Abstract
During the last century, the Eurasian otter (Lutra lutra) suffered a dramatic decline in Europe. In France, the same pattern of sharp decline was observed with local extinctions in many regions. Before the recolonisation process, two main populations still remained along the Atlantic coast and in the Massif Central. To investigate the impact of this decline on the genetic diversity and structure of the French otter population, tissue samples of 144 otter carcasses from road kills that were found during 1992–2011 along the Atlantic coast and in the Massif Central were used. They were analysed using 10 microsatellites loci. Observed (H o = 0.64) and expected heterozygosity (H e = 0.62) were moderate, but consistent with results found in other European populations. The bottleneck test showed an excess of heterozygotes, providing evidence of a recent decline. There was evidence for weak but significant allelic frequencies divergence between otters from the Atlantic coast and those from the Massif Central (F st = 0.040, p < 0.05), probably resulting from their isolation prior to the recolonisation process. As the French otter population has been expanding for several years, genetic intermixing is now occurring. Although this expansion has not yet genetically homogenised all populations, this is may be a matter a time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Eurasian otter Lutra lutra (Linnaeus, 1758) is a highly mobile species from the Mustelidae family and a top predator of aquatic food webs. Although it is a fish specialist, this mammal is able to adjust its diet to local conditions (Libois and Rosoux 1989, 1991; Libois 1997). Once widespread throughout Europe, otter populations began to decline significantly in the mid-twentieth century, especially in the industrialised countries of the western part of its range (Macdonald and Mason 1992). Otters were extirpated from many European regions, leading to the fragmentation and isolation of its remaining populations. The main causes were hunting and trapping (bounties), habitat destruction and water pollution (Ansorge et al. 1997; Mason 1995). This species has been protected since 1979 under the Berne Convention and is listed as ‘near threatened (NT)’ by the IUCN Red List (Reuther and Hilton-Taylor 2004). Despite this initially dramatic decline, the signs of natural recovery were detected in most European countries during the 1980s. Otters are, however, still threatened by poaching or accidental trapping, the continuous development of road networks (Rosoux and Libois 1994; Rosoux and Tournebize 1995; Koelewijn et al. 2010) and habitat degradation (Gascuel 1985).
In France, the same pattern of sharp decline has been observed, with local extinction occurring in many regions. Until recently, the otter population in France was fragmented, and the last remaining populations were restricted mainly to the prey-rich Atlantic coastal wetlands in the west of France and to the quiet rivers of the Massif Central. Since 1981, the French legal protection status has allowed Eurasian otter populations to slowly recover. The first signs of re-colonisation were detected in the Massif Central in the mid-1980s (Bouchardy and Boulade 1999). All remaining populations have progressively started to grow and range re-expansion has been observed. Since no reintroduction project has been implemented in continental European countries and no captive otters have been released, with the exception of Alsace (France) where the reintroduction program failed, these movements can be considered to be natural. In the 1990s, the French population represented less than 2 % (approximately 1000 individuals) of the estimated 1930s population (Rosoux and Libois 1994). Today, there is an estimated population of 5000 otters (Lemarchand, pers. comm.), and half of the country is occupied by the Eurasian otter (Rosoux and Green 2004; Kuhn 2009). A national action plan for the conservation of otters was implemented in 1999 (Rosoux et al. 1999; Bouchardy et al. 2001; Kuhn 2009).
Genetic studies have been carried out in several European countries, in order to detect population structure, identify gene flow between populations and follow the recolonisation process of this elusive species. Until now, only three studies focusing on the genetic characteristics of the French population have been published. An initial study investigated the mtDNA (CytB) variations of otter populations in the western and central parts of Europe (Spain, France, Ireland and Czech Republic), and found little diversity among these populations (Morales 2002). A second study focused on the recolonisation pattern of the Cevennes National Park in the Massif Central, where it detected at least two genetically distinct clusters with low genetic diversity (microsatellite DNA; Janssens et al. 2008). The most recent study focused on the landscape genetic structure of otters in Europe. In the case of the French population, this study focused on individuals from the Atlantic coastline only, and found that there are probably three genetically distinct populations along this coastline (microsatellite DNA; Mucci et al. 2010).
Based on these prior results, the main objectives of the present study are to detect whether the French otter population is genetically substructured and to investigate the influence of the sharp decline in this population on the genetic variability of the French Eurasian otter.
Materials and methods
Sample collection
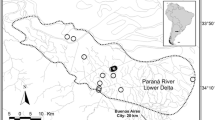
For the present study, tissue samples were used from of a total of 145 roadkill otter carcasses, opportunistically collected between 1992 and 2011. These consisted of muscle tissue samples that were collated by Rene Rosoux, Marie-des-Neiges de Bellefroid and Charles Lemarchand. They were collected in 13 departments along the Atlantic coastline and in the centre of France: 122 otters from marshes along the French Atlantic coastline, three otters from Aquitaine in the south-west of France, 16 otters from the Massif Central and two from the La Brenne marshes (Fig. 1). The geographic locations of these collected tissues represent the full extent of the current range of otters in France. All of the samples were stored in 96 % ethanol and frozen at −20 °C prior to DNA extraction.
Age and sex determination
During post mortem examination, all individuals were sexed and categorised in various classes as cub, young, subadult and adult based on a combination of morphometric criteria (Simpson 2001). For a subsample of 21 individuals, odontochronologic analysis was carried out using mainly the canine (transverse and longitudinal cuts). Estimated age was proven accurate in 90 % of cases.
DNA extraction, amplification and genotyping
DNA extraction was carried out from February to March 2012 in Liège, Belgium, using the Dneasy Tissue kit (Qiagen) in accordance with the manufacturer’s instructions. Gloves and aerosol-resistant pipette tips were used during all manipulations. The quality of the extracted DNA was controlled with a UV-VIS BioSpec-nano spectrophotometer (Shimadzu). The controlled was checked by means of a measurement without sample.
The multilocus genotypes of 145 otters were amplified using the polymerisation chain reaction (PCR) method. Ten microsatellite loci, identical to those used in the study of Janssens et al. (2008), were selected: Lut435, Lut604, Lut701, Lut715, Lut717, Lut733, Lut782, Lut818, Lut832 and Lut902 (Dallas and Piertney 1998; Dallas et al. 1999). One primer from each pair was labelled with fluorescent dye (6-FAM). The microsatellite DNA was amplified in a total reaction volume of 10 μl, with 1 μl of DNA extracted from each sample, 2 μM of each primer and 6.5 μl of PCR Master Mix (Qiagen).
Amplification was performed in a GeneAmp PCR System 2700 thermal cycler (Applied Biosystems) according to the following thermocycling protocol: initial denaturation at 95 °C for 15 min, 40 cycles at 94 °C for 30 s, 60 °C for 90 s for hybridization and 72 °C for 60 s, followed by a final extension step at 72 °C for 10 min.
The PCR products were genotyped at the GIGA-Genotranscriptomics Platform (University of Liège).
Microsatellite analysis
GeneMarker 2.2.0 was used to examine and determine the microsatellite alleles. The microsatellite quality was checked with Microchecker 2.2.3 (Van Oosterhout et al. 2004), to detect potential genotyping errors such as null alleles, allele drop-out or stuttering.
In order to assess the genetic diversity of the samples, the number of alleles (n A), allelic richness and inbreeding coefficient (F is Weir and Cockerham 1984) were measured for each microsatellite locus using FSTAT 2.9.3.2 (Goudet 2002). The observed (H o) and expected (H e) heterozygosities (Nei 1978), deviation from Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD) were estimated using GENEPOP, version 4 (Raymond and Rousset 1995). Bonferroni corrections were applied (Rice 1989; Sokal and Rohlf 1998).
In order to investigate the spatial pattern of genetic variation and determine the genetic structure of French otter populations, several approaches based on individual multilocus genotypes were used. Bayesian clustering analysis was performed using STRUCTURE 2.3.1 (Pritchard and Wen 2004; Falush et al. 2003, 2007). This software uses a Markov Chain Monte Carlo (MCMC) simulation to partition the individuals into K distinct genetic populations (clusters) with maximum posterior probability. Each simulated cluster was characterised by a set of allele frequencies at each locus, and deviations from Hardy-Weinberg equilibrium and marker linkage disequilibrium were minimised. The simulations were performed with K ranging from 1 to 8, and each run was repeated 5 times. The parameters were the admixture model with correlated allelic frequencies. The prioritisation was assigned to a sampling location in order to assist the clustering of datasets of a relatively small number of samples (LOCPRIOR model; Atlantic coast (n = 122), Aquitaine (n = 3), Massif Central (n = 16) and La Brenne (n = 2)). The LOCPRIOR model could be used when the amount of data is limited or when the differentiation between subpopulations is low (Hubisz et al. 2009). Each run consisted of an initial burn-in period of 2.5×105 iterations, followed by 3×106 MCMC iterations. In order to correctly assign the otters to simulated clusters, the assignment probability of their individual genotype (q i ) was used with a threshold value of 0.80. The STRUCTURE HARVERSTER program (Earl and vonHoldt 2012), based on the Evanno’s ΔK (Evanno et al. 2005), was used for selecting the most appropriate number of clusters that best fits the dataset.
This Bayesian clustering method was also compared with a descriptive statistical method. Factorial correspondence analysis (FCA; Benzecri 1973) was performed using GENETIX software (Belkhir et al. 2001). Individual multilocus genotypes were plotted on a 2D chart.
We calculated pairwise F st-values (based on allele frequencies) with FSTAT software for the clusters inferred with STRUCTURE.
BOTTLENECK 1.2.2 (Cornuet and Luikart 1996) was used to detect the presence of a recent genetic bottleneck signature, resulting from the decline in the French population. We used an infinite allele model (IAM), a stepwise mutation model (SMM) and a two-phase mutation model (TPM, with 90 % of SMM, variance 12.00; Hajkova et al. 2006). Significance was tested using the Wilcoxon signed rank test (Luikart 1997).
Results
A total of 145 otters were successfully genotyped with 10 microsatellite loci. The overall sex ratio was 51 % males and 49 % females. The age class structure comprised 100 adults (55 males and 45 females), 26 young (11 males and 15 females), 15 subadults (6 males and 9 females) and 4 otters of unknown age.
Genetic diversity
The estimated genetic diversity of the full set of samples is provided in Table 1. Microchecker did not detect any allele dropout or stuttering. However, three microsatellite markers (Lut435, Lut604 and Lut818) were characterised by a high frequency of null alleles, and the genotype data from those loci were not included in the subsequent genetic analysis. All of the remaining analysed loci were polymorphic. The allelic richness ranged from four to six alleles per locus, and the average allele number was A = 4.7 (Table 1). A private allele was found at locus Lut902 in individual otters from Aquitaine, La Brenne and the Massif Central only.
None of the loci were in linkage disequilibrium. The expected and observed heterozygosities were moderately high (respectively H e = 0.62 ± 0.08 and H o = 0.64 ± 0.13) with no significant deviation from the Hardy-Weinberg equilibrium. However, when calculating each locus independently, a weak but significant heterozygote deficiency was detected at two loci (Lut715 and Lut782), suggesting the existence of a genetic structure within the French population resulting from the Wahlund effect (Wahlund 1928).
Population genetic structure
The ΔK statistics applied to the Bayesian clustering performed with STRUCTURE suggested the presence of two clusters (K = 2; Table 2).
With the LOCPRIOR model, one cluster corresponded to the subpopulation from the Atlantic coast and the second cluster corresponded to the Massif Central and the Brenne subpopulation (Figure 2a). Surprisingly, an outsider was found in the Atlantic group. This corresponded to an otter found dead in the Marais Poitevin area (marshlands of the Vendée region), which could be assigned to the cluster from the Centre of France (q i = 0.83). The two individuals from La Brenne were clearly assigned to the Centre of France cluster (proportion of membership of 92 % for the pre-defined La Brenne group). Concerning the pre-defined group from the Massif Central, the proportion of membership to the Centre of France cluster was 0.89. Finally, the three otters from the Aquitaine area could not be clearly assigned to one or the other of the two simulated clusters, but seemed to be closer to the otters from the Centre of France (proportion of membership of 0.67 to the Centre cluster and 0.33 to the Atlantic coast cluster, although none of the three individuals had q > 0.8).
Bar plots based on results obtained with STRUCTURE for K = 2. a With prior information on the individual’s location (LOCPRIOR model). b Without prior information on the individual’s location. Each individual is represented by a vertical bar with visualisation of the probability of its genotype belonging to each cluster (q i). Upper arrow indicates the genetic outsider in the population from the Atlantic coast. 1 Aquitaine, 2 Brenne, 3 Massif Central, 4 Atlantic coast
However, without the LOCPRIOR model, samples could not be grouped into distinct genetic clusters (Figure 2b).
The results of the FCA based on allelic variations at seven microsatellites are shown in Fig. 3. The first axis accounts for 8.17 % of the total variance and the second accounts for 7.81 %. The FCA individual genotype plot showed that the two clusters inferred with STRUCTURE using the LOCPRIOR model; Centre and Atlantic coasts, are overlapping. The two otters from the Aquitaine area that could not be assigned to one or the other of the two clusters are nested at the centre of the overall group.
The two inferred clusters showed a weak but significant allelic frequency divergence among them (F st = 0.040; 95 % confidence interval = 0.009–0.073, computed after 1000 bootstraps on loci).
The procedure proposed by Cornuet and Luikart (1996) was used to test for a recent decline in population size. The French population showed a significant heterozygote excess with respect to the expected numbers, under the assumption of an IAM (p = 0.004) or a TPM (p = 0.004), but not under the assumption of an SSM (p = 0.148). This result suggests that French otters have suffered a recent decline.
Discussion
The various geographical locations from which samples were collected include all of the main areas occupied by stable and viable French otter populations (with the exception of Brittany). However, as the sample is quite unbalanced between regions (with the biggest part of the sample corresponding to the Atlantic coast region), results should be analysed with caution.
All individuals analysed in this study were roadkill. As dispersers are thought to be more likely to be injured or killed by cars, it has thus been proposed that this kind of individual genotype might lead to a distorted genetic analysis (Dallas et al. 2002). However, seasonal conditions are also known to increase otter road mortality. During autumn and winter, there are more mortalities, perhaps due to higher river levels, or the coincidence of rush hour with otters crepuscular activity. Roads may also be located within areas of good habitat with higher otter population densities. In France, the road network passing through the Poitevin Marshland and other Atlantic coast wetlands increases the risk of otter casualties (Rosoux and Tournebize 1995). Similarly, studies of otter mortality have indicated that deaths resulting from human activities (such as road traffic) are male-biased (Philcox et al. 1999; Heggberget 1991). This is not the case in the present study, in which the sample sex ratio was 51 % males. This number lies within the range observed in other European studies using this kind of sample (between 50 and 61 %; Hauer et al. 2002; Dallas et al. 2003; Hung et al. 2004).
Genetic diversity
Although only 7 loci were analysed, rather than 10, the results are in agreement with those reported elsewhere in the literature.
The mean number of alleles per locus (A = 4.7) is low, but similar to that previously reported for the Cevennes National Park (A = 4; Janssens et al. 2008) and for the Atlantic coast (A = 4.8) otter populations. This result is close to that reported for other European populations (A = 4.9; Mucci et al. 2010).
The observed and expected heterozygosities, respectively H o = 0.64 and H e = 0.62, are considered to be moderate and in line with those observed in other European populations. The value of H e is similar to that found in otter populations from nearby European countries: Germany (H e = 0.65; Honnen et al. 2010), Spain (H e = 0.64) and Portugal (H e = 0.60; Mucci et al. 2010), but slightly higher than that reported in other studies of French otters: H e = 0.52 in the Massif Central population (Janssens et al. 2008) and H e = 0.59 in the Atlantic coast population (Mucci et al. 2010). However, the low value reported by Janssens et al. (2008) was a specific outcome resulting from a recent colonisation event, caused by otters forming a newly established population in the Cevennes, with a typically lower genetic diversity. Although the overall value of F is indicates that there is no evidence of significant inbreeding (Table 1), the heterozygote deficiency observed at two loci are suggestive of the existence of genetic structure in French otter populations.
Population structure
The Bayesian clustering, without information on individual’s location, and FCA methods used to determine the genetic structure of the French otters provided convergent results, suggesting that the structure is very weak. At the lowest population level, gene flow between populations from the Atlantic coast and Massif Central could have been reduced, thereby increasing their regional genetic differences. Nevertheless, the genetic distance between these subpopulations is very low compared to other studied populations (Table 3), with the presence of genetic admixture indicated by the FCA (Fig. 3). The overlapping between the two subpopulations could explain why the Bayesian clustering did not find a structure without prior information on an individual’s location. This is in line with the idea that there was possibly a certain degree of connectivity between them before the recovery (Robitaille and Laurence 2002). The last available historical data from Limousin, Poitou-Charentes and Auvergne indicate that the disconnection between the two subpopulation occurred late compared to the rest of the population, probably explaining the low genetic distance (Lemarchand, pers. comm).
The Bayesian assignment results including prior information on the individuals’ geographic origin are partly in agreement with the pan-European study of Mucci et al. (2010), who found at least three genetically distinct subpopulations exist in France. Mucci et al. (2010) did not take samples from the Centre of France, and their three clusters were located along the Atlantic coast, in Brittany to the north-west and in the Aquitaine region to the south-west. In the present study, no samples were taken from Brittany (north-west) where one cluster is known to exist, and the cluster located in the Atlantic coast area corresponds to the second inferred cluster described by Mucci et al. (2010). The third cluster described by Mucci et al. (2010) corresponds geographically to the Aquitaine. The individuals from the latter region that were analysed in the present study cannot be clearly assigned to the Atlantic coast population or to the centre population.
Population decline
The bottleneck tests using the IAM and TPM models yield evidence of a recent decline of otter populations in France. The latter model is known to provide the best fit with microsatellite data and their mutation process (Williamson-Natesan 2005). Although it is known that otter populations have declined in recent decades, in most areas of Europe, few studies have documented genetic evidence of this event. It has been suggested that European otters suffered from a historical decline, approximately 4000 years ago, due to post-glacial founder events and the re-colonisation process of Northern Europe (Randi et al. 2003). Pertoldi et al. (2001) suggested that the historical decline of Danish otters that took place in Denmark 2000–3000 years ago could have resulted from human disturbance. Nevertheless, the genetic signature of a recent decline has been found in the Czech Republic and Slovak populations (Hajkova et al. 2006), and in the Schleswig-Holstein population, a recently recolonised area in north-west Germany (Honnen et al. 2010).
Conclusion
As in all other European countries, French otters experienced a severe decline up until the 1980s but started to recover following their legal protection in Europe. The results of this study suggest that the French otters are not strongly substructuring into geographically distinct subpopulations. The two inferred clusters, corresponding to the last two populations remaining in France, are partially admixed and appear to be genetically close (F st = 0.040). The natural re-colonisation process has been ongoing for more than 30 years, and otters from the Atlantic coast and the Massif Central regions do not appear to have remained completely isolated from each other. Although genetic intermixing is certainly underway, as has been suggested in previous studies (Reuther and Krekemeyer 2004; Rosoux and Green 2004), this expansion may still be insufficient to genetically homogenise all populations. As otters are now observed in many locations in France, more complete genetic homogenization is likely to be observed in the future.
Although the Eurasian otter has expanded its range to half of the country, it remains an endangered species and is thus a concern for conservation. Over the longer term, human activities (e.g. water pollution, road networks, intensive land-use (Robitaille and Laurence 2002; Reuther and Krekemeyer 2004; Lemarchand et al. 2010; Lemarchand et al. 2011)) could slow this expansion, or prevent otters from establishing and maintaining stable populations. In particular, as the Eurasian otter has weak demographic dynamics (Kruuk 2006), further expansion of this species must be carefully monitored.
References
Ansorge H, Schipke R, Zinke O (1997) Population structure of the otter, Lutra lutra; parameters and model for a Central European region. Mamm Biol 62:142–151
Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F (2001) GENETIX 4.02, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier II, Montpellier, France; http://www.univ-montp2.fr/~genetix/genetix
Benzecri JP (1973) L’analyse des données : L’analyse des correspondances. Dunod, Paris
Bouchardy C, Boulade Y (1999) Rapport de visite des passages à loutre dans les ouvrages hydrauliques sous l’autoroute A89 dans la section 7 Ussel Ouest-Le Sancy. Catiche Productions / Seyssinet-Pariset, Libris, Nohanent
Bouchardy C, Rosoux R, Boulade Y, Gouilloux N (2001) La loutre d’Europe, histoire d’une sauvegarde. Catiche Productions - Libris, Nohanent
Cohen TM, Narkiss T, Dolev A, Ben-Ari Y, Kronfeld-Schor N, Guter A, Saltz D, Bar-Gal GK (2013) Genetic diversity of the Eurasian otter (Lutra lutra) population in Israel. J Heredity 104:192–201
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Dallas JF, Piertney SB (1998) Microsatellite primers for the Eurasian otter. Mol Ecol 7:1248–1251
Dallas JF, Bacon PJ, Carss DN, Conroy JWH, Green R, Jefferies DJ, Kruuk H, Marshall F, Piertney SB, Racey PA (1999) Genetic diversity in the Eurasian otter, Lutra lutra, in Scotland. Evidence from microsatellite polymorphism. Biol J Linn Soc 68:73–86
Dallas J, Marshall F, Piertney S, Bacon P, Racey P (2002) Spatially restricted gene flow and reduced microsatellite polymorphism in the Eurasian otter Lutra lutra in Britain. Conserv Genet 3:15–29
Dallas J, Coxon K, Sykes T, Chanin P, Marshall F, Carss D, Bacon P, Piertney S, Racey P (2003) Similar estimates of population genetic composition and sex ratio derived from carcasses and faeces of Eurasian otter Lutra lutra. Mol Ecol 12:275–282
Earl DA, vonHoldt BM (2012) Structure harvester: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genet Resour 4:359–361
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Gascuel D (1985) Contribution à l’étude écologique des estuaires du littoral atlantique français: la faune accompagnatrice de la civelle. Dissertation, Université de Rennes-1
Goudet J (2002) FSTAT: a program to estimate and test gene diversities and fixation indices.Version 2.9.3.2. Available at http://www.unil.ch/izea/softwares/fstat.html
Hajkova P, Pertoldi C, Zemanova B, Roche K, Hajek B, Bryja J, Zima J (2006) Genetic structure and evidence for recent population decline in Eurasian otter populations in Czech and Slovac Republics: implications for conservation. J Zool 272:1–9
Hauer S, Ansorge H, Zinke O (2002) Mortality patterns of otters (Lutra lutra) from eastern Germany. J Zool 256:361–368
Heggberget TM (1991) Sex and Age distribution in European otters (Lutra lutra) killed by human activity. In: Reuther C. & Röchert R. (eds), Proc V Int. Otter Colloqu., Habitat 6: 123–125
Hobbs GI, Chadwick EA, Bruford MW, Slater FM (2011) Bayesian clustering techniques and progressive partitioning to identify population structuring within a recovering otter population in the UK. J Appl Ecol 48:1206–1217
Honnen AC, Petersen B, Kabler L, Elmeros M, Roos A, Sommer R, Zachos F (2010) Genetic structure of Eurasian otter (Lutra lutra, Carnivora : Mustelidae) populations from the western Baltic sea region and its implications for the recolonization of north-western Germany. J Zool Syst Evol Res 49:169–175
Honnen AC, Roos A, Stjernberg T, Zachos FE (2015) Genetic analysis of Eurasian otters (Lutra lutra) reveals high admixture in Finland and pronounced differentiation in Sweden. Mamm Biol 80:47–53
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol 9:1322–1332
Hung CM, Li SH, Lee LL (2004) Faecal DNA typing to determine the abundance and spatial organisation of otters (Lutra lutra) along two stream systems in Kinmen. Anim Conserv 7:301–311
Janssens X, Fontaine M, Michaux J, Libois R, De Kermabon J, Defourny P, Baret P (2008) Genetic pattern of the recent recovery of European otters in southern France. Ecography 31:176–186
Koelewijn H, Perez-Haro M, Jansman H, Boerwinkel M, Bovenschen J, Lammertsma D, Niewold F, Kuiters A (2010) The reintroduction of the Eurasian otter (Lutra lutra) into the Netherlands: hidden life revealed by noninvasive genetic monitoring. Conserv Genet 11:601–614
Kruuk H (2006) Otter: ecology, behaviour and conservation. Oxford University Press, New-York
Kuhn R (2009) Plan National d’Actions pour la Loutre d’Europe (Lutra lutra), 2010–2015. Societe Francaise pour l’Etude et la Protection des Mammiferes/Ministere de l’Ecologie, de l’Energie, du Developpement Durable et de la Mer, Paris
Lemarchand C, Rosoux R, Berny P (2010) Organochlorine pesticides, PCBs, heavy metals and anticoagulant rodenticides in tissues of Eurasian otters (Lutra lutra) from upper Loire river catchment (France). Chemosphere 80:1120–1124
Lemarchand C, Rosoux R, Berny P (2011) Ecotoxicology of the Eurasian otter (Lutra lutra) along Loire River (France) and predictable trends due to global change. Proceedings of XIth International Otter Colloquium, IUCN Otter Spec Group Bull 28B: 5–14
Libois R (1997) Régime et tactique alimentaires de la loutre (Lutra lutra) dans le Massif Central. Vie Milieu 47:33–45
Libois R, Rosoux R (1989) Ecologie de la loutre (Lutra lutra) dans le Marais Poitevin. I. Etude de la consommation d’Anguilles. Vie Milieu 39:191–197
Libois R, Rosoux R (1991) Ecologie de la loutre (Lutra lutra) dans le Marais Poitevin. II. Aperçu général du régime alimentaire Mammalia 55:35–47
Luikart G (1997) Usefulness of molecular markers for detecting population bottlenecks and monitoring genetic change. Dissertation, University of Montana, Missoula. 1211–1216
Macdonald DM, Mason C (1992) Status and conservation needs of the otter (Lutra lutra) in the Western Palearctic. Rapport de conseil de l’Europe, Strasbourg, pp 1–65
Mason CF (1995) Habitat quality, water quality and otter distribution. Hystrix 7(1–2):195–207
Morales CF (2002) Phylogéographie de la loutre d’Europe (Lutra lutra) dans la partie occidentale de son aire de répartition. DES Sciences naturelles (zoologie) Université de Liège
Mucci N, Arrendal J, Ansorge H, Bailey M, Bodner M, Delides M, Ferrando A, Fournier P, Fournier C, Godoy J, Hajkova P, Hauer S, Heggberget T, Heidecke D, Kirjavainen H, Krueger HH, Kvaloy K, Lafontaine L, Lanszki J, Lemarchand C, Liukko UM, Loeschcke V, Ludwig G, Madsen A, Mercier L, Ozolins J, Paunovic M, Pertoldi C, Piriz A, Prigioni C, Santos-Reis M, Luis ST, Stjernberg T, Schmid H, Suchentrunk F, Teubner J, Tornberg R, Zinke O, Randi E (2010) Genetic diversity and landscape genetic structure of otter (Lutra lutra) populations in Europe. Conserv Genet 11:583–599
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Pertoldi C, Hansen MM, Loeschcke V, Madsen AB, Jacobsen L, Baagoe H (2001) Genetic consequences of population decline in the European otter (Lutra lutra): an assessment of microsatellite DNA variation in Danish otters from 1883 to 1993. Proc R Soc B Biol Sci 268:1775–1781
Philcox CK, Agrogan AL, Macdonald DW (1999) Patterns of otter Lutra lutra road mortality in Britain. J Appl Ecol 36:748–761
Pritchard JK, Wen W (2004) Documentation for STRUCTURE software version 2. Dept of Human Genetics, Univ. of Chicago, USA
Randi E, Davoli F, Pierpaoli M, Pertoldi C, Madsen A, Loeschcke V (2003) Genetic structure in otter (Lutra lutra) populations in Europe : implications for conservation. Anim Conserv 6:93–100
Raymond M, Rousset F (1995) An exact test for population differentiation. Evolution 49:1280–1283
Reuther C, Hilton-Taylor C (2004) Lutra lutra. In: IUCN 2007. 2007 IUCN Red List of Threatened Species. Website : www.iucnredlist.org
Reuther C, Krekemeyer A (2004) On the way towards an Otter Habitat Network Europe (OHNE). Method and results of an assessment on the European and the German level. Habitat 15:1–308
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Robitaille JF, Laurence S (2002) Otter, Lutra lutra, occurrence in Europe and in France in relation to landscape characteristics. Anim Conserv 5:337–344
Rosoux R, Green J (2004) La Loutre. Belin Eveil Nature, Paris
Rosoux R, Libois R (1994) Statut, écologie et devenir des populations de loutre d’Europe (Lutra lutra) en France. (In: Actes Séminaire international: La loutre au Luxembourg et dans les pays limitrophes. Groupe loutre luxembourgeois, eds) Luxembourg : 6–12
Rosoux R, Tournebize T (1995) Analyse des causes de mortalité chez la Loutre d’Europe dans le Centre Ouest atlantique (France). Cah Ethol 15(2-3-4):337–350
Rosoux R, Libois R, de Bellefroid MN (1999) Rapport d’expertise : Programme de conservation de la loutre d’Europe dans la Réserve de Biosphère de Trebon (République tchèque). Conseil de l’Europe. Convention relative a la conservation de la vie sauvage et du milieu naturel de l’Europe T-PVS. Strasbourg
Simpson V (2001) Post mortem protocol for otters. In: Proceedings of the First Otter Toxicology Conference. Conroy JWH, Yoxon P. and Gutleb AC (eds.). Journal of the International Otter Survival Fund. Vol 1: 159–166
Sokal RR, Rohlf FJ (1998) Biometry. Fourth edition. W.H. Freeman, New-York, USA
Stanton DWG, Hobbs GI, McCafferty DJ, Chadwick EA, Philbey AW, Saccheri IJ, Slater FM, Bruford MW (2014) Contrasting genetic structure of the Eurasian otter (Lutra lutra) across a latitudinal divide. J Mammal 95:814–823
Tison JL, Blennow V, Palkopoulou E, Gustafsson P, Roos A, Dalen L (2015) Population structure and recent temporal changes in genetic variation in Eurasian otters from Sweden. Conserv Genet 16:371–384
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley PF (2004) Microchecker user guide. Dept of Biological Sciences, University of Hull
Wahlund S (1928) Composition of populations from the perspective of the theory of heredity. Hereditas 11:65–105
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Williamson-Natesan EG (2005) Comparison of methods for detecting bottlenecks from microsatellite loci. Conserv Genet 6:551–562
Acknowledgments
We would like to acknowledge the Natural History Museum of Orleans for the collection, the centralisation and the storage of otter tissue samples, the departmental services of the ONCFS (Loire-Atlantique, Vendée, Charente-Maritime, Deux-Sèvres, Gironde, Aveyron, Lot, Lozère, Cantal, Allier, Puy-de-Dôme, Corrèze, Creuse, Vienne, Haute-Vienne, Indre) to have facilitated this collection in many regions of France, the LPO (Vendée, Charente-Maritime, Charente, Pays de la Loire), the Natural Hstory Museum of Nantes et M. Didier Monfort, Catiche Productions, GREGE, the Parc Interregional of Marais Poitevin and PRO LUTRA association for their active collaboration in the survey, collection and storage of dead otters. Willy Dabin CNRS/ Pelagis for the odontochronological analyses. For their advice in laboratory and statistical analyses, we would like to thank especially Laurent Gohy, Virginie Hutsemeykers and Clotilde Lambinet, Marie-France Larigauderie, Marie-des-Neiges de Bellefroid and Amina Brahimi. For the map of France, we wish to thank Arnaud Beckers. We also thank the two anonymous reviewers for their careful revision and comments. This study has been partly supported by the Natural History Museum of Orleans, the DREAL Poitou-Charentes, the regional direction Centre/Ile-de-France of the ONCFS and Catiche Productions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Karol Zub
Rights and permissions
About this article
Cite this article
Geboes, AL., Rosoux, R., Lemarchand, C. et al. Genetic diversity and population structure of the Eurasian otter (Lutra lutra) in France. Mamm Res 61, 121–129 (2016). https://doi.org/10.1007/s13364-015-0258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-015-0258-5