Abstract
Transmission efficiencies of capsicum chlorosis virus (CaCV) were investigated for four thrips species (Thysanoptera: Thripidae), Frankliniella occidentalis (Pergande), Frankliniella intonsa (Trybom), Thrips palmi (Karny), and Thrips tabaci Lindeman infesting green pepper in Japan. The first-instar larvae of the thrips were allowed to feed on CaCV-infected green pepper leaves for 24 h. The transmission of CaCV by thrips was examined using a petunia leaf disk assay. CaCV was transmitted by four male (8.70%) and four female (3.88%) T. palmi, but the virus was not transmitted by adults of any other species. In a second transmission assay, 10 adult T. palmi that had fed on infected leaves as larvae were released to healthy green pepper seedlings. After 21 days, the seedlings were examined and 50% of them showed symptoms of CaCV infection. RT-PCR confirmed that they were infected with the virus. The viruliferous rate of adult T. palmi was 60%, while CaCV was not detected in adults of the other three species. These results suggest that T. palmi is capable of being a new vector of CaCV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viruses of the genus Orthotospovirus, within the family Tospoviridae, infect more than 1000 species of plants, including many important food crops and ornamental plants. They have a significant economic impact and many species are distributed globally (Rotenberg et al. 2015). The Orthotospovirus genome consists of three single-strand, negative-sense RNA (RNA-L), and ambisense RNAs (RNA-S and -M). In general, the viruses are transmitted by thrips in a persistent and propagative manner. They replicate within their insect host, circulate through its body, and persist through the various developmental stages (Rotenberg et al. 2015). After the first-instar larvae acquire viruses from infected plants (Ullman et al. 1993; Wijkamp et al. 1993), second-instar larvae and adults transmit the viruses to other plants (German et al. 1992; Ullman et al. 1995).
Capsicum chlorosis virus (CaCV) is a species of the genus Orthotospovirus (Abudurexiti et al. 2019). CaCV was first detected from capsicum, chili, and tomato in Australia in 1999 (McMichael et al. 2000, 2002). CaCV was later reported in Thailand (Knierim et al. 2006), Japan (Okuda 2016), India (Krishnareddy et al. 2008; Kunkalikar et al. 2007), Taiwan (Chen et al. 2007a; Huang et al. 2010), China (Chen et al. 2007b), and the United States (Melzer et al. 2014). The full genomes of some isolates have been sequenced (Knierim et al. 2006; Zheng et al. 2011). CaCV was detected from green pepper (Capsicum annuum) in Kochi Prefecture, Japan, for the first time in 2003. Subsequently, CaCV was detected from green pepper in Ibaraki Prefecture in 2005 (Okuda 2016) and in Oita Prefecture in 2016, and from tomato in Tochigi Prefecture in 2007.
Ceratothripoides claratris (Shumsher) (Thysanoptera: Thripidae), whose occurrence has not yet been confirmed in Japan, is a species of thrips that was shown to be a vector of CaCV in a transmission assay using tomato leaf disks in Thailand (Premachandra et al. 2005). Thrips palmi (Karny) and Frankliniella schultzei (Trybom) (Thysanoptera: Thripidae) are regularly found in areas with CaCV symptoms, so both species are considered to be CaCV vectors in Australia, but the experimental data have not been shown (McMichael et al. 2002; Persley et al. 2006).
In the present study, we conducted CaCV transmission assays using petunia leaf disks or green pepper seedlings for four thrips species infesting green pepper plants in Japan.
Materials and methods
Thrips
We used four thrips species that are known to be pests of green pepper in Japan (Thysanoptera: Thripidae): Frankliniella occidentalis (Pergande), Frankliniella intonsa (Trybom), T. palmi, and Thrips tabaci Lindeman. Only T. tabaci is a thelytokous strain which derived from multiple females. Specimens of each species were collected from various areas and crops in Japan (Table 1). The thrips species were reared and maintained in the laboratory following a previously described protocol (Sakurai et al. 2004).
Virus sample
CaCV-OITA isolate was collected from green pepper cultivar ‘Sarara’ plants in a greenhouse in Oita Prefecture, Japan, in 2016. This plant showed mosaic, yellowing, and chlorosis symptoms on the leaves. After conducting single local lesion isolation with Chenopodium quinoa, the virus was propagated with Nicotiana benthamiana by mechanical inoculation. Subsequently, CaCV-OITA isolate was inoculated back to green pepper ‘Sarara’ plants that were approximately 4 weeks old and 15 cm tall. After the mechanical inoculation, the plants were grown in an insect-free greenhouse maintained at 25 °C under natural light conditions until they were used for virus acquisition by thrips.
Transmission assay
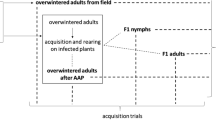
To survey the transmission efficiency of the four thrips species, we used a petunia leaf disk assay (Wijkamp and Peters 1993). Newly hatched thrips larvae up to 12 h old from each species were allowed to feed on CaCV-infected green pepper detached leaflets showing the same symptoms as the original plant for 24 h as an acquisition access period (AAP). After AAP, the larvae were grown on germinated broad beans at 25 °C under 16 h light/8 h dark (16L8D) conditions until they became adults. After adult eclosion, the transmission efficiencies were assessed by inoculating to a single petunia leaf disk (6 mm in diameter) with an adult thrips up to 2 days old in 2-ml microtubes for 24 h. After the inoculation access period (IAP), the leaf disks were floated on water for 2 days in 96-well ELISA plates for symptom development. Leaf disks that developed necrosis were analyzed using reverse transcription-polymerase chain reaction (RT-PCR) to determine whether they were infected with CaCV. RNA extraction from petunia leaf disks was conducted using an RNeasy Plant Mini Kit (QIAGEN) according to the manufacturer’s instructions. RT-PCR was performed with PrimeScript™ One-Step RT-PCR Kit Ver.2 (TaKaRa BIO), using a specific primer pair for the N gene of CaCV designed in this study (CaCV-N F:5′-CACACTTCAATAGATGTACT-3′ and CaCV-N R:5′-ATGTCTACCGTCAGGCAACT-3′). The estimated PCR product size is 827 bp. The one-step RT-PCR conditions consisted of one cycle of 50 °C for 30 min, one cycle of 94 °C for 2 min, and 35 cycles of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 45 s, followed by a final extension for 5 min at 72 °C. All PCR products were analyzed by electrophoresis in 2% agarose gel.
To prove that the result of the transmission assay using petunia leaf disk was not due to the feeding preference of thrips, a petunia leaf disk feeding test using four species of thrips was performed. Healthy thrips of each species larvae were reared to adulthood on germinated broad beans at 25 °C under 16L8D. After adult eclosion, an adult thrips was put in 2-ml microtube with one petunia leaf disk (6 mm in diameter), and the survival rate and feeding scar were confirmed visually after 24 h and 48 h. As a result, although the survival rate of males of F. intonsa was 80% (n = 10), all others showed over 90% survival (n = 10) after 48 h. Furthermore, feeding scars were observed from all leaf disks from which the surviving thrips were collected. Feeding scars by all females were observed after 24 h, while feeding scars by all males were not visible after 24 h, but were observed after 48 h.
The single thrips species (T. palmi) that was confirmed to transmit CaCV by the petunia leaf disk assay was then tested by another transmission assay with green pepper seedlings. Newly hatched larvae up to 12 h old were set on CaCV-infected green pepper detached leaflets for AAP of 24 h and grown on germinated broad beans at 25 °C under 16L8D. After adult eclosion, 10 adult thrips up to 2 days old were placed on a green pepper seedling (approximately 4 weeks old and 15 cm tall) in a rearing plastic cage (340 × 260 × 340 mm) for the IAP of 5 days at 25 °C under 16L8D. Ten replicates (10 green pepper plants) were established for transmission assay. After the IAP, thrips were killed by nitenpyram insecticide in the rearing cage. Then, the green peppers inoculated with CaCV by viruliferous thrips were grown in a greenhouse maintained at 25 °C under natural light condition. On the 21st day after viruliferous thrips were placed on the green pepper seedlings, we assessed the infection of CaCV by observing symptoms on leaves and conducting RT-PCR. RNA extraction from green pepper seedlings and RT-PCR was performed using the same method described above.
Viruliferous rate of each thrips species
After the AAP of 24 h to acquire CaCV by thrips larvae up to 12 h old, they were reared to adulthood on germinated broad beans at 25 °C under 16L8D as described above. From each thrips species, 10 adults were collected randomly, and RT-PCR was used to detect CaCV in each thrips species to determine the viruliferous rate of each species. The extraction of RNA from individual thrips was conducted as described in Kimura et al. (2016).
Results
Transmission of CaCV by four thrips species
The efficiencies of CaCV transmission to petunia leaf disks by four thrips species are presented in Table 2. CaCV was transmitted by 8.70% of male (n = 46) and 3.88% of female (n = 103) T. palmi thrips. There was no significant difference between the rates of transmission by male and female thrips (Fisher’s exact probability test, p > 0.05). The virus was not transmitted to petunia leaf disks by any of the other thrips species (Table 2). In case of inoculating CaCV by 10 T. palmi adults to a green pepper seedling, 50% of the green pepper seedlings expressed CaCV-infected symptoms (n = 10). Viral infection was apparent from an observation of plants, and the presence of CaCV in those plants was confirmed from positive leaf disks and seedlings by RT-PCR.
Viruliferous rates of CaCV in four thrips species
The viruliferous rates of CaCV in four thrips species are presented in Table 3. CaCV was detected in 60% of T. palmi adults (n = 10). However, the virus was not detected in the other thrips species.
Discussion
Persley et al. (2006) indicated that T. palmi and F. schultzei could be vectors of CaCV in Australia. However, no experimental evidence has confirmed that they are vectors. In the present study, only adult T. palmi transmitted CaCV to petunia leaf disks and green pepper plants; CaCV was not transmitted by adult F. occidentalis, F. intonsa, or T. tabaci. In addition, CaCV was detected only in adult T. palmi. These results suggested that T. palmi is capable of being a vector species of CaCV and that the other three species are not vectors.
Our results showed that the transmission rate of CaCV by T. palmi to petunia leaf disks was extremely low (average 5.4%). In contrast, Permachandra et al. (2005) reported that up to 87% of adult C. claratris transmitted CaCV to tomato leaf disks. The lower infection rate to petunia leaf disks may result from low feeding preference to petunia plants of T. palmi compared with tomato plants. However, our feeding test showed that at least these four thrips were able to survive for over 48 h when they were fed on petunia leaf disks. In addition, the petunia leaf disk assay for these four thrips species has been performed in previous studies (Inoue et al. 2004; Sakurai et al. 2004). This indicates that the low transmission rate of CaCV is not related to the low feeding preference to petunia plants by T. palmi.
In the transmission analysis using green pepper seedlings, 50% of plants were infected with CaCV by inoculating the virus by 10 T. palmi adults and showed typical symptoms of CaCV infection. Since the transmission rate of a single thrips to petunia leaf disks was 5.4%, the results of the experiment using seedlings almost agree with that of the petunia leaf disk assay. Although the transmission rate of CaCV by individual T. palmi may be low, high infestation of this thrips species to green pepper plants may spread CaCV infection rapidly. Thus, we suggest that appropriate control of T. palmi is necessary in green pepper fields.
It is well known that several thrips species transmit the same virus, with varying transmission rates. For example, F. occidentalis and F. intonsa transmit impatiens necrotic spot virus (INSV) in Japan, and F. occidentalis transmits the virus at a higher rate than does F. intonsa (Sakurai et al. 2004). The same phenomenon has been also observed in tomato spot wilt virus (TSWV), tomato chlorosis spot virus, and chrysanthemum stem necrosis virus (Inoue et al. 2004; Okuda et al. 2013; Wijkamp et al. 1995). As the present study showed, T. palmi may be a vector of CaCV in Japan, but its transmission rate is much lower than that of another vector of the virus, C. claratris. This low rate may be the reason that the virus has not yet become widespread in Japan. CaCV was also detected in tomatoes from Tochigi Prefecture, Japan. However, T. palmi does not generally infest tomatoes. Other unknown vector species infesting tomatoes, such as C. claratris, may be present in Japan, or CaCV may be transmitted by T. palmi after opportunistically feeding on tomatoes.
Several previous studies reported differences in tospovirus transmission by male and female thrips. Male F. occidentalis thrips showed a higher rate of transmission of TSWV than did females (Sakurai et al. 1998; van de Wetering et al. 1999), and male F. intonsa showed a higher transmission rate of INSV than did females (Sakurai et al. 2004). Although Premachandra et al. (2005) showed that male and female C. claratris did not differ in the efficiency of CaCV transmission; T. palmi males transmitted CaCV at more than twice the rate of females in the present study. However, the rates were low and there was no significant difference between them. Further research is necessary to elucidate the intersexual variation of CaCV transmission by T. palmi.
In conclusion, we propose that T. palmi is capable of being a vector species of CaCV, while F. occidentalis, F. intonsa, and T. tabaci are not vectors. Although the transmission competency of T. palmi was low in the present study, other populations of T. palmi should be investigated to more accurately assess the risk of CaCV transmission. In India, at least three cryptic species of T. palmi have been discovered by DNA barcoding analysis (Tyagi et al. 2017). In addition, we must guard against the invasion of the efficient vector C. claratris to prevent the spread of CaCV in Japan.
References
Abudurexiti A, Adkins S, Alioto D et al (2019) Taxonomy of the order Bunyavirales: update 2019. Arch Virol 164:1949–1965. https://doi.org/10.1007/s00705-019-04253-6
Chen CC, Huang CH, Chen TC, Yeh SD, Cheng YH, Hsu HT, Chang CA (2007a) First report of Capsicum chlorosis virus causing yellow stripes on calla lilies in Taiwan. Plant Dis 91:1201. https://doi.org/10.1094/PDIS-91-9-1201C
Chen K, Xu Z, Yan L, Wang G (2007b) Characterization of a new strain of Capsicum chlorosis virus from peanut (Arachis hypogaea L.) in China. J Phytopathol 155:178–181. https://doi.org/10.1111/j.1439-0434.2007.01217.x
German TL, Ullman DE, Moyer JW (1992) Tospoviruses: diagnosis, molecular biology, phylogeny, and vector relationships. Annu Rev Phytopathol 30:315–348. https://doi.org/10.1146/annurev.py.30.090192.001531
Huang CH, Zheng YX, Cheng YH, Lee WS, Jan FJ (2010) First report of Capsicum chlorosis virus infecting tomato in Taiwan. Plant Dis 94:1263. https://doi.org/10.1094/PDIS-04-10-0275
Inoue T, Sakurai T, Murai T, Maeda T (2004) Specificity of accumulation and transmission of tomato spotted wilt virus (TSWV) in two genera, Frankliniella and Thrips (Thysanoptera: Thripidae). Bull Entomol Res 94:501–507. https://doi.org/10.1079/BER2004326
Kimura K, Usugi T, Hoshi H, Kato A, Ono T, Koyano S, Kagiwada S, Nishio T, Tsuda S (2016) Surveys of viruliferous alate aphid of Plum pox virus in Prunus mume orchards in Japan. Plant Dis 100:40–48. https://doi.org/10.1094/PDIS-05-15-0540-RE
Knierim D, Blawid R, Maiss E (2006) The complete nucleotide sequence of a capsicum chlorosis virus isolate from Lycopersicum esculentum in Thailand. Arch Virol 151:1761–1782. https://doi.org/10.1007/s00705-006-0749-4
Krishnareddy M, Rani RU, Kumar KA, Reddy KM, Pappu HR (2008) Capsicum chlorosis virus (genus Tospovirus) infecting chili pepper (Capsicum annuum) in India. Plant Dis 92:1469. https://doi.org/10.1094/PDIS-92-10-1469B
Kunkalikar S, Poojari S, Rajagopalan P, Zehr UB, Naidu RA, Kankanallu RS (2007) First report of Capsicum chlorosis virus in tomato in India. Plant Health Prog. https://doi.org/10.1094/PHP-2007-1204-01-BR
McMichael LA, Persley DM, Thomas JE (2000) The first record of a serogroup IV tospovirus in Australia. Australas Plant Pathol 29:149. https://doi.org/10.1071/AP00023
McMichael LA, Persley DM, Thomas JE (2002) A new tospovirus serogroup IV species infecting capsicum and tomato in Queensland, Australia. Australas Plant Pathol 31:231–239. https://doi.org/10.1071/AP02016
Melzer MJ, Shimabukuro J, Long MH, Nelson SC, Alvarez AM, Borth WB, Hu JS (2014) First report of Capsicum chlorosis virus infecting waxflower (Hoya calycina Schlecter) in the United States. Plant Dis 98:571. https://doi.org/10.1094/PDIS-06-13-0588-PDN
Okuda M (2016) Tospoviruses occurring in and outside Japan. Jpn J Phytopathol 82:169–184. https://doi.org/10.3186/jjphytopath.82.169(in Japanese with English summary)
Okuda S, Okuda M, Matsuura S, Okazaki S, Iwai H (2013) Competence of Frankliniella occidentalis and Frankliniella intonsa strains as vectors for Chrysanthemum stem necrosis virus. Eur J Plant Pathol 136:355–362. https://doi.org/10.1007/s10658-013-0169-8
Persley DM, Thomas JE, Sharman M (2006) Tospoviruses—an Australian perspective. Australas Plant Pathol 35:161–180. https://doi.org/10.1071/AP06015
Premachandra WTSD, Borgemeister C, Maiss E, Knierim D, Poehling HM (2005) Ceratothripoides claratris, a new vector of a Capsicum chlorosis virus isolate infecting tomato in Thailand. Phytopathology 95:659–663. https://doi.org/10.1094/PHYTO-95-0659
Rotenberg D, Jacobson AL, Schneweis DJ, Whitfield AE (2015) Thrips transmission of tospoviruses. Curr Opin Virol 15:80–89. https://doi.org/10.1016/j.coviro.2015.08.003
Sakurai T, Murai T, Maeda T, Tsumuki H (1998) Sexual differences in transmission and accumulation of tomato spotted wilt virus in its insect vector Frankliniella occidentalis (Thysanoptera: Thripidae). Appl Entomol Zool 33:583–588. https://doi.org/10.1303/aez.33.583
Sakurai T, Inoue T, Tsuda S (2004) Distinct efficiencies of Impatiens necrotic spot virus transmission by five thrips vector species (Thysanoptera: Thripidae) of tospoviruses in Japan. Appl Entomol Zool 39:71–78. https://doi.org/10.1303/aez.2004.71
Tyagi K, Kumar V, Singha D, Chandra K, Laskar BA, Kundu S, Chakraborty R, Chatterjee S (2017) DNA Barcoding studies on Thrips in India: cryptic species and species complexes. Sci Rep 7:4898. https://doi.org/10.1038/s41598-017-05112-7
Ullman DE, German TL, Sherwood JL, Westcot DM, Cantone FA (1993) Tospovirus replication in insect vector cells: immunocytochemical evidence that the nonstructural protein encoded by the S RNA of tomato spotted wilt tospovirus is present in thrips vector cells. Phytopathology 83:456–463. https://doi.org/10.1094/Phyto-83-456
Ullman DE, Westcot DM, Chenault KD, Sherwood JL, German TL, Bandla MD, Cantone FA, Duer HL (1995) Compartmentalization, intracellular transport, and autophagy of tomato spotted wilt tospovirus proteins in infected thrips cells. Phytopathology 85:644–654. https://doi.org/10.1094/Phyto-83-456
van de Wetering F, van der Hoek M, Goldbach R, Peters D (1999) Differences in tomato spotted wilt virus vector competency between males and females of Frankliniella occidentalis. Entomol Exp Appl 93:105–112. https://doi.org/10.1046/j.1570-7458.1999.00567.x
Wijkamp I, Peters D (1993) Determination of the median latent period of two tospoviruses in Frankliniella occidentalis, using a novel leaf disk assay. Phytopathology 83:986–991. https://doi.org/10.1094/Phyto-83-986
Wijkamp I, van Lent J, Kormelink R, Goldbach R, Peters D (1993) Multiplication of tomato spotted wilt virus in its insect vector, Frankliniella occidentalis. J Gen Virol 74:341–349. https://doi.org/10.1099/0022-1317-74-3-341
Wijkamp I, Almarza N, Goldbach R, Peters D (1995) Distinct levels of specificity in thrips transmission of tospoviruses. Phytopathology 85:1069–1074. https://doi.org/10.1094/Phyto-85-1069
Zheng YX, Chen CC, Jan FJ (2011) Complete nucleotide sequence of capsicum chlorosis virus isolated from Phalaenopsis orchid and the prediction of the unexplored genetic information of tospoviruses. Arch Virol 156:421–432. https://doi.org/10.1007/s00705-010-0874-y
Acknowledgements
This work was supported by Cabinet Office, Government of Japan, Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for creating next-generation agriculture, forestry and fishers” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO). We would like to thank Dr. Shuichi Yamasaki (Oita Prefectural Agriculture, Forestry and Fisheries Research Center) for providing the CaCV-OITA isolate, and Dr. Takeshi Ohya (Kanagawa Agricultural Technology Center) and Dr. Kazuhiro Komi (Kochi Agricultural Research Center) for providing cultures of thrips populations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are affiliated with NARO. The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiaki, Y., Kubota, K., Tomitaka, Y. et al. Transmission of capsicum chlorosis virus by Thrips palmi (Thysanoptera: Thripidae). Appl Entomol Zool 55, 31–35 (2020). https://doi.org/10.1007/s13355-019-00649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00649-7