopredator release”; Ritchie and Johnson 2009; Ripple et al. 2014).
Mesopredator spread has been related to the decline of several prey species (Palomares et al. 1995; Crooks and Soulé 1999; Galetti et al. 2009) and may facilitate the transmission of pathogens to domestic carnivores and humans (Whiteman et al. 2007). Nonetheless, the expansion of autochthonous mesopredators can also have positive effects, e.g. by reducing the number of alien species, as it is the case of American grey squirrel (Sciurus carolinensis) population crash following the recovery of pine marten in Ireland (Sheehy and Lawton 2014). Less attention has been devoted to the intra-guild effects of expanding medium-sized carnivores (Hersteinsson and Macdonald 1992; Tannerfeldt et al. 2002).
Long considered a “forest-specialist”, in recent years, the pine marten Martes martes has been repeatedly reported to occur also in largely fragmented landscapes (reviewed by Virgós et al. 2012), suggesting a greater ecological flexibility than previously believed. In heterogeneous landscapes, individuals are confined to wood patches (Vergara et al. 2015), resulting in smaller home ranges compared to forested habitats (Mergey et al. 2011). Habitat fragmentation is also a major factor producing strong genetic differentiation between pine marten populations over short geographical distances (Ruiz-González et al. 2015).
In Italy, the pine marten and the closely related stone marten (Martes foina) occur sympatrically in mountainous areas, while in lowlands, only the latter has been reported (Prigioni et al. 2001; Genovesi and De Marinis 2003a, b; Sindaco and Carpegna 2010). The large plain crossed by the River Po is one of the most intensively cultivated and densely populated areas of Italy, where residual forest cover is <5 % (Falcucci et al. 2007). According to the analysis of available museum data (De Marinis and Lapini 1994; unpubl. data) and ca. 350 marten records collected between 1973 and 2007 (Balestrieri et al. 2010; Sindaco and Carpegna 2010; unpubl. data of Piedmont region Data Bank), the first record of the pine marten in the Po plain dates back to the end of 1988. In the following two decades, records of road-killed pine martens in the western section of this apparently unsuitable area (Piedmont region) grew exponentially (Balestrieri et al. 2010), with evidence of its stable occurrence in two small protected wooded areas (Remonti et al. 2012) and in riparian woodland on the banks of the River Ticino (Balestrieri et al. 2015a), a north-bank tributary of the River Po (Fig. 1). In the same period, in Piedmont, the stone marten showed an opposite trend (as assessed by comparing the number of records in 1991–1999 and 2000–2007), although being still considered widespread throughout the region (Sindaco and Carpegna 2010; unpubl. data of Piedmont region Data Bank). In contrast, except for a few recent records on the River Oglio (Lombardy region; Mantovani 2010), no evidence of pine marten presence is available for the central (Prigioni et al. 2001) and eastern (Bon et al. 1995; Mezzavilla F., pers comm. Oct. 2015) sections of the Po plain, where the stone marten is widespread. Although the more generalist and synanthropic stone marten should be more adapted to human-impacted, fragmented landscapes than the pine marten (Sacchi and Meriggi 1995; Rondinini and Boitani 2002; Santos and Santos-Reis 2010; Vergara et al. 2015), on the River Ticino, pine marten range increase seems to have been accompanied by a sharp restriction in stone marten range (Balestrieri et al. 2015a).
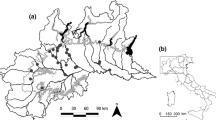
Distribution of both the pine (MM) and stone marten (MF) in the western Po plain (a) as assessed by faecal mDNA analysis; the 300 m a.s.l. contour line, which marks the limit of the River Po plain, and sampling grid (10 × 10 km) are shown; sampled squares are in grey. The stone marten occurs in the whole peninsula; its past distribution in the study area (data collected in 1980–2010) is shown (b). The map of Italy (c) shows the location of the study area with respect to pine marten range (in grey)
To determine both the overall level of occupancy for pine marten and current stone marten distribution in the western Po plain, we applied molecular methods on non-invasively collected faecal samples. DNA analysis was necessary because the faeces of the Martes species, while being relatively abundant and easy to collect, thus facilitating the extensive sampling of these elusive species, cannot be distinguished from each other visually and can also be confused with those of other carnivores (Davison et al. 2002). Faecal DNA-based genotyping has proven to be a cost-effective way to reliably verify and monitor these elusive species’ presence (O’Reilly et al. 2008; Ruiz-González et al. 2013a). Consequently, we either applied a polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) method for mtDNA, which allows effective identification of the two marten species and reliable monitoring of their distribution patterns (Ruiz-González et al. 2008), or sequenced a mtDNA fragment of the control region (D-loop) that has been previously shown to be sufficiently informative to both allow the identification of Martes spp. and identify the main haplotypes and haplogroups described in previous studies (Davison et al. 2001; Pertoldi et al. 2008; Jordan et al. 2012; Ruiz-Gonzalez et al. 2013a).
The second method was applied following the unexpected discovery on the River Ticino of two haplotypes (i.e. Mm47 and Mm48) from the central-northern European (CNE) lineage (Ruiz-González et al. 2013b). By analysing an ∼1600-bp long fragment of the mtDNA of 287 individuals sampled across the entire distribution range of the pine marten, Ruiz-González et al. (2013b) provided evidence for the occurrence in continental Europe of two main phylogroups: the CNE phylogroup and the Mediterranean (MED) phylogroup. As the latter was the only phylogroup recorded in the whole Mediterranean basin, the two CNE haplotypes found in NW Italy have been hypothesised to come from a recent colonisation event from Alpine or trans-Alpine CNE populations (Balestrieri et al. 2015a). MtDNA sequencing was then applied to get more detailed information about the distribution of main haplotypes and phylogroups in this contact zone between the Mediterranean area and continental Europe.
Study area
The Po-Venetian alluvial plain is the largest in Italy (ca. 46,000 km2). The pedogenetic and micro-morphological characteristics of the soils of the lower plain, crossed from west to east by the River Po (652 km in length), support high levels of agricultural productivity and are intensively managed for cattle husbandry and the production of rice, maize and wheat.
Since the second half of the nineteenth century, widespread urbanisation and industrialisation have led to a progressive depletion of soil resources, and built-up areas currently cover ca. 9 % of the area (Gherardi et al. 2009). About 70 % of residual forests are in the western and central plain (Camerano et al. 2010) and either consist of small, isolated fragments (mean patch size =4.5 ha; Lassini et al. 2007) scattered within the agricultural matrix or, as in most European lowlands (Coles et al. 1989), cover the banks of major rivers.
Based on previous pine marten records (see Balestrieri et al. 2010), we focused on the western sector of the Po plain (< 300 m above sea level, Piedmont region), covering an area of ca. 7000 km2 (Fig. 1). The Eastern limit of the study area was marked by the valley of the River Ticino, including the largest and best-conserved riparian forests of the Po-Venetian plain. In Piedmont, the mean annual discharge of the River Po ranges between 43 and 121 m3/s (sampling station of Turin, period 1995–2013; ARPA 2015).
Climate was sub-continental temperate, with mean yearly temperature of 12.0 °C and mean yearly rainfall of 1000 mm.
Methods
To assess the overall level of occupancy for both marten species in the western Po plain, surveys were carried out in a 10 × 10-km grid, superimposed on the kilometric grid of digitised, 1:10,000 Regional Technical Maps. We did not assume that occupancy and detection probabilities were constant across space (MacKenzie et al. 2003; Royle and Nichols 2003), as in Mediterranean agricultural landscapes, the occurrence of both the pine marten (Pereboom et al. 2008; Balestrieri et al. 2015a) and stone marten (Virgós and Garcia 2002; Mortelliti and Boitani 2008) depends on forest availability, i.e. size and degree of connectivity of residual wood patches. As in fragmented habitats carnivores concentrate in the remnant forest patches (Barrull et al. 2014; Šálek et al. 2014), where they are more often detected than into the surrounding agricultural matrix (Santos et al. 2016), faecal samples were searched for along linear transects (mean length of each transect ± SE = 2.6 ± 0.17 km) placed along wood/field margins, paths and country roads as to cover available wood patches and their surrounding open habitats.
Because faeces can be washed away by rain, surveys were not carried out in rainy periods, being delayed by at least 1 week after heavy rain.
To determine how many surveys should be conducted per square, we followed the recommendations by Balestrieri et al. (2015a), who suggested that genetic survey protocols must involve multiple visits (between 1 and 3.9 per sampling unit, depending on marking intensity). We then applied a “hybrid” sampling design, which, for large study areas, represents a good compromise between the robustness of standard designs (all sites surveyed K times) and cost efficiency (Mackenzie and Royle 2005). About 60 % of sampling squares were surveyed only once and the others up to a maximum of four times, depending on the number (N) of “marten-like faeces found per kilometre of transect during each survey (N > 1 and N < 0.5, respectively; Balestrieri et al. 2015a). Repeated surveys of a same square were conducted as multiple discrete visits (i.e. a minimum of two transects per square surveyed on up to four different days) and/or multiple transects within a single visit (up to four transects in a single day, all or partially re-surveyed on one to three different days; Mackenzie and Royle 2005).
Sampling was conducted between January 2013 and December 2014. Hypothesising that river corridors may facilitate and drive pine marten colonisation of the interior of the plain (Balestrieri et al. 2015a), surveys were mainly conducted from north to south (i.e. squares at approx. The same latitude were sampled in the same sub-period), so as to follow the course of the major tributaries of the River Po (Fig. 1).
A portion (ca. 1 cm) of each faeces suspected of belonging to a Martes spp. was picked up using sticks, stored in autoclaved tubes containing ethanol 96 % and frozen at −20 °C until processed (Ruiz-González et al. 2008). All samples were georeferenced and projected onto a GIS (Arcview, ESRI).
DNA was isolated using the QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturer’s instructions. The specific identification of faecal samples was accomplished either by a polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) method (N = 35), according to Ruiz-González et al. (2008), or by amplifying and sequencing a 330-bp mtDNA fragment of the control region (D-loop) (N = 46).
By the first method, two primers—Mm_L1 (5′- CCC AAA GCT GAC ATT CTA AC −3′) and Mm_H1 (5′ - ATG GGC CCG GAG CGA GAA GAG GTA CAC-3′)—amplify the mtDNA from Martes martes and Martes foina and four Mustela species. The simultaneous digestion of amplified mtDNA by two restriction enzymes (RsaI and HaeIII) generates different restriction patterns for each mustelid species (i.e. DNA fragments differing in both number and length), allowing the unambiguous identification of faecal samples (see Ruiz-González et al. 2008 for further details).
By the second method, a 330-bp mtDNA fragment of the control region (D-loop) was amplified using the primers MFABDL228 (5′-AGA CTC AAG GAA GAA GCA ATA GCC-3′) and CLUDH350 (5′-GGG CCT GAA GTA AGA ACC AGA TGC C-3′) (Randi et al. 2000). This DNA fragment was selected as it contains the main diagnostic mutations which reliably identify both the samples at species level and previously described pine marten haplotypes and/or haplogroups (Davison et al. 2001; Pertoldi et al. 2008; Rozhnov et al. 2010; Jordan et al. 2012; Ruiz-González et al. 2013a), thus allowing for comprehensive comparisons. The standard polymerase chain reaction (PCR) amplifications were conducted in 15-μL reactions containing 3 μL of diluted (1:10) template DNA, 3.2 pmol of each primer, 1.75 μM dNTP, 1.33 μM MgCl2, 1.56 μL of Gold STAR 10× buffer, and 0.6 U Taq DNA polymerase, using the following cycling conditions: an initial denaturing step at 94 °C for 5 min, 42 cycles of denaturing at 94 °C for 50 s, annealing at 58.5 °C for 45 s, and extending at 72 °C for 90 s, with a final extending step of 72 °C for 10 min. The PCR products were purified using EXO-SAP IT (USB, Cleveland, OH, USA) and sequenced using the BigDye Terminator Kit V1.1 (Applied Biosystems, Foster City, CA, USA) in an ABI PRISM Model 3130 Genetic Analyser (Applied Biosystems). Electropherograms were visually inspected and edited using SEQSCAPE 2.5 (Applied Biosystems), and nucleotide sequences were further aligned and edited in BIOEDIT 5.0.9 (Hall 1999). Identical haplotypes were matched using DNASP v.5 (Librado and Rozas 2009). We then used BLAST software (Altschul et al. 1990) to determine (1) species’ identity and (2) the correspondence of each discovered haplotype with already published haplotypes in GenBank and the pine marten phylogroups described by Ruiz-González et al. (2013b) (Table S1).
Additionally, we included unequivocal species records from road-killed individuals collected in 2013–2014.
Results
We surveyed 44 100-km2 squares, for a total of 180.9 km of transects, collecting 107 faecal samples. To maximise the cost effectiveness of genetic analyses, a subsample of 81 samples was selected (discarding apparently “old” and dubious surplus samples) and analysed. Identification success was 93.7 % by the PCR-RFLP method (24 Martes martes and 9 Martes foina out of 35 samples) and 71.7 % by DNA sequencing (33 out of 46). By means of DNA sequencing, 21 samples were identified as Martes martes, 8 as Martes foina, 3 as Vulpes vulpes, and 1 as Mustela putorius. Overall, by the application of both molecular methods, 45 samples were assigned to the pine marten and 17 to the stone marten. Additionally, two pine and seven stone marten records from road kills were included. Thirty-six squares (81.8 %) were found to be positive for at least one marten species (Fig. 1a). All squares surveyed more than once were found to be positive (Table 1).
The distributional range of the two species scarcely overlapped, both species occurring in only four squares, two at foot hills and two in the valley of the River Ticino. Overall, pine marten dominated the north of the River Po, while the stone marten was the only Martes species occurring south of the river (Fig. 1a). North of the River Po, the stone marten was not found in 11 out of 13 surveyed squares where it has been previously recorded (Fig. 1b). In general, few samples were found in the central part of the study area, which was intensively cultivated for rice.
In total, three different pine marten haplotypes and six stone marten haplotypes were identified (Table S1 in Electronic Supplementary Material). Five Martes foina haplotypes were novel (Mf_It_1–5; GenBank accession nos.: KX649915–KX649919), while Mf_It_6 had been previously identified in Bulgarian samples (Table S1). All the three pine marten haplotypes retrieved in this study had already been found in previous studies (see Table S1). Using BLAST, haplotype Mm_It_1 was found to be identical (pairwise identity = 100 %) to previously identified haplotypes of the CNE phylogroup, while Mm_It_2 and Mm_It_3 belong to the Mediterranean phylogroup.
Fifteen out of the 21 pine marten samples (71.5 %) assigned to the pine marten through DNA sequencing belonged to the Mediterranean phylogroup (MED), while six samples (28.5 %) were from the central-northern European phylogroup (CNE). Pine martens from the CNE occurred throughout the northern sector of the study area (Fig. 2).
Distribution of pine marten samples belonging to the Mediterranean (MED) and central-northern European (CNE) phylogroups; the 300 m a.s.l. contour line, which marks the limit of the River Po plain, and sampling grid (10 × 10 km) are shown; the map of Italy shows the study area and pine marten distribution in grey, while the stone marten occurs in the whole peninsula
Discussion
Considering the overall high species identification success, faecal DNA analysis confirmed to be an effective method for landscape-scale sampling of sympatric martens (Ruiz-González et al. 2008, 2013a; O’Reilly et al. 2008).
Based on sampling efficacy, i.e. the relatively high probability of marten detection and the 100 % positivity of sites which were surveyed repeatedly, pine marten can be considered to have colonised all the northern section of the study area, at least wherever wood patches still occur.
The current pine marten distribution in the western Po plain suggests that expansion has mainly followed the main watercourses flowing southwards from the Alps, with the River Po, which crosses the study area from the south-west to the east, acting as a natural barrier. Considering that the inner part of the plain has been reached by the pine marten only recently (Balestrieri et al. 2010), the crossing of this barrier may occur in the near future as south of the river, there is large availability of areas suitable for the pine marten (Balestrieri et al. 2016).
Spatial tracking of the environmental niche in response to changing environmental conditions has been often invoked as an explanation for range variation (Davis and Shaw 2001; Svenning et al. 2008). Variations in either climate (Sexton et al. 2009) or land use (Brooks et al. 2002; Cousins et al. 2015) are unanimously considered major determinants of species’ range limits.
As a consequence of gradual global warming, in the northern hemisphere, the ranges of temperate species are shifting northwards or upwards in elevation (Parmesan and Yohe 2003). In this context, pine marten expansion in arable lands would follow a counter-intuitive direction.
Woodlands are key features for pine marten (Virgós et al. 2012); accordingly, in lowland, riparian areas, its abundance has been related to the size and degree of fragmentation of wood patches (Balestrieri et al. 2015a). There is no evidence that in recent decades, forest cover has increased in the western Po plain, favouring pine marten colonisation. In contrast, the analysis of land cover change between 1960 and 2000 at country scale showed that both intensive agriculture and, particularly urbanisation have gradually increased in lowland areas of the whole peninsula (Falcucci et al. 2007). An opposite pattern was recorded for European mountain areas, where following widespread abandonment of low-intensity farming and livestock rearing, forest cover has increased (MacDonald et al. 2000), with a positive effect on forest-dwelling species, such as roe deer Capreolus capreolus (Vernesi et al. 2002; Jepsen and Topping 2004). Alpine pine marten populations may have taken advantage of this increase in forest cover (ca. 50 % between the 1960s and 2000; Falcucci et al. 2007), re-colonising mountain districts from which pine martens had disappeared in the twentieth century (Balestrieri et al. 2016) and expanding in less suitable lowland areas. Similarly, the recent expansion of the pine marten in Ireland and Britain has been related to increased rates of afforestation (O’Mahony et al. 2012; Croose et al. 2013).
The relatively high percentage of CNE martens found in the western Po plain, which currently represents a unicum in southern Europe (Ruiz-González et al. 2013b), is consistent with the hypothesis of an on-going expansion of Alpine and trans-Alpine pine marten populations.
The negligible occurrence of stone marten records north of the River Po agreed with the trend reported for the valley of the River Ticino, where pine marten expansion has coincided with a contraction of stone marten range (Balestrieri et al. 2015a). In contrast, on the south bank of the river, the stone marten is still widespread, as demonstrated by genetically identified faecal samples (this study) and available road kill records (Sindaco and Carpegna 2010). Interestingly, and in spite of small sample size and surveyed area, we identified a high number of closely related stone marten haplotypes. These findings will require additional analysis encompassing a longer DNA fragment and a European-scale survey (currently underway).
Within Mustelidae, there are several sympatric species with similar ecological requirements that have evolved mechanisms to coexist (e.g. Mustela frenata–Mustela erminea, St-Pierre et al. 2006; Mustela erminea–Mustela nivalis, Aunapuu and Oksanen 2003; Lutra lutra–Mustela vison, Bonesi et al. 2004). Pine and stone martens are sympatric across a large part of continental Europe (Proulx et al. 2004) and have also been reported to be syntopic (Pilot et al. 2007; Ruiz-González et al. 2008). In fact, these two martens are quite similar in size, morphology and feeding habits (Marchesi et al. 1989; Balestrieri et al. 2011, 2013) and, when syntopic, their trophic niches can overlap extensively (Posluszny et al. 2007). There is still little information about the factors that may facilitate their coexistence, although some degree of spatial segregation (i.e. differential habitat use) has been reported to potentially reduce interspecific competition (Vergara et al. 2015; Wereszczuk and Zalewski 2015). In sympatry, the pine marten predominates in woodland, while the stone marten mainly occurs in rocky areas and suburban areas (Virgós et al. 2012). This shift in habitat use by the stone marten has been associated to interspecific interactions with the more competitive pine marten (Delibes 1983). Accordingly, in the western Po plain, pine marten expansion may have forced the stone marten to restrict itself to less suitable agricultural and urban areas, where food diversity is expected to be lower—and human disturbance higher—than in residual wood patches (Benton et al. 2003; Balestrieri et al. 2015b).
It is still not clear why pine martens should be able to force stone martens to use suboptimal habitats (Larroque et al. 2015). Remonti et al. (2012) compared the diets of martens and red fox Vulpes vulpes in northern Italy, reporting that the food niche of each marten species highly overlapped with that of the fox. They concluded that the entry of the pine marten could have altered the existing ecological relationship within the carnivore guild to the detriment of the stone marten, the diet of which overlapped to a larger extent that of the red fox (Remonti et al. 2012). Recently, it has been proposed that the lower ability of the stone marten to avoid interference competition at community-level may play a major role in determining its exclusion from forested areas by the pine marten (Balestrieri 2016). Whatever the mechanism determining the lower competitive ability of the stone marten, as sampling was focused on residual forest patch segregation of space use, may explain the small number of stone marten records found north of the River Po.
Nonetheless, we cannot exclude a priori that stone marten population is declining, as the current distribution patterns of the two species and the negative trend of stone marten road kill records (Sindaco and Carpegna 2010) suggest that the pine marten may being actually replacing the stone marten in the lowlands of NW Italy. As population declines are often dictated by complex combinations of environmental and biotic factors (Sinclair and Byrom 2006), particularly for same-sized Mustelidae (Powell and Zielinski 1983), further studies on niche partitioning among species belonging to the same guild are needed to predict short- and long-term outcomes of their interactions.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andow DA, Kareiva PM, Levin SA, Okuba A (1990) Spread of invading organisms. Landscape Ecol 4:177–188
ARPA (2015) L’idrologia in Piemonte—2014. ARPA Piemonte, Torino
Aunapuu M, Oksanen T (2003) Habitat selection of coexisting competitors: a study of small mustelids in northern Norway. Evol Ecol 17:371–392
Balestrieri A (2016) Distribution and ecology of lowland pine marten Martes martes L. 1758. PhD thesis in Natural and Environmental Sciences, University of Milan
Balestrieri A, Remonti L, Ruiz-González A, Gómez-Moliner BJ, Vergara M, Prigioni C (2010) Range expansion of the pine marten (Martes martes) in an agricultural landscape matrix (NW Italy. Mamm Biol 75:412–419
Balestrieri A, Remonti L, Ruiz-González A, Vergara M, Capelli E, Gómez-Moliner BJ, Prigioni C (2011) Food habits of genetically identified pine martens (Martes martes) expanding in agricultural lowlands (NW Italy. Acta Theriol 56:199–207
Balestrieri A, Remonti L, Capra RB, Canova L, Prigioni C (2013) Food habits of the stone marten (Martes foina) (Mammalia: Carnivora) in plain areas of northern Italy prior to pine marten (M. martes) spreading. It J Zool. doi:10.1080/11250003.2012.730067
Balestrieri A, Remonti L, Morotti L, Saino N, Prigioni C, Guidali F (2015a) Multilevel habitat preferences of Apodemus sylvaticus and Clethrionomys glareolus in an intensively cultivated agricultural landscape. Ethol Ecol Evol doi. doi:10.1080/03949370.2015.1077893
Balestrieri A, Remonti L, Ruiz-González A, Zenato M, Gazzola A, Vergara M, Dettori EE, Saino N, Capelli E, Gómez-Moliner BJ, Guidali F, Prigioni C (2015b) Distribution and habitat use by pine marten Martes martes in a riparian corridor crossing intensively cultivated lowlands. Ecol Res 30:153–162
Balestrieri A, Mosini A, Saino N (2016) Distribuzione ed ecologia di martora e faina nel Parco Nazionale della Val Grande. Tecnica report, Dept. of Biosciences, University of Milan.
Barrull J, Mate I, Ruiz-Olmo J, Casanovas JG, Gosàlbez J, Salicrú M (2014) Factors and mechanisms that explain coexistence in a Mediterranean carnivore assemblage: an integrated study based on camera trapping and diet. Mamm Biol 79(2):123–131
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188
Bon M, Paolucci P, Mezzavilla F, Battisti R, Vernier E (1995) Atlante dei Mammiferi del Veneto. Lav Soc Ven Sci Nat 21(Suppl)
Bonesi L, Chanin P, Macdonald DW (2004) Competition between Eurasian otter Lutra lutra and American mink Mustela vison probed by niche shift. Oikos 106:19–26
Brooks TM, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldifeld S, Magin G, Hilton-Taylor C (2002) Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol 16:909–923
Camerano P, Grieco C, Terzuolo PG (2010) I boschi planiziali: conoscenza, conservazione e valorizzazione. Regione Piemonte e Blu edizioni, Torino
Cianfrani C, Le Lay G, Maiorano L, Satizábal HF, Loy A, Guisan A (2011) Adapting global conservation strategies to climate change at the European scale: the otter as a flagship species. Biol Conserv 144:2068–2080
Coles TF, Southey JM, Forbes I, Clough T (1989) River wildlife data bases and their value for sensitive environmental management. Regul Rivers: Res Mgmt 4(2):179–189
Cousins SAO, Auffret AG, Lindgren J, Tränk L (2015) Regional-scale land-cover change during the 20th century and its consequences for biodiversity. Ambio 44:S17–S27
Crooks KR, Soulé ME (1999) Mesopredator release and avifauna extinctions in a fragmented system. Nature 400:563–566
Croose E, Birks JDS, Schofield HW (2013) Expansion zone survey of pine marten (Martes martes) distribution in Scotland. Scottish Natural Heritage Commissioned Report No. 520
Davis MB, Shaw RG (2001) Range shifts and adaptive responses to quaternary climate change. Science 292:673–679
Davison A, Birks JD, Brookes RC, Messenger JE, Griffiths HI (2001) Mitochondrial phylogeography and population history of pine martens Martes martes compared with polecats Mustela putorius. Mol Ecol 10(10):2479–2488
Davison A, Birks JDS, Brookes RC, Braithwaite TC, Messenger JE (2002) On the origin of faeces: morphological versus molecular methods for surveying rare carnivores from their scats. J Zool (London) 257:141–143
De Marinis A, Lapini L (1994) Collections of Italian Mustelidae (Mammalia, Carnivora) housed in Italian museums. Boll Mus reg Sci nat Torino 12:255–325
Delibes M (1983) Interspecific competition and the habitat of the stone marten Martes foina (Erxleben, 1777) in Europe. Acta Zool Fenn 174:229–231
Falcucci A, Maiorano L, Boitani L (2007) Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landscape Ecol 22:617–631
Galetti M, Bovendorp RS, Fadini R, Gussoni CA, Rodrigues M, Alavarez AD, Guimarães P Jr, Alves K (2009) Hyper abundant mesopredators and non-random bird extinction in an Atlantic forest island. Rev Bras Zool 26:288–298
Gaston KJ (2003) The structure and dynamics of geographic ranges. Oxford Univ, Press, Oxford
Genovesi P, De Marinis AM (2003a) Martes foina. In: Boitani L, Lovari S, Vigna Taglianti A (eds) fauna d’Italia Mammalia III, Carnivora—Artiodactyla. Calderini, Bologna, pp. 117–128
Genovesi P, De Marinis AM (2003b) Martes martes. In: Boitani L, Lovari S, Vigna Taglianti A (eds) Fauna d’Italia. Mammalia III, Carnivora—Artiodactyla. Calderini, Bologna, pp. 106–117
Gherardi M, Lorito S, Vianello G, Vittori Antisari L (2009) Qualitative and quantitative evaluation of soil depletion due to urbanisation in the areas near the Po River. EQA – Environmental quality 2:29–38
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nuc Acid S41:95–98
Hastings A (1996) Models of spatial spread: is the theory complete? Ecology 77:1675–1679
Hersteinsson P, Macdonald DW (1992) Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus. Oikos 64:505–515
Jepsen JU, Topping CJ (2004) Modelling roe deer (Capreolus capreolus) in a gradient of forest fragmentation: behavioural plasticity and choice of cover. Can J Zool 82:1528–1541
Jordan NR, Messenger J, Turner P, Croose E, Birks J, O’Reilly C (2012) Molecular comparison of historical and contemporary pine marten (Martes martes) populations in the British Isles: evidence of differing origins and fates, and implications for conservation management. Conserv Genet 13:1195–1212
Larroque J, Ruette S, Vandel J-M, Devillard S (2015) Where to sleep in a rural landscape? A comparative study of resting sites pattern in two syntopic Martes species. Ecography 38:1–12
Lassini P, Monzani F, Pileri P (2007) A green vision for the renewal of the Lombardy landscape. In: Pedroli B, Van Doorn A, De Blust G, Paracchini ML, Wascher D, Bunce F (eds), Europe’s living landscapes. Essays on exploring our identity in the countryside, Landscape Europe/KNNV
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Macdonald D, Crabtree JR, Weisinger G, Dax T, Stamou N, Fleury P, Gutierrez Lazpita J, Gibon A (2000) Agricultural abandonment in mountain areas of Europe: environmental consequences and policy response. J Environ Manag 59:47–69
MacKenzie DI, Royle JA (2005) Designing occupancy studies: general advice and allocating survey effort. J Appl Ecol 42:1105–1114
MacKenzie DI, Nichols JD, Hines JE, Knutson MG, Franklin AD (2003) Estimating site occupancy, colonization and local extinction probabilities when a species is not detected with certainty. Ecology 84:2200–2207
Mantovani S (2010) Recenti segnalazioni della martora, Martes martes, in provincia di Cremona. Pianura 25:95–107
Marchesi P, Lachat N, Lienhard R, Debieve PH, Mermod C (1989) Comparaison des régimes alimentaires de la fouine (Martes foina Erxl) et de la martre (Martes martes L) dans une région du Jura suisse. Rev Suisse Zool 96:127–146
Mergey M, Helder R, Roeder J-J (2011) Effect of forest fragmentation on space-use patterns in the European pine marten (Martes martes. J Mammal 92:328–335
Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR (2008) Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322:261–264
Mortelliti A, Boitani L (2008) Interaction of food resources and landscape structure in determining the probability of patch use by carnivores in fragmented landscapes. Landscape Ecol 23:285–298
O’Mahony D, O’Reilly C, Turner P (2012) Pine marten (Martes martes) distribution and abundance in Ireland: a cross-jurisdictional analysis using non-invasive genetic survey techniques. Mamm Biol 77(5):351–357
O’Reilly C, Statham M, Mullins J, Turner PD, O’Mahony D (2008) Efficient species identification of pine marten (Martes martes) and red fox (Vulpes vulpes) scats using a 5¢ nuclease real-time PCR assay. Conserv Genet doi. doi:10.1007/s10592-007-9371-6
Palomares F, Gaona P, Ferreras P, Delibes M (1995) Positive effects on game species of top predators by controlling smaller predator populations: an example with lynx, mongooses, and rabbits. Conserv Biol 9:295–305
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Pereboom V, Mergey M, Villerette N, Helder R, Gerard JF, Lodé T (2008) Movement patterns, habitat selection, and corridor use of a typical woodland-dweller species, the European pine marten (Martes martes), in a fragmented landscape. Can J Zool 86:983–991
Pertoldi C, Muñoz J, Madsen A, et al. (2008) Genetic variability in the mitochondrial DNA of the Danish pine marten (Martes martes. J Zool (London) 275:68–175
Pilot M, Gralak B, Goszczynski J, Posluszny M (2007) A method of genetic identification of pine marten (Martes martes) and stone marten (Martes foina) and its application to faecal samples. J Zool London 271:140–147
Posluszny M, Pilot M, Goszczynski J, Gralak B (2007) Diet of sympatric pine marten (Martes martes) and stone marten (Martes foina) identified by genotyping of DNA from faeces. Ann Zool Fenn 44:269–284
Powell RA, Zielinski WJ (1983) Competition and coexistence in mustelid communities. Acta Zool Fenn 174:223–227
Prigioni C, Cantini M, Zilio A (2001) Atlante dei Mammiferi della Lombardia. Regione Lombardia e Università degli Studi di Pavia, Milano, 325 pp
Proulx G, Aubry KB, Birks J, Buskirk SW, Fortin C, Frost HC, Krohn WB, Mayo L, Monakhov V, Payer D, Saeki M, Santos-Reis M, Weir R, Zielinski WJ (2004) World distribution and status of the genus Martes in 2000. In: Harrison DJ, Fuller AK, Proulx G (eds) Martens and fishers (Martes) in human-altered environments: an international perspective. Springer-Verlag, New York, pp. 21–76
Randi E, Lucchini V, Christensen MF (2000) Mitochondrial DNA variability in Italian and east European wolves: detecting the consequences of small population size and hybridization. Conserv Biol 14:464–473
Remonti L, Balestrieri A, Ruiz-González A, Gómez-Moliner BJ, Capelli E, Prigioni C (2012) Intraguild dietary overlap and its possible relationship to the coexistence of mesocarnivores in intensive agricultural habitats. Popul Ecol 54:521–532
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ (2014) Status and ecological effects of the world’s largest carnivores. Science 343 1241484
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Rondinini C, Boitani L (2002) Habitat use by beech martens in a fragmented landscape. Ecography 25:257–264
Royle JA, Nichols JD (2003) Estimating abundance from repeated presence–absence data or point counts. Ecology 84:777–790
Rozhnov VV, Meschersky IG, Pishchulina SL, Simakin LV (2010) Genetic analysis of sable (Martes zibellina) and pine marten (M. martes) populations in sympatric part of distribution area in the northern Urals. Russ J Genet 46:488–492
Ruiz-González A, Rubines J, Berdiόn O, Gomez-Moliner BJ (2008) A non-invasive genetic method to identify the sympatric mustelids pine marten (Martes martes) and stone marten (Martes foina): preliminary distribution survey on the northern Iberian peninsula. Eur J Wildl Res 54(2):253–261
Ruiz-González A, Madeira MJ, Randi E, Urra F, Gómez-Moliner BJ (2013a) Non-invasive genetic sampling of sympatric marten species (Martes martes and Martes foina): assessing species and individual identification success rates on faecal DNA genotyping. Eur J Wildl Res 59:371–386
Ruiz-González A, Madeira MJ, Randi E, Abramov AV, Davoli F, Gomez-Moliner BJ (2013b) Phylogeography of the forest-dwelling European pine marten (Martes martes): new insights into cryptic northern glacial refugia. Biol J Linn Soc 109:1–18
Ruiz-González A, Cushman SA, Madeira MJ, Etore R, Gómez-Moliner BJ (2015) Isolation by distance, resistance and/or clusters? Lessons learned from a forest-dwelling carnivore inhabiting a heterogeneous landscape. Mol Ecol 24:5110–5129
Sacchi O, Meriggi A (1995) Habitat requirements of the stone marten (Martes foina) on the Tyrrhenian slopes of the northern Apennines. In: Prigioni C (ed) Proc III Symp on Carnivores. Hystrix 7, pp. 99–104
Šálek M, Červinka J, Padyšáková E, Kreisinger J (2014) Does spatial co-occurrence of carnivores in a central European agricultural landscape follow the null model? Eur J Wildl Res 60:99–107
Santos MJ, Santos-Reis M (2010) Stone marten (Martes foina) habitat in a Mediterranean ecosystem: effects of scale, sex, and interspecific interactions. Eur J Wildl Res 56:275–286
Santos MJ, Rosalino LM, Matos HM, Santos-Reis M (2016) Riparian ecosystem configuration influences mesocarnivores presence in Mediterranean landscapes. Eur J Wildl Res. doi:10.1007/s10344-016-0984-2
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Sheehy E, Lawton C (2014) Population crash in an invasive species following the recovery of a native predator: the case of the American grey squirrel and the European pine marten in Ireland. Biodivers Conserv DOI. doi:10.1007/s10531-014-0632-7
Sinclair ARE, Byrom AE (2006) Understanding ecosystem dynamics for conservation of biota. Jl of Anim. Ecol 75:64–79
Sindaco R, Carpegna F (2010) Segnalazioni Faunistiche Piemontesi. III. Dati preliminari sulla distribuzione dei Mustelidi del Piemonte (Mammalia, Carnivora, Mustelidae). Rivista piemontese di Storia naturale 31:397–422
St-Pierre C, Ouellet JP, Crệte M (2006) Do competitive intraguild interactions affect space use and habitat use by small carnivores in a forested landscape? Ecography 29:487–496
Svenning JC, Normand S, Skov F (2008) Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography 31:316–326
Svenning J-C, Gravel D, Holt RD, Schurr FM, Thuiller W, Münkemüller T, Schiffers KH, Dullinger S, Edwards TC Jr, Hickler T, Higgins SI, Nabel JEMS, Pagel J, Normand S (2014) The influence of interspecific interactions on species range expansion rates. Ecography 37:1198–1209
Tannerfeldt M, Elmhagen B, Angerbjörn A (2002) Exclusion by interference competition? The relationship between red and arctic foxes. Oecologia 132:213–220
Vergara M, Cushman SA, Urra F, Ruiz-González A (2015) Shaken but not stirred: multiscale habitat suitability modeling of sympatric marten species (Martes martes and Martes foina) in the northern Iberian peninsula. Landscape Ecol. doi:10.1007/s10980-015-0307-0
Vernesi C, Pecchioli E, Caramelli D, Tiedemann R, Randi E, Bertorelle G (2002) The genetic structure of natural and reintroduced roe deer (Capreolus capreolus) populations in the alps and Central Italy, with reference to the mitochondrial DNA phylogeography of Europe. Mol Ecol 11:1285–1297
Virgós E, Garcia FJ (2002) Patch occupancy by stone martens (Martes foina) in fragmented landscapes of Central Spain: the role of fragment size isolation and habitat structure. Acta Oecol 23:231–237
Virgós E, Zalewski A, Rosalino LM, Mergey M (2012) Habitat ecology of genus Martes in Europe: a review of the evidences. In: Aubry KB, Zielinski WJ, Raphael MG, Proulx G, Buskirk SW (eds) Biology and conservation of marten, sables, and fisher: a new synthesis. Cornell University Press, New York, pp. 255–266
Von Holle B, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218
Wereszczuk A, Zalewski A (2015) Spatial niche segregation of sympatric stone Marten and Pine Marten—avoidance of competition or selection of optimal habitat? PLoS One. doi:10.1371/journal.pone.0139852
Whiteman CW, Matushima ER, Confalonieri UEC, Palha MDC, Silva ASL, Monteiro VC (2007) Human and domestic animal populations as a potential threat to wild carnivore conservation in a fragmented landscape from the eastern Brazilian Amazon. Biol Conserv 138:290–296
Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, Dormann CF, Forchhammer MC, Grytnes JA, Guisan A, et al. (2013) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev 88:15–30
Acknowledgments
This study has been partially funded by the Basque Government through the Research group on “Systematics, Biogeography and Population Dynamics” (Ref. IT317-10; GIC10/76; IT575/13), the SAIOTEK research programme (Ref: S-PE11UN028). Ruiz-González holds a post doc fellowship awarded by the Dept. of Education Universities and Research of the Basque Government (Ref. DKR-2012-64). A. Balestrieri was supported by a Ph.D. fellowship awarded by the Dept. of Biosciences of the University of Milan. Lesley C. Wright kindly revised the English language. The comments and suggestions of two anonymous referees helped to greatly improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by: Allan McDevitt
Electronic supplementary material
ESM 1
(DOCX 21.0 kb)
Rights and permissions
About this article
Cite this article
Balestrieri, A., Ruiz-González, A., Capelli, E. et al. Pine marten vs. stone marten in agricultural lowlands: a landscape-scale, genetic survey. Mamm Res 61, 327–335 (2016). https://doi.org/10.1007/s13364-016-0295-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-016-0295-8