Abstract
We provide the initial performance evaluation of a 21 Tesla Fourier transform ion cyclotron resonance mass spectrometer operating at the Environmental Molecular Sciences Laboratory at the Pacific Northwest National Laboratory. The spectrometer constructed for the 21T system employs a commercial dual linear ion trap mass spectrometer coupled to a FTICR spectrometer designed and built in-house. Performance gains from moving to higher magnetic field strength are exemplified by the measurement of peptide isotopic fine structure, complex natural organic matter mixtures, and large proteins. Accurate determination of isotopic fine structure was demonstrated for doubly charged Substance P with minimal spectral averaging, and 8158 molecular formulas assigned to Suwannee River Fulvic Acid standard with root-mean-square (RMS) error of 10 ppb. We also demonstrated superior performance for intact proteins; namely, broadband isotopic resolution of the entire charge state distribution of apo-transferrin (78 kDa) and facile isotopic resolution of monoclonal antibody under a variety of acquisition parameters (e.g., 6 s time-domains with absorption mode processing yielded resolution of approximately 1 M at m/z = 2700).

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fourier-transform ion cyclotron resonance mass spectrometry (FTICR MS) is the highest performance MS technique, and thus uniquely valued as a research tool for analytical applications requiring ultra-high resolution and mass measurement accuracy [1, 2]. Increasing magnetic field strength enables multiplicative improvements in FTICR MS performance, including improved resolution (for the same acquisition period), acquisition speed, mass measurement accuracy, dynamic range, and resistance to peak coalescence that will have transformative impact in a variety of scientific applications [3, 4]. Unequivocal ability to discern, identify, and quantify diverse molecular species in complex systems will enable researchers to tackle current analytical grand challenges in omics and systems biology, including characterization of intact proteins (i.e., top-down proteomics) and metabolites in complex environmental mixtures (i.e., soil organic matter).
For instance, identification and quantitation of intact molecular structures derived from higher plants and soil microbiome within their native inorganic soil matrix are essential for advancing molecular-scale mechanistic understanding of the role of physical, geochemical, and biological processes below ground, which in turn is central to development of better predictive models of terrestrial carbon cycle [5] in the face of global and regional climate change [6]. Similarly, advanced MS performance will improve measurements of isotopic patterns in stable-isotope profiling experiments, including protein turnover and metabolic flux measurements, enabling scientists to address the key knowledge gaps in functional understanding of how complex microbiomes influence and are influenced by their environment.
Another area of research that will be significantly impacted by 21T FTICR MS capability is characterization and quantitation of intact proteins, so-called top-down proteomics. Identification of protein variation arising from sequence differences, proteolysis, and post-translational modifications (PTMs) is essential for understanding protein function and identification of novel biomarkers. Critical information, including stoichiometry of PTMs on a single proteoform [7], or presence of a combination of multiple PTMs on a single proteoform, can only be determined by the top-down approach. Hence, this approach is rapidly becoming an indispensable avenue for (functional) proteomic studies. Recent advances in MS instrumentation, separation, and bioinformatics significantly increased the throughput of top-down proteomics, allowing the identification of hundreds of intact proteoforms [8, 9]. Yet many challenges persist, including effective MS characterization of large proteins on a chromatographic time scale. Because all key measures of FTICR MS performance improve with increased magnetic field strength, a 21T FTICR spectrometer will arguably provide that next level of performance needed to bring top-down proteomics to the mainstream.
Herein, we present the initial performance evaluation of the 21T FTICR MS at the Environmental and Molecular Sciences Laboratory, a US DOE supported national user facility located at the Pacific Northwest National Laboratory. 21T magnetic field strength enables the unprecedented ability to routinely analyze large proteins (>100 kDa), measure isotopic fine structure with high fidelity, and extract more information from complex natural organic matter mixtures. The user facility setting allows access to this highly specialized capability to a range of qualified scientific users.
Experimental
Materials and Sample Preparation
Substance P (AnaSpec Inc., Freemont, CA, USA) and human apo-transferrin (Sigma-Aldrich, St. Louis, MO, USA) were used without further purification. Monoclonal antibody (mouse IgG1, intact mAb mass check standard) was purchased from Waters Corporation (Milford, MA, USA). Monoclonal antibody (mAb) was reconstituted in water and divided into 100 μg aliquots at 1 μg/μL. Prior to use, the mAb aliquots were rinsed five times with water using Amicon 0.5 mL centrifugal 30 kDa molecular weight cutoff filters (EMD Millipore, Darmstadt, Germany). Suwannee River Fulvic Acid (SRFA) standard was acquired from the International Humic Substances Society and stored as a 1 mg/mL stock solution. Twenty ppm working solutions of SRFA were prepared by dilution with LCMS grade methanol and analyzed with negative ion mode electrospray ionization (ESI). All peptide and protein samples were prepared at 1 μM in 50:50 acetonitrile:water with 0.1% formic acid and analyzed by direct infusion at 0.5 μL/min for positive ion mode electrospray ionization ESI. Pierce LTQ Velos ESI positive and negative ion calibration solutions and all other chemicals were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
21T Magnet
The EMSL 21 Tesla magnet was specified and designed in concert with Agilent Technologies in Yarnton, UK (former Magnex and Varian Magnet Technology Centre manufacturing site). The magnet is a 22-ton, 2.53 m long × 2.44 m wide × 3.36 m high system, with a room temperature bore of 112 mm. The magnet cryostat is a directly pumped, single reservoir system holding approximately 4050 L of liquid helium cryogen that is held at ~55 mbar and 2.2 K, using two Sumitomo dual-stage GM (Gifford-McMahon) type cryo-coolers (Model RDK 408D2) to maintain an inner radiation shield temperature of 8–9 K and an outer radiation shield temperature of 27–28 K. The cryogenics system is a simple, low-loss design that results in a cryogen consumption of ~1 L/d, necessitating a <10% refill of the system only once per year.
The magnet itself features a multi-coil, actively shielded design using both NbTi and Nb3Sn superconducting wire. Compensation and shim coil assemblies are inherent to the magnet/cryostat design; no room-temperature shims are utilized. Fringe magnetic fields of ≤5 Gauss are located at 3.5 m radially and 3.2 m axially. The magnetic field drift rate was measured at 2 ppb/h over a 7 day period 4–5 weeks after installation. The magnetic field homogeneity was measured at 9.2 ppm over a cylinder region of 60 mm diameter by 100 mm length at the same time. Total stored energy in this magnet is approximately 32.7 MJoules.
FTICR Mass Spectrometer
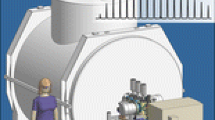
The mass spectrometer designed and built for the 21 T magnet builds upon the foundation of previous generations of hybrid FTMS instrumentation [10–12]. A Velos Pro dual linear quadrupole ion trap mass spectrometer (ThermoFisher Scientific, San Jose, CA, USA) was chosen for the front end of the hybrid platform (Figure 1). The Velos Pro provides high sensitivity, efficient ion isolation, multistage MS/MS capabilities through ion trap collision induced dissociation (CID), and modulation of the ion population sent to the ICR cell via automatic gain control (AGC). An additional 75 mm long linear quadrupole ion trap (6.35 mm rod diameter, 2.76 mm inscribed radius, 2.5 mm entrance and exit lens aperture) acts as an intermediate storage device during transfer of ions from the Velos Pro to the ICR cell and as a collision cell for beam type CID. Additionally, the external trap facilitates conversion between the ion charge capacity of the Velos Pro and ICR cell through multiple fills of the external trap per FTICR acquisition. The external trap is typically operated with 1–3 mTorr of nitrogen bath gas as measured by a flange mounted Pirani gauge connected to the ion trap shroud by 0.25 in. tubing. The flow of nitrogen into the external ion trap through 0.0625 in. tubing is regulated by an electronic flow controller (MKS Instruments, Inc., Andover, MA, USA). Two short rf-only quadrupole ion guides (6.35 mm rod diameter with 2.76 mm inscribed radius) transmit ions from the Velos Pro to the external trap and from the external trap to the conductance limit (2.5 mm aperture) prior to the first stage of ultra-high vacuum. The three stages of ultra-high vacuum are traversed by two quadrupole ion guides (6.35 mm rod diameter with 2.76 mm inscribed radius) and a 2.5 mm conductance limit separating the first two stages from the analyzer stage. The external ion trap and the first three quadrupole ion guides are driven at ~3 MHz and 300–800 Vp-p. The final quadrupole ion guide that transmits ions through the magnetic field gradient is driven at ~4.4 MHz and up to 500 Vp-p. This corresponds to a low mass cutoff of m/z ~150 for ions transmitted to the ICR cell due to resonant excitation of ion cyclotron motion within the quadrupole ion guide by the rf drive [13]. The external linear quadrupole ion trap and all four rf-only quadrupole ion guides are driven by AMPS boxes (Pacific Northwest National Laboratory, Richland, WA, USA) and MIPS RF drivers (GAA Custom Engineering, Benton City, WA, USA) [14].
The vacuum chamber that houses the external linear quadrupole ion trap is an extension of the Velos Pro vacuum chamber, and it is pumped by the Velos Pro vacuum system. A 14-way stainless steel block comprises the three stages of ultra-high vacuum, and each stage is pumped by a magnetically shielded turbo molecular pump (EXT556HF; Edwards Vacuum, Tewksbury, MA, USA). Each turbo pump is spaced down from the UHV chamber by some distance to place the turbo pump in areas of fringe magnetic field less than 500 gauss. The titanium flight tube (Sharon Vacuum, Brockton, MA, USA) is 1778 mm flange to flange and allows spacing of the spectrometer back from the face of magnet to accommodate magnetic fringe fields. The three stages of differential pumping reduce pressure from ~2 × 10−5 Torr at the external ion trap to ~1 × 10−10 Torr or less in the analyzer pumping stage with a 24 h bakeout at 120 °C.
Our laboratory has recently developed and implemented a harmonized ICR cell that utilizes external shimming to closely approximate an ideal quadrupolar trapping electric field (i.e., the window cell) [15]. The window cell consists of two coaxially nested cylinders. The outer cylinder is formed by 19 stacked ring electrodes (each 4.8 mm wide, 0.9 mm spacing) used for external shimming. The inner cylinder is split into four sectors used for dipolar excitation and detection and has a 46 mm inner diameter and 49 mm outer diameter. Each sector has five rectangular openings (43.7 mm long, 2.8 mm wide, 3.4 mm separation), or windows, which allow penetration of external shimming electric field into the trapping volume of the ICR cell. The gap between the four excite and detect electrodes is equal to the spacing between adjacent windows in the electrodes (i.e., 3.4 mm). The trapping volume is enclosed axially by two 42 mm diameter end caps with 6 mm inner diameter apertures. The inner surface of the end caps are spherical (76.3 mm radius) and approximate the ideal hyperbolic boundary. The spacing between the end caps is 69 mm, or 1.5 times the trapping volume inner diameter.
Instrument Control, Data Acquisition, and Data Processing
Modifications to the Velos Pro tune software enabled integration of DC power supplies and the FT acquisition computer from an LTQ-FT Ultra (ThermoFisher Scientific, San Jose, CA, USA). Time-dependent control of the DC offsets for the external ion trap, lenses, all quadrupole ion guides, and the ICR cell was integrated into the Velos Pro tune software and enabled automated tuning of ion transfer to the ICR cell. All experiments were performed with 1 V applied to the endcaps, 289 V on the front and back shim electrodes, and −38 V on the center shim electrode. Stored waveform inverse Fourier transform (SWIFT) excite waveforms were generated by the FT acquisition computer and amplified by a linear power amplifier (T&C Power Conversion, Inc., Rochester, NY, USA). For magnitude mode spectra, all time-domain data processing was performed on the FT acquisition computer, and spectra were stored as .raw files. For absorption mode spectra, time-domain data were saved as .dat files and processed off-line using Autophaser [16, 17]. Absorption mode resolution enhancement of greater than 2× reported here is a result of the use of different apodization routines during calculation of magnitude mode and absorption mode spectra. Generation of theoretical isotopic distributions and protein spectral deconvolution using the Xtract algorithm was performed using Thermo Xcalibur 2.2 (ThermoFisher Scientific, San Jose, CA, USA).
For the analysis of SRFA, peak picking in the range 200 < m/z <1000 was performed using the ICIS algorithm within Thermo Xcalibur, and peaks with S/N > 3 were considered for molecular formula assignment. Molecular formula assignment was performed using an in-house refactored version of Compound Identification Algorithm (CIA) developed by Kujawinski and Behn [18] and modified by Minor et al. [19]. Previously reported molecular formula assignments by Stenson et al. [20] from SRFA samples were used as internal calibration targets with initial matching tolerance of 3 ppm resulting with 125 calibration peaks with mean post-calibration mass measurement accuracy of 0.00 ppm and standard deviation of 0.05. Based on this assessment, a full CIA database search with 0.5 ppm mass tolerance was performed limiting non-oxygen hetero-atom counts to 0–1 for N and P and 0–2 for S. The database was searched starting from m/z = 200 with molecular formula matches propagated using CH2, H2, and O building blocks. In the case of multiple candidates, the formula with the lowest N+S+P count was selected. If this criterion did not bring resolution, formula with smaller absolute mass error was selected. The matching formulas from this search were filtered using 3-sigma rule from calculated mean mass measurement accuracy.
Results and Discussion
The performance metrics of the FTICR MS directly relate to magnetic field strength. Linear improvements in resolution and acquisition rate and quadratic improvements in mass measurement accuracy, dynamic range, and charge capacity are expected when moving to ever increasing magnetic fields. The transformative effects of higher magnetic field strength are most evident during the analysis of complex mixtures, such as natural organic matter. For instance, ultra-high resolution and mass accuracy, charge capacity, and large dynamic range are essential for observation of isotopic fine structure and unambiguous speciation of aerosols or soil organic matter via accurate molecular formula assignment. Additionally, higher magnetic field strength provides significant advances in the sensitivity and throughput for characterization and quantitation of large intact proteins (i.e., >100 kDa) enabling physiologically relevant studies of microbes and higher eukaryotes.
Supplementary Figure S1 shows the observed resolution (in magnitude mode) for time domain acquisitions ranging from 24 ms to 6.144 s using a calibration mixture. Instrument manufacturers commonly specify resolution at m/z = 200, 400, and/or 1000; therefore we have included the observed resolution at m/z 195, 393, 524, and 1022 for easy comparison. Additionally, Figure S1 illustrates the high linearity of resolution as a function of acquisition time achieved with a relatively high AGC target of one million. With internal calibration, mass accuracy of 0.02 ppm or less is typically observed for a wide range of AGC targets from 5E4 to 3E6.
Isotopic Fine Structure for Complex Mixture Analysis
Routine observation of isotopic fine structure is a very demanding feat, especially when moving to higher m/z and multiply charged analytes, requiring large dynamic range, ultra-high resolution and resistance to peak coalescence (i.e. reduced space charge effects). Figure 2 shows the A + 2 fine isotopic structure obtained for doubly charged Substance P (C63H98N18O13S, Mr = 1,346.72815 Da). The spectrum was generated from the average of four 12 s transient acquisitions and yielded a magnitude mode resolution of 3.2 million (and 7.9 million in absorption mode). The theoretical isotopic distribution simulated for a resolution of 3 million is overlaid for comparison. Quite remarkable agreement (with minimal averaging) in both the isotopes observed and the relative abundance of the isotopes is achieved between the experimental magnitude mode spectrum and the theoretical isotopic fine structure. All isotopes were observed at S/N > 3, and the 13C2 peak was observed at S/N = 85. When normalized to the 13C2 abundance, the relative abundances of all isotopic fine structure peaks were within 3% relative abundance of the theoretical distribution. The ability to observe isotopic fine structure and accurately measure isotope abundances is critical for the unambiguous assignment of molecular formulas based on intact mass alone, a standard practice in analysis of complex mixtures, and for precise stable isotope based measurements. This information is critically needed to e.g., address the key knowledge gaps in functional understanding of how complex microbiomes influence and are influenced by their environment.
Figure 3 shows a negative ion mode ESI mass spectrum of SRFA standard. The spectrum was obtained from one thousand 3 s transient averages with an AGC target of two million (corresponding to the ion injection time of approximately 2 ms per acquisition). A single fill of the external ion trap and a single time of flight setting was used during transfer of ions from the Velos Pro to the ICR cell (i.e., stitching of spectra acquired with multiple time of flight settings was not used to increase the observed mass range). The spectrum contains 11,893 peaks at S/N > 3. The inset spectrum in Figure 3 illustrates the high resolution (1.2 million in magnitude mode at m/z = 367) obtained with a relatively short time-domain acquisition and the ability to resolve peaks separated by as little as 0.00042 Da. After filtering molecular formula assignments, 5712 formulas were obtained with a mean mass measurement accuracy of −0.01 ppm and standard deviation of 0.09 ppm. Resolved by elemental composition, there was 4577 CHO, 882 CHON, 270 CHOS, and 3 CHOP formula, respectively; with 13C peaks observed for 2405, 19, 2, and 0 formula of the CHO, CHON, CHOS, and CHOP, respectively. In total, 8158 of the 11,893 peaks were assigned molecular formula. While SRFA is a relatively simple mixture allowing us to clearly demonstrate the advantage provided by the higher magnetic field strength, we hypothesize the gains will be even more significant when analyzing environmental mixtures of ever increasing complexity (e.g., soil organic matter, organic aerosols, petroleum, or biofuels).
Intact Protein Analysis
The utility of top-down proteomics is rapidly expanding because of recent wide availability of commercial high resolution and mass measurement accuracy instrumentation, such as time of flight and Orbitrap mass analyzers. However, routine analysis is generally limited to intact proteins with molecular mass less than approximately 30 kDa. A number of factors contribute to this limitation, including sample preparation and inefficient on-line separations, but poor sensitivity for high resolution mass analysis is likely one of the current major limitations. Moving to higher magnetic field strength in FTICR enables greater charge capacity and reduces the acquisition time (i.e., ensures greater resolution per unit time) needed to accurately measure the broad isotopic distributions of large proteins. To the best of our knowledge, there has been only one report of isotopic resolution and accurate mass determination for a protein with a molecular mass greater than 100 kDa using instrumentation other than FTICR MS [21]. This can be attributed to the limited resolution of time of flight mass analyzers (generally <100,000) and the high energy injection of ions into Orbitrap mass analyzers [22]. 21T magnetic field strength and the supporting instrumentation described in this report have enabled routine analysis of large proteins, including facile isotopic resolution of intact monoclonal antibodies (Mr ~150 kDa).
High resolution mass analysis of large proteins is typically performed using an isolated charge state of the protein in order to minimize Coulombic interactions between ions with closely spaced frequencies. However, with sufficient charge capacity and proper control of the number of charges within the ICR cell, broadband detection of the entire charge state distribution for large proteins becomes possible. Figure 4 shows a magnitude mode mass spectrum of the entire charge state distribution of apo-transferrin (Mr ~78 kDa) acquired using 3 s transient acquisitions and 64 averages with an AGC target of 1E6. Baseline isotopic resolution was achieved as illustrated for the 35+ charge state (Figure 4 inset). Magnitude mode and absorption mode processing yielded resolution of 225,000 and 560,000 at m/z = 2274, respectively. The most abundant charge state was observed at S/N = 200 in magnitude mode. These results indicate the potential to efficiently analyze proteins much larger than 30 kDa (i.e., current practical limit) with time domain acquisitions and averaging that is compatible with online separations.
To demonstrate the capabilities of the 21T FTICR MS system for the analysis of proteins with molecular mass greater than 100 kDa, we attempted to measure a monoclonal antibody (mAb) standard. Owing to the large size of mAbs and the presence of multiple glycoforms, we elected to isolate all of the glycoforms for a particular charge to minimize space charge effects and achieve isotopic resolution with relatively short time-domain acquisitions. The lower spectrum in Figure 5 is a magnitude mode spectrum generated from two hundred 6 s transient averages of the 55+ charge state mAb glycoform distribution. The isotopic distribution of the most abundant glycoform (G0F/G1F) is shown in the upper left corner of Figure 5. High quality isotopic distributions were obtained and S/N of 1000 was achieved for the G0F/G1F glycoform. The neutral monoisotopic mass spectrum is shown in the upper right corner of Figure 5. The mass errors for the calculated neutral monoisotopic masses for all the glycoforms, except G1F/G2F, reflect a common 1 Da mass shift attributable to incorrect fitting of a theoretical averagine distribution to the observed isotopic distribution by the Xtract algorithm. When the averagine distribution was properly assigned to the observed isotopic distribution, excellent mass accuracy was achieved as reflected by the 0.47 ppm error observed for the G1F/G2F glycoform. Manual assignment of the center isotope of the isotopic distributions of the glycoforms and comparison to theoretical masses yielded mass measurement errors between 0.74 ppm and 1.15 ppm for all glycoforms.
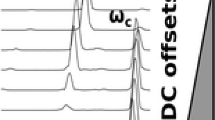
Isotopic resolution was achieved for the mAb in several acquisition modes. Three s transient acquisitions yielded a partially resolved spectrum in magnitude mode (R = 130,000 at m/z = 2699; Figure 6a) and baseline isotopic resolution in absorption mode (R = 490,000 at m/z = 2699; Figure 6c). Extending the transient acquisition to 6 s resulted in baseline isotopic resolution in magnitude mode (R = 350,000 at m/z = 2699; Figure 6b) and absorption mode (R = 980,000 at m/z = 2699; Figure 6d). For compatibility with liquid chromatography timescale, 3 s transient acquisitions with absorption mode post-processing offer the best compromise between resolution and spectral acquisition rate. Due to the characteristic broad elution profiles of large protein with reversed phase liquid chromatography, the most common approach to top-down proteomics, 3 s acquisitions should enable sufficient averaging to obtain high S/N and baseline isotopic resolution on a chromatographic timescale. Future implementation of ICR cell geometries that enable detection at multiples of the cyclotron frequency (i.e., 4× resolution per unit time) [23] holds the potential to make characterization of large proteins (>100 kDa) on chromatographic timescale routine operation.
Zoomed in mass spectra of the 55+ charge state monoclonal antibody G0F/G1F glycoform acquired using 3 s transients with magnitude mode (a) or absorption mode (c) processing, or 6 s transients with magnitude mode (b) or absorption mode (d) processing. Spectra were acquired using 200 transient averages and an AGC target of 1E6 for the isolated 55+ charge glycoform distribution
The magnitude mode resolution values reported here are for apodized time domain signals and yield approximately half the resolution as the corresponding non-apodized time domain signals. Non-apodized time domain signals for apo-transferrin and mAb were processed to produce magnitude mode resolution values for comparison to the theoretical low pressure maximum resolution given by [1]
wherein m/z is u per elementary charge, B0 is magnetic field strength in Tesla, and Tacqn is acquisition time in seconds. The non-apodized magnitude mode spectrum yielded resolution of 346,700 (3.072 s acquisition) for the 35+ charge state of apo-transferrin (m/z = 2274) and is 98% of the theoretical resolution. Supplementary Figure S2A shows the time domain signal for the full mass spectrum of apo-transferrin. Extracted time domain signals for frequency ranges corresponding to the 32+ and 39+ charges states are shown in Supplementary Figure S2B and S2C, respectively. The higher frequency and likely more extended gas-phase structure of the 39+ charge state yields a greater decay rate compared with the 32+ charge state as a result of more scattering collisions with residual gas in the ICR cell. For the 55+ charge state of mAb, the non-apodized magnitude mode observed and theoretical resolution are 298,600 and 304,500, respectively, for 3.072 s acquisition. However, the observed resolution drops to 85% of the theoretical resolution of 609,000 for 6.144 s acquisitions. The same reasoning explains the non-linearity of the resolution as a function of acquisition time for the mAb. The large collisional cross-section of the mAb yields more frequent collisions with residual gas resulting in lower resolution than predicted at longer acquisition times. This is evident in the 6.144 s time domain signal for isolated 55+ mAb (Supplementary Figure S3) showing near complete signal decay by the end of the acquisition. In contrast, the relatively small and singly charged calibration compounds exhibit near perfect linearity in resolution with increasing acquisition time (Supplementary Figure S1). This is consistent with previous experimental and theoretical results [24]. The pressure in the analyzer vacuum stage of the spectrometer is ~1E-10 Torr. Our results indicate that this is suitable for analytes ranging from small molecules to moderately sized proteins (e.g., <50 kDa); however, ultimate resolution for large proteins is limited by pressure in the analyzer vacuum stage. We have investigated the use of getter pumps placed in the flight tube to increase pumping in the analyzer vacuum stage and near the ICR cell. Initial results in a test vacuum chamber indicate the use of three getter pumps (D 400; SAES Getters) distributed along the length of the flight tube yields approximately one order of magnitude reduction in pressure at the ICR cell.
High resolution and mass measurement accuracy is vital to the success of tandem MS of intact proteins as well. The Velos Pro provides CID capabilities. Additionally, we have implemented beam-type CID in the external quadrupole ion trap and ultraviolet photodissociation (UVPD) within the ICR cell similar to what has been described for lower field instruments [25]. Modifications are under way that will incorporate a 90° bend after the external quadrupole ion trap. This will enable UVPD to be performed within the external quadrupole ion trap as previously described [26].
Conclusions
Initial performance characterization of the 21 T FTICR system at the Environmental Molecular Sciences Laboratory at the Pacific Northwest National Laboratory demonstrates enormous potential for future application to the extremely complex molecular mixtures and systems frequently encountered in environmental, biological, atmospheric, and energy research. The transformative effects of higher magnetic field strength are realized in the ability to acquire high S/N and resolution spectra of the entire charge state distribution of a 78 kDa protein (apo-transferrin), facile isotopic resolution of the proteoforms of a 150 kDa monoclonal antibody, precise measurement of the isotopic fine structure of the peptide Substance P, and the assignment of 8158 molecular formulas in a natural organic matter mixture (SRFA) with mass measurement accuracy within 0.1 ppm. Hence, as hypothesized, FTICR at 21T offers the unprecedented ability to routinely analyze large proteins (>100 kDa), measure isotopic fine structure with high fidelity, and extract more information from complex natural organic matter mixtures. This highly specialized capability is located within the user facility and thus available to the researchers worldwide.
References
Marshall, A.G., Hendrickson, C.L., Jackson, G.S.: Fourier transform ion cyclotron resonance mass spectrometry: a primer. Mass Spectrom. Rev. 17, 1–35 (1998)
Scigelova, M., Hornshaw, M., Giannakopulos, A., Makarov, A.: Fourier transform mass spectrometry. Mol. Cell. Proteom. 10, (2011). doi:10.1074/mcp.M111.009431
Marshall, A.G., Guan, S.: Advantages of high magnetic field for Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 10, 1819–1823 (1996)
Karabacak, N.M., Easterling, M.L., Agar, N.Y.R., Agar, J.N.: Transformative effects of higher magnetic field in Fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 21, 1218–1222 (2010)
Wieder, W.R., Grandy, A.S., Kallenbach, C.M., Bonan, G.B.: Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 11, 3899–3917 (2014)
Moss, R.H., Edmonds, J.A., Hibbard, K.A., Manning, M.R., Rose, S.K., van Vuuren, D.P., Carter, T.R., Emori, S., Kainuma, M., Kram, T., Meehl, G.A., Mitchell, J.F.B., Nakicenovic, N., Riahi, K., Smith, S.J., Stouffer, R.J., Thomson, A.M., Weyant, J.P., Wilbanks, T.J.: The next generation of scenarios for climate change research and assessment. Nature 463, 747–756 (2010)
Smith, L.M., Kelleher, N.L., Consortium for Top Down Proteomics: Proteoform: a single term describing protein complexity. Nat. Methods 10, 186–187 (2013)
Tran, J.C., Zamdborg, L., Ahlf, D.R., Lee, J.E., Catherman, A.D., Durbin, K.R., Tipton, J.D., Vellaichamy, A., Kellie, J.F., Li, M., Wu, C., Sweet, S.M.M., Early, B.P., Siuti, N., LeDuc, R.D., Compton, P.D., Thomas, P.M., Kelleher, N.L.: Mapping intact protein isoforms in discovery mode using top-down proteomics. Nature 480, 254–258 (2011)
Ansong, C., Wu, S., Meng, D., Liu, X., Brewer, H.M., Kaiser, B.L.D., Nakayasu, E.S., Cort, J.R., Pevzner, P., Smith, R.D.: Top-down proteomics reveals a unique protein S-thiolation switch in Salmonella typhimurium in response to infection-like conditions. Proc. Natl. Acad. Sci. 110, 10153–10158 (2013)
Michalski, A., Damoc, E., Lange, O., Denisov, E., Nolting, D., Müller, M., Viner, R., Schwartz, J., Remes, P., Belford, M., Dunyach, J.-J., Cox, J., Horning, S., Mann, M., Makarov, A.: Ultra high resolution linear ion trap Orbitrap mass spectrometer (Orbitrap Elite) facilitates top down LC MS/MS and versatile peptide fragmentation modes. Mol. Cell. Proteom. 11, (2012). doi:10.1074/mcp.O111.013698
Weisbrod, C.R., Hoopmann, M.R., Senko, M.W., Bruce, J.E.: Performance evaluation of a dual linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer for proteomics research. J. Proteome 88, 109–119 (2013)
Hendrickson, C.L., Quinn, J.P., Kaiser, N.K., Smith, D.F., Blakney, G.T., Chen, T., Marshall, A.G., Weisbrod, C.R., Beu, S.C.: 21 Tesla Fourier transform ion cyclotron resonance mass spectrometer: a national resource for ultrahigh resolution mass analysis. J. Am. Soc. Mass Spectrom. 26, 1626–1632 (2015)
Beu, S.C., Hendrickson, C.L., Marshall, A.G.: Excitation of radial ion motion in an rf-only multipole ion guide immersed in a strong magnetic field gradient. J. Am. Soc. Mass Spectrom. 22, 591–601 (2011)
Lin, T.-Y., Anderson, G.A., Norheim, R.V., Prost, S.A., LaMarche, B.L., Leach, F.E., Auberry, K.J., Smith, R.D., Koppenaal, D.W., Robinson, E.W., Paša-Tolić, L.: An adaptable multiple power source for mass spectrometry and other scientific instruments. Rev. Sci. Instrum. 86, 94102 (2015)
Tolmachev, A.V., Robinson, E.W., Smith, R.D., Leach, F.E., Futrell, J.H., Paša-Tolić, L.: A conceptual approach for FT-ICR cell harmonization utilizing external shim electrodes. Int. J. Mass Spectrom. 325/327, 45–50 (2012)
Kilgour, D.P.A., Wills, R., Qi, Y., O’Connor, P.B.: Autophaser: an Algorithm for automated generation of absorption mode spectra for FT-ICR MS. Anal. Chem. 85, 3903–3911 (2013)
Kilgour, D.P.A., Van Orden, S.L.: Absorption mode Fourier transform mass spectrometry with no baseline correction using a novel asymmetric apodization function: Absorption mode FTMS with no baseline correction. Rapid Commun. Mass Spectrom. 29, 1009–1018 (2015)
Kujawinski, E.B., Behn, M.D.: Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Anal. Chem. 78, 4363–4373 (2006)
Minor, E.C., Steinbring, C.J., Longnecker, K., Kujawinski, E.B.: Characterization of dissolved organic matter in Lake Superior and its watershed using ultrahigh resolution mass spectrometry. Org. Geochem. 43, 1–11 (2012)
Stenson, A.C., Marshall, A.G., Cooper, W.T.: Exact masses and chemical formulas of individual Suwannee River Fulvic Acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal. Chem. 75, 1275–1284 (2003)
Shaw, J.B., Brodbelt, J.S.: Extending the isotopically resolved mass range of Orbitrap mass spectrometers. Anal. Chem. 85, 8313–8318 (2013)
Makarov, A., Denisov, E.: Dynamics of ions of intact proteins in the Orbitrap mass analyzer. J. Am. Soc. Mass Spectrom. 20, 1486–1495 (2009)
Nagornov, K.O., Gorshkov, M.V., Kozhinov, A.N., Tsybin, Y.O.: High-resolution Fourier transform ion cyclotron resonance mass spectrometry with increased throughput for biomolecular analysis. Anal. Chem. 86, 9020–9028 (2014)
Tolmachev, A.V., Robinson, E.W., Wu, S., Paša-Tolić, L., Smith, R.D.: FT-ICR MS optimization for the analysis of intact proteins. Int. J. Mass Spectrom. 287, 32–38 (2009)
Shaw, J.B., Robinson, E.W., Paša-Tolić, L.: Vacuum ultraviolet photodissociation and FT-ICR mass spectrometry: revisited. Anal. Chem. 88, 3019–3023 (2016)
Shaw, J.B., Li, W., Holden, D.D., Zhang, Y., Griep-Raming, J., Fellers, R.T., Early, B.P., Thomas, P.M., Kelleher, N.L., Brodbelt, J.S.: Complete protein characterization using top-down mass spectrometry and ultraviolet photodissociation. J. Am. Chem. Soc. 135, 12646–12651 (2013)
Acknowledgments
This research is part of the “High Resolution and Mass Accuracy Capability” development project at EMSL, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory in Richland, WA. The authors thank David P. A. Kilgour, Spencer A. Prost, Kenneth J. Auberry, Randolph V. Norheim, Gordon A. Anderson, Jean H. Futrell, James R. Ewing, and Mark R. Townsend for expert help with bioinformatic analyses, electronics, data acquisition workflows, and mechanical design and fabrication throughout this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 313 kb)
Rights and permissions
About this article
Cite this article
Shaw, J.B., Lin, TY., Leach, F.E. et al. 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer Greatly Expands Mass Spectrometry Toolbox. J. Am. Soc. Mass Spectrom. 27, 1929–1936 (2016). https://doi.org/10.1007/s13361-016-1507-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-016-1507-9