Abstract
The mode of life of the turricones and colchicones are considered against the background of functional–morphological analyses of their shell modifications during ontogeny. Among turricones, there were more or less differently adapted (nektobenthos, planktobenthos, demersal) habitants. In colchicones transformation from helical to planispiral coiling indicates a transition to a more active, free-swimming mode of life. At the adult stage, with U-shaped shaft–hook morphology, colchicones could passively float or migrate up and down, not in far distance from the seafloor in a near-vertical position of the shaft, with the aperture facing upward. Though, they were able to maneuver and change the orientation of the shell to establish contact with the substrate.
Similar content being viewed by others
Introduction
The difficulties in assessing the swimming abilities of heteromorph ammonites, their shell orientation, stability and buoyancy during ontogeny have attracted the attention of many specialists (e.g., Diener 1912; Berry 1928; Davitashvili 1949; Kakabadze 1971; Okamoto 1988, 1996; Klinger 1981). Many articles address these topics, and the respective authors have employed morphological analyses, included valuable data from mock-ups, carried out computer calculations, etc. Nevertheless, there are still many unsolved questions concerning the reconstruction of lifestyle of heteromorph ammonites. Little is known about the animal’s soft body shape, size, possible maneuvering abilities within and outside the body chamber, as well as about ability to change shell orientation, swimming direction and velocity by the regulation of phragmocone chambers liquid, the hyponome jet, and the tentacles (still very poor data about their size, number, etc.).
Data on the analytic geometry of the shell is important in ethological studies (e.g., Kaplan 2002), though insufficient for conclusions about maneuvering ability of different morphological groups of heteromorphs (e.g., turricones, colchicones) (Figs. 1, 2). The variability of parameters affecting buoyancy is in some cases high. Figure 2 shows that the colchicones possess a varying number of planispiral whorls (some having one whorl and others more than two whorls) and varying morphology of the shaft with hook. The length of the body chamber is also variable. Hence, besides evaluations of the U-shaped shaft–hook, data on the combined functional morphology of the shell (i.e., ontogenetic alternation in whorl coiling, cross section, length of body chamber, sculpture, muscle scars, orientation of the aperture and siphuncle, etc.), as well as the data of the isotopic composition of the shell together with facies and taphonomic analyses may yield new insights into poorly understood aspects of the mode of life of heteromorphs.
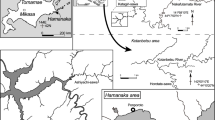
Variation in the morphology of the shell in turricone (Wright et al. 1996): a Proturrilitoides astierianum (Orbigny); b Turrilites costatus Orbigny; c Hypoturrilites gravesianus (Orbigny); d Ostlingoceras puzosianum (Orbigny); e Turrilitoides densicostatus Passendorfer
On mode of life of turricone and colchicone
Most heteromorph ammonites, including colchicones, are characterized by rapid and repeated ontogenetic changes in shell morphology. These were closely related to changes in mode of life of the animal during ontogeny. In most cases the shells become more complex in the adult stage. In view of this fact, ethological studies of heteromorphs need to be carried out for each morphological group, and major shell modifications throughout ontogeny analyzed. Functional–morphological analyses of shell modifications for each ontogenetic stage should be carried out with particular attention paid to features of correlative changes of the principal morphological shell parameters (cross section, sculpture, septa, orientation of the aperture and siphuncle, etc.), also where such changes were manifested between the transitional intervals of the ontogenetic stages (Kakabadze 1985, 1988).
The heteromorphs and monomorphs are characterized by more or less similar first ammonitella stage, and I share viewpoint that at this stage many representatives of both morphological types must have had more or less similar planktonic mode of life; to wit, presumably most of them easily migrated in a suspended state with water flows and they were characterized by wide pelagic distribution (Drushchitz et al. 1969; Mutvei and Reyment 1973; Drushchitz and Dogudzhaeva 1981; Tanabe et al. 1981; Kakabadze 1981; Landman 1985; Shigeta 1993; Barskov 2012).
Ecological differentiation of ammonites took place directly after ammonitella stage. Some representatives have adapted to nektonic or nektoplanktic mode of life, whereas others—to benthic or nektobenthic mode. It is possible that some forms continued a planktonic lifestyle during their earliest post-ammonitella stage (Tanabe et al. 1981; Kakabadze 1981).
Heteromorphic groups (turricone, colchicone) being under consideration are characterized by helical coiling directly after ammonitella stage. Turricones possess helically coiled contiguous whorls to the end of the adult stage. Most representatives of turricones, as well as colchicones in the helicoidal ontogenetic stage, probably maintained apex-upward shell orientation (Fig. 3a) (Diener 1912; Berry 1928; Trueman 1941; Kakabadze 1971; Wiedmann 1973). The diversity of marine life is at its highest on the seafloor and it can be assumed that turricones were adapted to a various seafloor niches. Some were lying on or moving along the hard substrate, others could “hover” over muddy or sandy substrates. Due to its helical coiling, the ammonite in case of need was able to rapidly turn about its vertical or subvertical axis without expending much energy (Klinger 1981). Thanks to its ability of regulating the cameral liquid (Ward 1979, etc.) and its presumably actively functioning hyponome (key for reactive locomotion) and tentacles, many of them were also able to swim in different directions (including vertically). In general, they were poor swimmers, though some were relatively mobile. Thus, based on variations in shell sculpture (fine ribbing, coarse ribbing, spines, etc.), differences in coiling (Fig. 1) and position of the siphuncle, there are reasons to believe that among turricones there were differently adapted habitants, such as nektobenthos, planktobenthos, also demersal forms living and feeding close to the seafloor (lying on or moving along the hard substrate).
Unlike turricones, the colchicones are characterized by a planispiral stage after the helicoidal stage, with loosely coiled or contiguous whorls (e.g., Colchidites, Kutatissites, Imerites, etc.) (Fig. 2). The transition from helical to planispiral coiling was fairly rapid and it is remarkable that not only the whorl coiling but also the sculpture, suture and the cross section became bilaterally symmetric already from the starting point of the first planispiral whorl. Thus, the shells of the colchicones became similar to the shells of monomorphic ammonites characterized by planispiral-evolute coiling. Taking all these data into account, it appears that the transition from helicoidal to planispiral indicates a change to a more active, free-swimming mode of life (Fig. 3b). In the planispiral ontogenetic stage there is a great variability in cross section and sculpture, as well as in number of whorls and in coiling peculiarities (Kakabadze 2004); this suggests differences in swimming speed and maneuverability. For example, in the planispiral stage, those colchicones, which are characterized by nearly contiguous, convex and strongly ornamented planispiral whorls, in all probability were comparatively slow swimmers (Kakabadze 1981).
After the planispiral stage the straight or nearly straight shaft was formed. There exist several hypotheses about mode of life in the shaft stage; for example, (1) during secretion of the shaft many heteromorphs were planktonic (Westermann 1993, 1996); (2) they were nektoplanktonic or nektobenthic, with only slight contact with the seafloor (Kaplan 2002). It is likely that during formation of the shaft colchicones became less active swimmers than in the planispiral stage, probably assuming a nektobenthic mode of life with the shaft in a nearly horizontal position at the beginning of the straightly uncoiling process. However, the explanatory mechanisms of these hypotheses are still unclear.
Later, during the formation of the knee bend, colchicones were most likely to have lived near the seafloor assuming a near-vertical position of the shaft supported by the buoyant chambers (Fig. 3c). Remarkable that such assumption is supported by the data of their facies-controlled distribution in the Caucasus (Kakabadze 1971, 1981), e.g., in the Lower Cretaceous of Georgia colchicones (as well as ancylocones) are not distributed in pelagic or hemipelagic facies. Separate groups of species of colchicones and ancylocones have rather restricted facies-controlled distribution in the various facies of the shelf area (shallow water, comparatively more or less deeper area of the shelf, etc.).
The adult shells of colchicones are similar to those of ancylocones, both possessing a shaft with a prominent hook and the body chamber occupying the entire hook and a part of the shaft. The length of the body chamber varies between genera and species. In colchicones the shaft and hook morphology is variable; in some species shaft is subparallel to the terminal part of the hook (Fig. 2a), whereas in other species the U-shaped hook is more open, with the angle between the shaft and terminal part of the hook exceeding 35°, in some cases reaching 50–55° (Fig. 2f). Based on data on functional morphology and facies-controlled distribution, it is reasonable to assume that adult colchicones and ancylocones could passively float or migrate up and down in the water column, presumably not in far distance from the seafloor, with the shaft in a vertical or near-vertical position (aperture facing upward) supported by the buoyant chambers above. Such a shell orientation appears suitable for the requirements for passive floating with neutral buoyancy (Fig. 3c). This same orientation allows the ammonite to repose with the bottom of the hook resting on the substrate,Footnote 1 or to float passively near the seafloor (Klinger 1981; Ebel 1999, etc.). However, such a position, hovering above seafloor, may have hampered its feeding from the substrate (Ward 1976, 1979) and, thus, was not an optimal life position. But the need for contact of animal with the seafloor, in all probability, was dictated by optimal feeding conditions on the seafloor.
In contrast to the viewpoint that the U-shaped adult heteromorphs (e.g., ancylocones) could not change significantly the vertical position of their shaft and accordingly had no direct contact with the substrate (Kaplan 2002), it is more likely that adult colchicones and ancylocones, when needed, were able to adjust their buoyancy and shell orientation to reach the substrate (Kakabadze 1981; Kakabadze and Sharikadze 1993, etc.). Figure 3d shows that in Colchidites riosuaresi Kakabadze and Hoedemaeker, the animal’s contact with the substrate was achieved at a tilt angle of 40° from the vertical position of the shaft. In relation to this question it should be noted that dorsal and ventral muscle scars of U-shaped Ancyloceras were studied/analyzed by Dogudzhaeva and Mikhailova (1991). They noted that dorsal muscle scars are of larger sizes, than they are in other Cretaceous heteromorphic genera (Ptychoceras or Pseudocrioceratites) and they are located in the front part of the body chamber, indicating that a considerable part of the soft body was beyond the living chamber.
It is obvious that the ammonite was not able to readjust its buoyancy and thus orientation of the shell only by acting with protruding parts of the soft body out of the aperture, or by cameral fluid adjustments, or by action of the tentacles and hyponome. On the contrary, it seems more likely that the ammonite could alter its orientation only by the simultaneous action of all these mechanisms.
Conclusions
Thus, representatives of turricones, as well as colchicones in the helicoidal ontogenetic stage, were habitant of the seafloor with apex-upward shell orientation. Based on variability in shell ornamentation, differences in helically coiling peculiarities, siphuncle position and ability of liquid regulation within the cameras there are reasons to assume that among turricones there were more or less differently adapted mobile benthic, planktobenthic and perhaps also nektobenthic habitants.
Transformation from helicoidal to planispiral coiling in colchicones indicates a transition to a more active, free-swimming mode of life. At the adult ontogenetic stage with U-shaped shaft–hook morphology they could passively float or migrate up and down in the water, presumably not in far distance from the sea floor, with the aperture facing upward. Moreover, the animal could readjust its buoyancy and thus orientation of the shell thanks to simultaneous coordinated, combined action of protruding parts of the soft body out of the aperture, cameral fluid adjustments, as well of tentacles and hyponome.
It is not the goal of the present paper to reconstruct the lifestyle of each turricone or colchicone species. Among e.g., colchicones, there are species which differ from each other by having fine (smooth whorls, fine ribs) or significantly strong (coarse ribs with spines, etc.) ornamentation. Moreover, there can be differences also in whorl cross section, whorl coiling, peculiarities of muscle scars, etc. Most probably these animals had more or less different mode of life. Certainly, such detailed studies require a combined analytical approach (functional morphology during ontogeny, litho- and biofacial dependence, etc.). Unfortunately, lack of sufficiently well-preserved material hampers such investigations. No doubt that in future, finds of well-preserved shells, as well as remnants of soft body (of turricones, colchicones, ancylocones) will promote checking of some disputable ideas of this article with application of new methods for buoyancy control calculation (Lemanis et al. 2015) and stable isotope studies (e.g., Moriya et al. 2003, Lukeneder et al. 2010, Ritterbush et al. 2014), especially with analysis through ontogeny. As far as I know, such material for ancylocones exists in Volga region, near Ulyanovsk (see Dogudzhaeva and Mikhailova 1991), and for ptychocones (e.g., muscle scars, injury)—in NW Caucasus (see e.g., Kakabadze and Sharikadze 1993).
Notes
Klinger (1981) noted about signs of wear on the U-shaped hook of some representatives of ancylocone, reflecting their rest on the bottom.
References
Barskov, I.S. (2012). Evolution of the Ectocochlear Heteromorphs. Materials of Conference. Moscow, 9–12 April, 2012—Modern problems of study of Cephalopod Mollusks. Morphology, systematic, evolution, ecology and biostratigraphy. Issue 3, Moscow, 29–34 (in Russian).
Berry, E.W. (1928). Cephalopod adaptations—the record and its interpretations. The Quaternery Review of Biology (Baltimore) 39–108.
Davitashvili, L. Sh. (1949). Course of Palaeontology. Moscow-Leningrad. p. 835 (in Russian).
Diener, C. (1912). Lebensweise und Verbreitung der Ammoniten. Neues Jahrbuch für Mineralogie, Geologie und Paläontologie, 2, 67–89.
Dogudzhaeva, L.A., Mikhailova, I.A. (1991). New data on muscle system of Heteromorph ammonites. In: Proceedings of the USSR Academy of Sciences (Dokladi Academii Nauk SSSR), t. 318 (4), 981–985 (in Russian).
Drushchitz, V.V., Barskov, I.S., Khiami, N. (1969). Ultramicroscopic structure of the skeleton of two late Aptian ammonites (Zurcherella, Salfeldiella). Bull. MOIP. Geol. (Bulleten Moskovskikh Ispitateleyi Pripodi. Geologia) T. 44(2), 157–158 (in Russian).
Drushchitz, V.V., Dogudzhaeva, L.A. (1981). The Ammonites under the electronic microscop. Moscow, MGU. (Moskovskyi Gosudarstvennyi Universitet). 238 p. (in Russian).
Ebel, K. (1999). Hydrostatics of fossil ectocochleate cephalopods and its significance for the reconstruction of their lifestyle. Paläontologische Zeitschrift, 73(3–4), 277–288.
Kakabadze, M.V. (1971). Colchidites and their stratigraphical significance. In: Proceedings of Geological Institute Academy of Sciences of Georgian SSR (Trudy Geologicheskogo Instituta Academii Nauk Gruzinskoyi SSR). New Series. Vol. 26, p. 125 (in Russian).
Kakabadze, M.V. (1981). Ancyloceratids of the South USSR and their stratigraphical significance. In: Proceedings of Geological Institute Academy of Sciences of Georgian SSR (Trudy Geologicheskogo Instituta Academii Nauk Gruzinskoyi SSR). New Series. Vol. 71, p 197 (in Russian).
Kakabadze, M. V. (1985). On principles of the ethological investigations of Heteromorphic ammonites. Bulletin of Academy of Sciences of Georgia (Bulletin Akademii Nauk Gruzii), 118(2), 377–380. (in Russian).
Kakabadze, M.V. (1988). On the morphological classification of the Heteromorph ammonites. In J. Wiedmann, J. Cullmann (Eds.), Cephalopodes, Present and Past. E. Schweizerbart’sche Verlagbuchhandlung. Stuttgart, (pp. 447–452).
Kakabadze M.V. (2004). Intraspecies and intrageneric variabilities and their implications for the systematics of Cretaceous heteromorph ammonites: a review. In: S.K. Donovan (Ed.), Early Cretaceous ammonites from Colombia. Scripta Geologica, 128, December 2004, Leiden, pp. 17–37.
Kakabadze, M. V., & Sharikadze, M. Z. (1993). On the mode of life of Heteromorph ammonites (heterocone, ancylocone, ptychocone). Geobios. M. S., 15, 207–215.
Kaplan, P. (2002). Biomechanics as a Test of Functional Plausibility: testing the Adaptive Value of Terminal-Countdown Heteromorphy in Cretaceous Ammonoids. Abhandlungen der Geologischen Bundesanstalt., 57, 181–197.
Klinger, H.C. (1981). Speculations on buoyancy control and ecology in some Heteromorph Ammonites. In M.R. House, J.R. Senior (Eds.), The Ammonoidea. Systematics Association Special Volum 18 (pp. 337–355).
Landman, N.H. (1985). Preserved ammonitellas of Scaphites (Ammonoidea, Ancyloceratina). American Museum Novitates, 2815, 1–10.
Lemanis, R., Zachow, S., Fusseis, F., Hoffmann, R. (2015). A new approach using high-resolution computed tomography to test the buoyant properties of chambered cephalopod shells. Paleobiology, FirstView, 1–17.
Lukeneder, A., Harzhauser, M., Müllegger, S., & Piller, W. E. (2010). Ontogeny and habitat change in Mesozoic cephalopods revealed by stable isotopes (δ18O, δ13C). Earth and Planetary Science Letters, 296, 103–114.
Moriya, K., Nishi, H., Kawahata, H., Tanabe, K., & Takayanagi, Y. (2003). Demersal habitat of Late Cretaceous ammonoids: evidence from oxygen isotopes for the Campanian (Late Cretaceous) northwestern Pacific thermal structure. Geology, 31, 167–170.
Mutvei, H., & Reyment, R.A. (1973). Buoyancy control and siphuncle function in ammonoids. Palaeontology, 16, 623–636.
Okamoto, T. (1988). Changes in life orientation during the ontogeny of some heteromorph ammonoids. Palaeontology, 31(2), 281–294.
Okamoto, T. (1996). Theoretical modeling of Ammonoid morphology. In N. H. Landman, K. Tanabe, & R. A. Davis (Eds.), Ammonoid Paleobiology, Topics in Geobiology (Vol. 13, pp. 225–251). New York: Plenum Press.
Ritterbush, K.A., Hoffmann, R., Lukeneder, A., & De Baets, K. (2014). Pelagic palaeoecology: the importance of recent constraints on ammonoid palaeobiology and life history. Journal of Zoology, 292(4), 229–241.
Shigeta, Y. (1993). Post-hatching early life history of Cretaceous Ammonoidea. Lethaia, 26, 133–145.
Tanabe, K., Obata, I., Futakami, M. (1981). Early shell morphology in some upper Cretaceous heteromorph ammonites. Transactions and Proceedings of the Palaeontological Society of Japan. New Series 124, 215–234.
Trueman, A.E. (1941). The ammonite body chamber, with special reference to the buoyancy and mode of life of the living ammonite. Quarterly Journal Geological Society, 96, 339–383.
Ward, P.D. (1976). Upper Cretaceous ammonites (Santonian-Campanian) from Orcas Island, Washington. Journal of Paleontology, 50(3), 454–461.
Ward, P.D. (1979). Functional morphology of Cretaceous helically-coiled ammonite shells. Paleobiology, 5, 415–422.
Westermann, G.E.G. (1993). On alleged negative buoyancy in ammonoids. Lethaia, 26, 246.
Westermann, G.E.G. (1996). Ammonoid life and habit. In N. H. Landman, K. Tanabe, & R. A. Davis (Eds.), Ammonoid Paleobiology, Topics in Geobiology (Vol. 13, pp. 607–707). New York: Plenum Press.
Wiedmann, J. (1973). Upper Triassic heteromorph ammonites. In A. Hallam (Ed.), Atlas of Paleobiogeography (pp. 235–249). Elsevier: Amsterdam.
Wright, C.W., Callomon, J.H., Howarth, M.K. (1996). Treatise on Invertebrate Palaeontology, Part L, Mollusca 4 (Revised), Volume 4: Cretaceous Ammonoidea. The Geological Society of America and The University of Kansas, Boulder, Colorado and Lawrence, Kansas, i-xx + 362 pp.
Acknowledgments
I am grateful to Peter Bengtson (Heidelberg University) for his constructive remarks and suggestions. I would also like to thank both anonymous reviewers for critical comments and valuable advices, which really were a substantial help for improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kakabadze, M.V. Speculations on the ethology of some heteromorph ammonites. Swiss J Palaeontol 135, 63–68 (2016). https://doi.org/10.1007/s13358-015-0104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13358-015-0104-z