Abstract
Temperature is an important environmental factor for life-history traits in poikilothermic animals. Many of experiments on evolution have been conducted using Drosophila species, and effects on life-history traits vary depending on the study. On the other hand, few studies have been conducted on the effects of temperature on life-history traits in the other insect species. In the present study, we reared adzuki bean beetles under two different temperatures, high and low, for 2 years (20 generations), and compared life-history traits including body size of females, fecundity, egg size, rate of egg hatching, emergence rate, development time, and wing length. No differences in responses were found in these traits between selection strains, except the rate of egg hatching. That is, the rates of egg hatching in high-temperature (32 °C) selection strains were significantly higher than those in low-temperature (24 °C) selection strains. We discuss the cause of change in egg hatchability during successive generations under different temperature treatments from the following viewpoints including evolutionary adaptation to high temperature and the experimental protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperature is an important environmental factor for life-history traits in poikilothermic animals including insects (e.g., Atkinson 1994; Berger et al. 2017; Huey and Bennett 1987; Hoffmann and Parsons 1991). Previous studies of temperature adaptation have often been conducted to search for geographical variations in many animal taxa (e.g., Bedford and Hoekstra 2015; Boyle et al. 2016; Huey et al. 1991; James and Partridge 1995; James et al. 1997; Parsons and Joern 2014; Roitberg et al. 2015). However, previous comparative studies have been influenced by many ecological factors including parasitic relations, competition among species, and other environmental factors including day length, weather, and differences in host plant (Partridge et al. 1994; Stillwell and Fox 2005).
On the other hand, studies on experimental evolution have been also conducted to test adaptation of life-history traits due to long-term rearing under different temperatures, mainly using Drosophila populations (e.g., Azevedo et al. 1996; Bennett et al. 1990; Cavicchi et al. 1989, 1995; Huey et al. 1991; Huey and Kingsolver 1993; James and Partridge, 1995; Neat et al. 1995; Partridge et al. 1994, 1995; Service et al. 1985). These studies showed that long-term rearing under different temperatures altered many morphological and life-history traits, including development time (Huey et al. 1991; James and Partridge 1995), longevity and fecundity (Partridge et al. 1995), survival rate (Huey et al. 1991), wing length (Partridge et al. 1994), and egg size (Azevedo et al. 1996). Gilchrist et al. (1997) showed resistance to heat (38.5 °C) for adults and eggs that have evolved to higher temperatures for more than 4 years by rearing at 29 °C rather than 16.5 °C.
In seed beetles, however, few studies have been conducted on laboratory evolution, i.e., adaptation to different temperatures (but see, Stillwell et al. 2008). Seed beetles grow in a bean during their life from larvae to pupae, and thus, these stages might be less affected by heat compared to egg and adult stages. Therefore, this beetle may provide novel information concerning adaptation to a warm environment. We hypothesized that life-history traits could respond to experimental evolution. To test this hypothesis, we reared adzuki bean beetles, Callosobruchus chinensis (L.) (Coleoptera: Chrysomelidae), under different temperatures for 20 generations at 24 °C and 32 °C. At the 20th generation, we assayed the life-history traits, including the body size of females, fecundity, egg size, rate of egg hatching, emergence rate, development time, and wing length at different temperatures, i.e., 25 °C and 33 °C.
Materials and methods
Insects and experimental evolution
We used a population named isC strain of Callosobruchus chinensis, which was established with about two hundreds of adults collected from mung beans, Vigna angularis, in Ishigaki City, Ishigaki Island, belonging to Yaeyama Islands, of Japan in 1997 (Yanagi and Miyatake 2003). The population was divided, and each maintained in a separate incubator (LPH-6-400-MPZ, Nippon Medical & Chemical Instruments Co. Ltd., Osaka, Japan) kept at 24 °C (L strains) or 32 °C (H strains), respectively (14L10D, 60% humidity) for about 2 years (= 19 generations) with adzuki beans (Dainagon). In each temperature treatment, three populations were reared as replicate lines: H1, H2, H3 (H strains) and L1, L2, and L3 (L strains).
Each line was maintained by 20 males and 20 females randomly selected from emerged adult populations with 5 adzuki beans in a petri-dish (8.5 cm in diameter, 1.4 cm in height), and allowed to lay eggs. We controlled the number of eggs on a bean by scraping away excess eggs to control rearing density of larvae in a bean, and established three eggs per adzuki bean if females laid more than three eggs on the next day after oviposition. This protocol was repeated for 19 generations during 2005 and 2006.
Rearing for pre-assayed generation
At the next generation (20th), 20 females and 20 males were chosen from the 6 lines and were placed in an incubator kept at 25 °C for one generation to avoid potential maternal effects on the measured traits in the present study. The beetles from each line were kept with 50 adzuki beans and allowed to mate. The number of eggs per bean was limited to three. We controlled the number of eggs on a bean by scraping away excess eggs on the day after oviposition, and established three eggs per adzuki bean if a female laid more than three eggs. They were separated in each petri-dish described above per line placed with beans and placed in an incubator kept at 25 °C (14L10D, 60%RH). To gain virgin adults, each bean was transferred to one well of a 48-well tissue-culture plate (Falcon, Becton–Dickinson, NJ, USA) at 10 days after oviposition until these beetles emerged from the beans.
Assay of traits
We checked for newly emerged adults of each line and recorded their sex every day at the 21st generation. Five pairs of one male and one female were put together in a plastic petri-dish (90 mm diameter, 15 mm height) and allowed to mate for 1 day. On the next day, we removed the males, and females were allowed to lay eggs on adzuki beans. If a female laid more than one egg, we scraped away excess eggs and established one egg per bean to avoid the effects derived from any larval competition. Each bean was kept in a well of a 48-well tissue-culture plate (Falcon, Becton–Dickinson).
Each culture plate with 48 beans each (more than 100 for each line) was kept in two incubators set at 25 °C or 33 °C, and the rates of egg hatching and emergence from eggs were observed every day. Burrowing of the larva into each bean was observed every day (= rate of egg hatching). In C. chinensis, the hatched larvae burrow into beans, leaving the empty egg behind. Thus, we can discriminate hatched eggs from non-hatched eggs by the change in their color (see Yanagi and Miyatake 2002). The difference in temperature between at successive rearing (32 °C and 24 °C) and at bioassay (33 °C and 25 °C) depends on account of the adjustment of the temperature-controlled room.
Emerged adults were sexed and placed in the petri-dish with 5 adults for 1 h in an incubator. Then, males were removed and eggs on each bean were counted every day, i.e., the bean was replaced every day until the death of the females (life-time fecundity). We also recorded the development time (from eggs to adult emergence from beans using larvae at 22nd generation).
Dead adults were maintained in a deep freezer kept at − 20 °C. On a later date, right wings were removed and their length was measured as adult body size. The eggs on beans were measured directly with an optical micrometer on a dissecting microscope (VM-50N, Olympus, Tokyo, Japan). The length (L) and width (W) of around thirty randomly selected eggs laid by each female were measured. The size of each egg (V, in cubic millimeters) was calculated, assuming the egg to be an approximate semioval, by the formula V = (πLW2)/12 (Yanagi and Miyatake 2002). The assay of traits was conducted in 2007.
Statistics
All statistical tests were conducted using JMP Ver. 12.2.0 (SAS Institute 2015). Mixed-design ANOVAs were performed to test female body size, fecundity, and egg size as parental traits, and rate of egg hatching and emergence rate as offspring traits, using two separate selection regimes as a fixed-effects factor (i.e., H/L), replicate lines (i.e. 1, 2, and 3) as a random-effects factor. Mixed-design ANOVAs including the sex factor were performed to test development time and wing length. The Tukey-HSD tests were conducted as the post hoc test.
Results
Table 1 shows the means with SE and sample size (N) of rates of egg hatching and emergence for two assays of each line. Table 2 shows the means with SE and sample size of fecundity and egg size for two assays of each line. Table 3 shows the means with SE and sample size of development time and wing length of both sexes for two assays of each line.
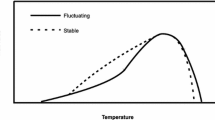
Mixed-design ANOVA showed a significant difference in rate of egg hatching, but no difference in emergence rate between strains (Table 4). Significant differences were found in both traits between assays, and in the interaction of strain and assay, suggesting different performance in the rate of egg hatching per strains in reared temperatures (Table 4). The Tukey-HSD test (a post hoc test) showed a significantly higher rate of egg hatching in the H strain at 33 °C of compared to other treatments: the L strain at 33 °C, and H and L strains at 25 °C (Fig. 1). This means a higher rate of egg hatching in H than L strains at 33 °C.
No differences were found in fecundity or egg size between strains, but significant differences were found for all traits between assays (Table 5). A significant interaction between strain and assay was found only in egg size (Table 5).
No difference was found in development time and wing length between strains (Table 6). A significant difference was found in development time between assays. A significant difference was also found between sexes and in the interaction between strain and assay in both strains (Table 6).
Discussion
In small-sized heterothermic animals with short-term generations, rapid evolution may occur to adapt to specific temperature (Huey and Stevenson 1979; Huey et al. 1991). Maharjan et al. (2017) found that different development times were observed in C. chinensis; the optimal temperature for development is around 35 °C compared with 25 °C or more than 37 °C, and thus, the temperatures in the present experiments would strongly affect life-history traits, including development time. However, the present result of experiments reared under different temperature regimes over 20 generations showed that most life-history traits did not show any responses (Tables 4, 5). Only the rate of egg hatching showed significant differences; lower egg hatching was observed under hot (33 °C) than usual (25 °C) temperatures in L strains (Fig. 1), suggesting adaptation for a higher penetration rate into a bean under high ambient temperature in H strains compared to L strains. However, this difference was not seen at the low temperature (24 °C). Three independent lines showed the same results (Table 1), suggesting that this response might be evolutionary. In the present experiment, three replications were prepared for each line, and we reared all beetles at the same temperature during the parent generation to avoid a maternal effect. Therefore, the present results of response to different rearing temperatures should depend on a genetic factor. However, we did not measure relative humidity in the incubators used, so the effect of humidity on egg hatchability might not be rejected as an explanation of the present results. Further studies are required to test it.
Bean beetles oviposit eggs on the surface of beans, and therefore, the most sensitive stage to ambient temperature might be the egg stage. The lack of a difference in the emergence rate may depend on the unique life style of bean beetles, because bean beetles grow within a bean where the effect of the ambient environmental factors might affect beetles grown within a bean affect them less. This might be a reason why different results have been reported for the bean beetle than for Drosophila (see Azevedo et al. 1996; Huey et al. 1991; James and Partridge 1995; Partridge et al. 1994, 1995). For example, a shorter development time (Huey et al. 1991), shorter female longevity, and fewer eggs were observed under high than low temperatures during long-term generations in Drosophila species in the assay at 25 °C, while the opposite trends were observed at the assay at 16.5 °C (Huey et al. 1991, Partridge et al. 1995).
Another possibility for the lack of a difference in many life-history traits in the present results might depend on shorter generations (even in 20 generations), because there was no time to show evolutionary responses.
Recently, adaptation to hot temperatures is an important aspect in studies in relation to global warming (e.g., Hughes 2000; Parmesan 2006; Tseng et al. 2018). Therefore, studies on adaptation to high temperatures in many taxa with a unique life style must be important. The present result suggests that performance in the egg stage may be important in species grown in enclosed spaces, such as in beans, due to the stress-less condition within the bean. It is required to determine stress resistance in the egg stage in seed beetles.
References
Atkinson D (1994) Temperature and organism size-a biological law for ectotherms? Adv Ecol Res 25:1–58
Azevedo RBR, French V, Partridge L (1996) Thermal evolution of egg size in Drosophila melanogaster. Evolution 50:2338–2345. https://doi.org/10.1111/j.1558-5646.1996.tb03621.x
Bedford NL, Hoekstra H (2015) Peromyscus mice as a model for studying natural variation. eLife 4:e06813. https://doi.org/10.7554/elife.06813
Bennett A, Dao FKM, Lenski RE (1990) Rapid evolution in response to high temperature selection. Nature 346:79–81
Berger D, Stångberg J, Grieshop K, Martinossi-Allibert I, Arnqvist G (2017) Temperature effects on life-history trade-offs, germline maintenance and mutation rate under simulated climate warming. Proc R Soc B 284:20171721. https://doi.org/10.1098/rspb.2017.1721
Boyle WA, Sandercock BK, Martin K (2016) Patterns and drivers of intraspecific variation in avian life history along elevational gradients: a meta-analysis. Biol Rev 91:469–482. https://doi.org/10.1111/brv.12180
Cavicchi S, Guerra V, Natali V, Pezzoli C, Giorgi G (1989) Temperature-related divergence in experimental populations of Drosophila melanogaster. II. Correlation between fitness and body dimensions. J Evol Biol 2:235–251. https://doi.org/10.1046/j.1420-9101.1989.2040235.x
Cavicchi S, Guerra D, LaTorre V, Huey RB (1995) Chromosomal analysis of heat-shock tolerance in Drosophila melanogaster evolving at different temperatures in the laboratory. Evolution 49:676–684
Gilchrist GW, Huey RB, Partridge L (1997) Thermal sensitivity of Drosophila melanogaster: evolutionary responses of adults and eggs to laboratory natural selection at different temperatures. Physiol Zool 70:403–414. https://doi.org/10.1086/515853
Hoffmann AA, Parsons PA (1991) Evolutionary genetics and environmental stress. Oxford University Press, Oxford
Huey RB, Bennett AF (1987) Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41:1098–1115. https://doi.org/10.1111/j.1558-5646.1987.tb05879.x
Huey RB, Kingsolver LG (1993) Evolution of resistance to high temperature in ectotherms. Am Nat 142:S21–S41. https://doi.org/10.1086/285521
Huey RB, Stevenson RD (1979) Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Amer Zool 19:357–366. https://doi.org/10.1093/icb/19.1.357
Huey RB, Partridge L, Fowler K (1991) Thermal sensitivity of Drosophila malanogaster responds rapidly to laboratory natural selection. Evolution 45:751–756. https://doi.org/10.1111/j.1558-5646.1991.tb04343.x
Hughes L (2000) Biological consequences of global warming: is the signal already apparent? Trends in Ecol Evol 15:56–61. https://doi.org/10.1016/S0169-5347(99)01764-4
James A, Partridge L (1995) Thermal evolution of rate of development in Drosophila melanogaster. J Evol Biol 8:315–330
James AC, Azevedo RBR, Partridge L (1997) Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics 146:881–890
Maharjan R, Ahn J, Park C, Yoon Y, Jang Y, Kang H, Bae S (2017) Effects of temperature on development of the adzuki bean weevil, Callosobruchus chinensis (Coleoptera: Bruchidae) on two leguminous seed. J Stored Prod Res 72:90–99. https://doi.org/10.1016/j.jspr.2017.04.005
Neat F, Fowler K, Frencg V, Partridge L (1995) Thermal evolution at low temperature reduces the nutritional requirement for growth in Drosophila melanogaster. Proc R Soc B 260:73–78. https://doi.org/10.1098/rspb.1995.0061
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–639. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Parsons SMA, Joern A (2014) Life history traits associated with body size covary along a latitudinal gradient in a generalist grasshopper. Oecologia 174:379–391. https://doi.org/10.1007/s00442-013-2785-6
Partridge L, Barrie B, Fowler K, French V (1994) Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48:1269–1276. https://doi.org/10.1111/j.1558-5646.1994.tb05311.x
Partridge L, Barrie B, Barton NH, Fowler K, French V (1995) Rapid laboratory evolution of adult life-history traits in Drosophila melanogaster in response to temperature. Evolution 49:538–544. https://doi.org/10.1111/j.1558-5646.1995.tb02285.x
Roitberg ES, Eplanova GV, Kotenko TI, Amat F, Carretero MA, Kuranova VN, Bulakhova NA, Zinenko OI, Yakovlev VA (2015) Geographic variation of life-history traits in the sand lizard, Lacerta agilis: testing Darwin’s fecundity-advantage hypothesis. J Evol Biol 28:613–629. https://doi.org/10.1111/jeb.12594
SAS Institute Inc. (2015) JMP® 12.2.0. SAS Institute Inc., Cary, NC, USA
Service PM, Hutchinson MD, MacKinley MD, Rose MR (1985) Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol Zool 58:380–389. https://doi.org/10.1086/physzool.58.4.30156013
Stillwell RC, Fox CW (2005) Complex patterns of phenotypic plasticity: interactive effect of temperature during rearing and oviposition. Ecology 86:924–934. https://doi.org/10.1890/04-0547
Stillwell RC, Moya-Laraňo J, Fox CW (2008) Selection does not favor larger body size at lower temperature in a seed-feeding beetle. Evolution 62:2534–2544. https://doi.org/10.1111/j.1558-5646.2008.00467.x
Tseng M, Kaur KM, Pari SS, Sarai K, Chan D, Yao CH, Porto P, Toor A, Toor HS, Fograscher K (2018) Decreases in beetle body size linked to climate change and warning temperatures. J Anim Ecol 87:647–659. https://doi.org/10.1111/1365-2656.12789
Yanagi S, Miyatake T (2002) Effects of maternal age on reproductive traits and fitness components of the offspring in the bruchid beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Physiol Entomol 27:261–266. https://doi.org/10.1046/j.1365-3032.2002.00294.x
Yanagi S, Miyatake T (2003) Costs of mating and egg production in female Callosobruchus chinensis. J Insect Physiol 49:823–827. https://doi.org/10.1016/S0022-1910(03)00119-7
Acknowledgements
We thank Dr. Shin-ichi Yanagi, Dr. Takuro Oikawa, and Mr. Kazuma Kuroda for helpful advices. This work was mainly supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Grants, KAKENHI 17H05976, and 18H02510 to T.M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Terada, K., Matsumura, K. & Miyatake, T. Effects of temperature during successive generations on life-history traits in a seed beetle Callosobruchus chinensis (Chrysomelidae: Coleoptera). Appl Entomol Zool 54, 459–464 (2019). https://doi.org/10.1007/s13355-019-00643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00643-z