Abstract
The fruit-piercing moth Oraesia excavata (Butler) only attacks ripe as opposed to immature peach fruits. The moth putatively uses volatile emissions from ripe peach fruits as a cue for food orientation. The volatiles emitted by ‘Hakutou’ peach (Prunus persica L.) fruit during maturation were analyzed by headspace solid-phase microextraction followed by gas chromatography and mass spectrometry. Although seven compounds increased remarkably during maturation, among them, only ethyl acetate and ethyl butanoate elicited electroantennographic responses. During a field-trap test, traps baited with both compounds captured moths, but significantly fewer moths were captured by the traps as compared to peach fruit. However, traps baited with a mixture of both compounds and lactones peculiar to ripe peach fruits captured more moths than did traps with ripe peach fruits only. The results of a seasonal survey using this mixture as an attractant suggest that seasonal forecasting of the fruit-piercing moth is possible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many phytophagous insects use odors as cues for orientation to their food resources. Attraction of the insects to their host plants is guided mainly by volatile phytochemicals, which are perceived by chemoreceptor neurons on the antenna (Van Loon 1996). However, knowledge of the mechanisms that guide insects to their host in the field remains limited.
Fruit-piercing moths are distributed worldwide (Muniappan et al. 1995). In Japan, Oraesia excavata (Butler), O. emarginata (Fabricius), and Adris tyrannus amurensis (Staudinger) are the main species that attack ripe fruits, including peaches, oranges, grapes, and pears (Nomura 1962; Omori and Mori 1962; Uchida et al. 1978). Fruit-piercing moths are known to be attracted to various fruit odors (Kohno 1962; Miyazaki et al. 1972; Saito and Munakata 1970; Uchida et al. 1978), in particular, among fruits of various kinds, the moths are strongly attracted to peach fruit (Uchida et al. 1978) and are most often captured in peach orchards (Park et al. 1988). In an earlier study, we clarified the olfactory responses of adult O. excavata males and females to lactones as specific components of ripe peach fruit aromas using electroantennographic (EAG) techniques on moths trapped in the field (Tian et al. 2008). The EAG response to 4-hexanolide, which was present at the highest concentration among the six lactones tested, was not as strong as the response to a mixture of five lactones when 10 % concentrations (v/v) of the lactones were used. In the field-trap test, a mixture of five lactones attracted the moths, but each lactone alone was insufficient to attract the moth. Nevertheless, the number of moths captured using the mixture was almost half that captured using ripe peach fruits. These results suggest that, although they are important as attractants in ripe peaches, other compounds are necessary to attract O. excavata. Actually, peach fruit volatiles include many compounds other than lactones (Derail et al. 1999; Horvat et al. 1990; Jennings and Sevenants 1964; Spencer et al. 1978). The compounds with increased expression in peach fruit during maturation are clearly important for attracting fruit-piercing moths because the moths attack only ripe fruit.
We studied the responses of O. excavata to volatile compounds released by ripe peach fruit. Specifically, we identified peach fruit headspace volatiles during maturation and examined the antennal responses to individual compounds and mixtures using EAG techniques and trap-capturing in the field.
Materials and methods
Insects
The O. excavata larvae were given an artificial diet composed mainly of dried host plant (Cocculus trilobus DC) leaf powder, bean powder, wheat germ, and dried brewer’s yeast at 25 °C under a photoperiod of 16 h light and 8 h day (Ohmasa et al. 1991). Pupae were sexed and placed in screen cages (30 × 30 × 30 cm) until adult emergence. Adult moths were kept in the same screen cages and provided with a whole apple fruit as food until use. The antennae of adult females and males 2–4 days after eclosion were used for the EAG experiments.
Plants and collection of headspace volatile compounds
The ‘Hakutou’ peach fruits (Prunus persica L.) were divided into three stages depending on the degree of maturation. Fruits containing less than 13 % sugar content were classified as immature; those with more than 13 % were classified as ripe. Moreover, ripe fruits kept at room temperature for 3 days were classified as overmature.
A solid-phase microextraction (SPME) triple-phase (divinylbenzene/carboxen/polydimethylsiloxane, 50/30 μm in thickness, 2 cm in length, Sigma-Aldrich Co., St. Louis, Mo, USA) StableFlex fiber was employed for the extraction of volatiles from peach fruit. Each whole fruit (approx. 250 g) was placed in a 500-mL Pyrex® glass jar, covered with aluminum foil, and equilibrated for 30 min at room temperature. The SPME fiber was inserted through the foil and exposed for 30 min. The collected volatiles were then analyzed by gas chromatography and mass spectrometry (GC/MS). The fiber was placed into the injection port of the GC/MS equipment and thermally desorbed for 10 min at 250 °C.
Volatile compound analyses
GC/MS analyses were performed on an Agilent 6890GC/5975MSD (Agilent Technologies, Palo Alto, CA, USA) with a fused silica DB-WAX capillary column (60 m × 0.25 mm, 0.25-μm film thickness, Agilent Technologies). The flow of the helium carrier gas was 1.6 mL/min. The oven temperature was programmed as 50 °C for the first 2 min, followed by an increase of 3 °C/min to 220 °C, then held at 220 °C for 20 min. The injection port, equipped with a 0.75-mm-internal-diameter liner (Sigma-Aldrich Co.), was maintained at 250 °C. The inlet was operated in the splitless mode, and the injection purge on the GC system was turned off for the first 1 min to allow for manual SPME injection. Volatile compounds were identified by comparison of their mass spectra and Kováts retention indices (RIs) with that of an authentic reference standard. When standards were not available, identifications were assigned on the basis of mass spectra and retention indices reported in the Wiley 7th/NIST05 (John Wiley, NY, USA), the NIST Chemistry WebBook (NIST 2011), and VCF Volatile Compounds in Food online database (Nijssen 1963–2014). Each analysis was performed with three repetitions.
Authentic chemicals
All standard compounds were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and Sigma-Aldrich Co. The following chemical purities were (Z)-3-hexenol, 98 %; ethyl acetate, 99.5 %; propyl acetate, 98 %; ethyl butanoate, 98 %; ethyl hexanoate, 99 %; 3-hydroxy-2-butanone, 95 %; ethyl octanoate, 98 %; 4-hexanolide, 98 %; 4-octanolide, 97 %; 4-decanolide, 96 %; 5-decanolide, 97 %; and 4-dodecanolide, 95 %. Paraffin oil and ethanol were obtained from Nacalai Tesque, Inc. (Kyoto, Japan).
EAG recordings
EAG was measured using the methods of Tian et al. (2008). An antenna was excised at its base and mounted between two metal electrodes using electrically conductive gel (Spectra® 360; Parker Laboratories, Inc., Fairfield, New Jersey 07004, USA). The EAG analyses were performed using a 16-bit analog to digital converter (IDAC-2; Syntech®, Germany) and a PC running EAG ver. 2.7 software (Syntech®). A green leaf volatile compound, (Z)-3-hexenol (1 % v/v), was used as the reference compound for normalization of all responses relative to the responses to the reference compound (100 %). For all experimental procedures, an interstimulus interval of 60–120 s was maintained between successive stimulations. Each antenna was used only once for each compound. Each chemical and the respective mixtures were dissolved in paraffin oil at concentrations of 1 % (v/v). One microliter of the solution was applied to a piece of filter paper (Toyo Roshi Kaisha, Ltd., Japan), which was then inserted into a glass Pasteur pipette. The same amount of paraffin oil was used as a blank control. The tip of the pipette was inserted into the side hole of the mixing tube, where continuous charcoal-filtered airflow (1.2 l/min) was blown through onto the antenna. Using a stimulus controller (Type CS-55, Syntech®), a 0.5-s puff of air (0.6 l/min) was injected through the pipette to simulate an antenna positioned 20 mm from the tube outlet.
Field-trap test
The field-trap test was conducted in areas surrounding peach orchards on a hill and in the peach orchards in Tamashima in Kurashiki city, Japan (34.3°N, 133.4°E). Nine plastic funnel traps (UNITRAP; Sankei Chemicals Co. Ltd., Japan), which had been baited with ripe peach fruit and 0.5 ml of 59.6 % (v/v) ethyl acetate, 0.2 % (v/v) ethyl butanoate, a five-lactone mixture, two-combination mixtures, a mixture of all compounds (the mixture ratio is shown in Table 1), and paraffin oil as a control contained in glass vials (0.8 mm dia. × 40 mm ht.), were hung on branches 1–1.5 m above the ground and at a distance of about 15 m (Tian et al. 2007). Three sets of these nine funnel traps were prepared for this experiment. The glass vial was placed in the bottom of the funnel trap. The ripe peach fruit was replaced three times per week. All chemical compounds were changed weekly. The hanging locations of the traps were randomly changed weekly. Traps were checked three times per week. After the number of captured moths was recorded, moths were removed from the traps. The field test was conducted during 1–31 August 2007 and during 1–31 July 2010.
Seasonal survey
A seasonal survey was conducted in the surroundings of peach and orange orchards in Matsuyama city, Japan (33.8°N, 132.8°E). A glass vial with 1 ml of a mixture of all compounds (the mixture ratio is shown in Table 1) was placed in each plastic funnel trap as bait. The traps were hung from tree branches at about 1–1.5 m above the ground around the peach orchard. Three traps were prepared for this experiment. The traps were set up initially in late May 2008 and were maintained until the end of October 2008. The traps were checked and the bait was changed on a weekly basis.
Data analysis
Normalized EAG responses (%) were analyzed using ANOVA after square root transformation of the data. The transformed values were compared using Tukey’s method when ANOVA indicated significance at the 5 % level. The quantities of moths captured by the traps on each collection day were analyzed using ANOVA with factors of the day and test samples (ripe peach fruit, ethyl acetate, ethyl butanoate, five-lactone mixture, two-combination mixtures, a mixture of all compounds, and paraffin oil as the control), followed by Bonferroni’s test.
Results
Peach fruit volatile compounds
In all, 76 compounds of peach fruits that were tested were identified using GC/MS in this study (Table 2). In ripe peach fruits, volatile compounds were found to be more than 0.1 %; relative levels of seven compounds (ethyl acetate, ethanol, propyl acetate, ethyl butanoate, ethyl hexanoate, 3-hydroxy-2-butanone, ethyl octanoate) increased more than five times compared with those in immature fruits. In overmature fruits, the respective ratios of ethyl acetate and ethanol increased. These comprised 90 % of all detected compounds (Table 2).
EAG responses
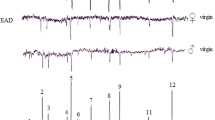
The electroantennogram responses elicited by paraffin oil were 0.88 ± 0.07 mV in females and 0.71 ± 0.06 mV in males (n = 10). The EAG amplitudes in response to (the standard stimulus) 1 μl of 1 % (Z)-3-hexenol were 2.96 ± 0.21 mV (mean ± SE, n = 10) and 2.70 ± 0.15 mV (mean ± SE, n = 10), respectively, on female and male antennae. The EAG responses to the seven compounds (ethyl acetate, ethanol, propyl acetate, ethyl butyrate, ethyl hexanoate, acetoin, and ethyl octanoate) were investigated; of these, only EAG responses to ethyl acetate and ethyl butanoate were stronger than those to paraffin oil (data not shown). Moreover, the EAG response to ethyl butanoate was stronger than that to ethyl acetate (Fig. 1). No significant difference was found in EAG response to ethyl butanoate and ethyl acetate between males and females.
EAG responses (mean ± SE) of O. excavata (n = 10) to 1 μl of ethyl acetate and ethyl butyrate at concentrations of 1 % (v/v) and paraffin oil. The EAG responses were normalized to 1 μl of 1 % (v/v) (Z)-3-hexenol. Different lowercase letters indicate significant differences (p < 0.05) with Tukey’s test after ANOVA. No significant difference was observed between males and females
Field-trap test
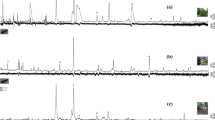
In the field-trap test conducted in August 2007, ethyl acetate, ethyl butanoate, the five-lactone mixture, and each two-combination mixture and a mixture of all compounds were used for bait. A trap baited with ripe peach fruit was also used. Traps baited with ethyl acetate and ethyl butanoate captured O. excavata moths, but fewer moths were captured by those traps than by those with ripe peach fruit (Fig. 2). All two-combination mixtures caught moths. The trap baited with a mixture of ethyl acetate and lactones captured almost as many moths as the ripe fruit. The trap baited with the mixture of all compounds captured the most moths of all traps tested. In the field-trap test conducted in July 2010, the trap baited with the mixture of all compounds captured many more moths than the trap baited with peach fruit before harvest week in the peach orchard. During the harvest week, moths were rarely captured by traps baited with the mixture of all compounds. On the other hand, moths were captured by the traps baited with peach fruit during this same week (Fig. 3).
Number of O. excavata moths captured by individual funnel traps baited with ripe peach fruit, ethyl acetate (ethyl-a), ethyl butanoate (ethyl-b), five-lactone mixture (lactones), two-combination mixtures, and a mixture of all compounds. Each value represents mean ± SE. Different lowercase letters indicate significant differences (p < 0.05) according to Bonferroni’s test after ANOVA
Seasonal survey
A seasonal survey was conducted from late May to the end of October 2008. In this experiment, the mixture of all compounds was used as the peach fruit volatile component for bait. Moths were first captured in mid-June. The first small peak was detected at the end of June (Fig. 4). The number of moths captured by the traps increased during the last 10 days of July and in the first 10 days of August with a large peak. Moths were captured continually until mid-October.
Discussion
Many studies of the attraction of adult moths by volatile emissions from a host plant have been related to the ovipositional selection of adult females (Fraser et al. 2003; Hern and Dorn 2001; Hern and Dorn 2004; Landolt and Guedot 2008; Natale et al. 2004; Pivnick et al. 1994). Research related to the food search behavior of adult moths that attack host plants directly, as fruit-piercing moths do, is limited. In the present study, we identified the headspace volatiles in ripe peach fruit, measured EAG responses by O. excavata moths to some of these volatile compounds, and verified our findings by field-trapping with these volatiles and mixtures for bait. Moths showed EAG responses to ethyl acetate and ethyl butanoate. They were captured by the trap baited with ethyl acetate and ethyl butanoate. The moths were also captured by the trap baited with a mixture of five lactones, but not by traps with individual lactones, although an EAG response to the individual lactones was shown (Tian et al. 2008). Moreover, much ethyl acetate and ethyl butanoate are contained in the aroma volatiles of the Asian pear cultivar ‘Kousui’ (Pyrus pyrifolia var. culta ‘Kousui’), which is attacked by O. excavata moths similarly to ripe peach fruits (data not shown). Another species of fruit-piercing moth, Adris tyrannus amurensis, which attacks ripe peach fruit, was captured using traps baited with the mixture of ethyl acetate and ethyl butanoate (data not shown). These results indicate that ethyl acetate and ethyl butanoate are more important than individual lactones for the fruit-piercing moth’s food search.
In a previous study, moths were captured using traps baited with a mixture of five lactones, but significantly fewer moths were captured than with ripe peach fruit (Tian et al. 2008). In the present study, when traps baited with the mixture of ethyl acetate, ethyl butanoate, and five lactones were used, about twice the number of moths were captured than when using ripe peach fruit. The mixture is useful year round as an attractant in place of ripe peach fruit. Actually, seasonal survey results obtained in the present study were comparable to those obtained using a trap baited with apple (Ogihara 1997). These results show that the seasonal forecasting of fruit-piercing moths can be performed using this mixture as an attractant. Consequently, it is useful for control of the fruit-piecing moth (e.g., decision at lamplight time of moth-repellent yellow lamp). However, at the time of the seasonal survey in 2008, few moths had been captured by the traps in mid-July. This was peach harvesting time; many ripe peaches and many moths were observed inside the peach orchard (data not shown). Moreover, at the trap test in 2010, the moths were rarely captured by traps baited with the mixture of all compounds inside the peach orchard during the harvesting season. On the other hand, many moths had been captured by the traps baited with ripe peach fruit during this same period. These results suggest that minor compounds in ripe peach volatiles participate in the final step of food orientation by O. excavata. In addition, in the present study, we did not perform a quantitative analysis of the volatile compounds. Therefore, the component ratio of the mixture of all compounds is slightly different from that in a peach fruit because of the characteristics of SPME fibers. Moreover, some lactones have stereoisomers and the ratio of these isomers differs depending on the types of lactone in the peach (Bernreuther et al. 1989; Werkhoff et al. 1993). In the present study, however, we could not determine the isomeric ratio. Therefore, the attraction effect may be improved by using the isomeric mixture whose ratio is similar to that of the natural product. To address these issues, we are planning to perform a quantitative analysis of peach volatile compounds.
The results of the present and previous studies suggest that lactones play an important role in the search for food by O. excavata. Although these lactones are peculiar to ripe peach fruits, they are not present in Asian pears, which are attacked by O. excavata. Moreover, the moths were captured by traps baited with ethyl acetate and ethyl butanoate only outside the orchard, but not inside the orchard. These results also indicate that the respective roles of ethyl acetate, ethyl butanoate, and lactones in the search for food by O. excavata differ. We are currently planning to investigate differences in the behavioral responses of O. excavata to each compound further using Y-tube olfactometers and wind-tunnel testing.
References
Bernreuther A, Christoph N, Schreier P (1989) Determination of the enantiomeric composition of γ-lactones in complex natural matrices using multidimensional capillary gas chromatography. J Chromatogr 481:363–367
Derail C, Hofmann T, Schieberle P (1999) Differences in key odorants of handmade juice of yellow-flesh peaches (Prunus persica L.) induced by the workup procedure. J Agric Food Chem 47:4742–4745
Fraser AM, Mechaber WL, Hildebrand JG (2003) Electroantennographic and behavioral responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J Chem Ecol 29:1813–1833
Hern A, Dorn S (2001) Induced emissions of apple fruit volatiles by the codling moth: changing patterns with different time periods after infestation and different larval instars. Phytochemistry 57:409–416
Hern A, Dorn S (2004) A female-specific attractant for the codling moth, Cydia pomonella, from apple fruit volatiles. Naturwissenschaften 91:77–80
Horvat RJ, Chapman GW Jr, Robertson JA, Meredith FI, Scorza R, Callahan AM, Morgens P (1990) Comparison of the volatile compounds from several commercial peach cultivars. J Agric Food Chem 38:234–237
Jennings WG, Sevenants MR (1964) Volatile components of peach. J Food Sci 29:796–801
Kohno M (1962) Studies of the bionomics of Adris tyrannus Guenée and control of fruit-piercing moths. In: Research on the control of fruit-piercing moths. Japan Plant Protection Association, Tokyo, pp 81–90 (in Japanese with English summary)
Landolt PJ, Guedot C (2008) Field attraction of codling moths (Lepidoptera: Tortricidae) to apple and pear fruit, and quantitation of kairomones from attractive fruit. Ann Entomol Soc Am 101:675–681
Miyazaki A, Honda H, Saito T, Munakata K (1972) Studies of the attractants of fruit-piercing moths. Jpn J Appl Entomol Zool 16:40–43 (in Japanese with English summary)
Muniappan R, Silva-Krott IU, Lali TS (1995) Distribution of larval host plants of the fruit piercing moth, Othreis fullonia. Chemoecology 5(6):75–77
Natale D, Mattiacci L, Pasqualini E, Dorn S (2004) Apple and peach fruit volatiles and the apple constituent butyl hexanoate attract female oriental fruit moth, Cydia molesta. Lab J Appl Entomol 128:22–27
Nijssen LM, Ingen-Visscher CA, van Donders JJH (1963–2014) VCF Volatile Compounds in Food online database ver. 15.2. http://www.vcf-online.nl. Accessed 15 Dec 2014
NIST Mass Spec Data Center (2011) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. http://webbook.nist.gov. Accessed 15 Dec 2014
Nomura K (1962) Ecological survey on fruit-piercing moths in Japan, with special references to their distribution, general bionomics and injury. In: Research on the control of fruit-piercing moths. Japan Plant Protection Association, Tokyo, pp 19–35 (in Japanese with English summary)
Ogihara H (1997) Bionomics, ecology and control methods of fruit-piercing moths, Oraesia emarginata Fabricus and Oraesia excavata Butler (Lepidoptera: Noctuidae). In: Bulletin of Ehime fruit tree experiment station. Ehime Fruit Tree Experiment Station, Ehime, pp 58–88 (in Japanese with English summary)
Ohmasa Y, Wakamura S, Kozai S, Sugie H, Horiike M, Hirano C, Mori S (1991) Sex pheromone of the fruit-piercing moth, Oraesia excavata (Butler) (Lepidoptera: Noctuidae): isolation and identification. Appl Entomol Zool 26:55–62
Omori H, Mori S (1962) Research on the control of fruit-piercing moths. In: Research on the control of fruit-piercing moths. Japan Plant Protection Association, Tokyo, pp 65–80 (in Japanese with English summary)
Park CG, Shin WK, Kim IG, Kim CH (1988) Fruit piercing moths collected at an orchard surrounded by forest in Gyeongnam province. Korean J Appl Entomol 27:111–116
Pivnick KA, Jarvis BJ, Slater GP (1994) Identification of olfactory cues used in host-plant finding by diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J Chem Ecol 20:1407–1427
Saito T, Munakata K (1970) Insect attractants of vegetable origin, with special reference to the rice stem borer and fruit-piercing moths. In: Wood DL, Silverstein RM, Nakajima M (eds) Control of insect behavior by natural products. Academic, New York, pp 225–236
Spencer MD, Pangborn RM, Jennings WG (1978) Gas chromatographic and sensory analysis of volatiles from cling peaches. J Agric Food Chem 26:725–732
Tian R, Izumi Y, Sonoda S, Yoshida H, Fukumoto T, Saito T, Tsumuki H (2007) Estimation of repellency of a volatile compound, sec-butyl β-styryl ketone, against fruit-piercing moths. Appl Entomol Zool 42:433–437
Tian R, Izumi Y, Sonoda S, Yoshida H, Takanashi T, Nakamuta K, Tsumuki H (2008) Electroantennographic responses and field attraction to peach fruit odors in the fruit-piercing moth, Oraesia excavata (Butler) (Lepidoptera: Noctuidae). Appl Entomol Zool 43:265–269
Uchida M, Fukuta H, Udagawa H (1978) Biology and control of fruit-piercing moths in pear orchards. Bull Tottori Tree Fruit Exp Stn 8:1–29 (in Japanese with English summary)
Van Loon JJA (1996) Chemosensory basis of feeding and oviposition behaviour in herbivorous insects: a glance at the periphery. Entomol Exp Appl 80:7–13
Werkhoff P, Brennecke S, Bretschneider W, Güntert M, Hopp R, Surburg H (1993) Chirospecific analysis in essential oil, fragrance and flavor research. Z Lebensm Unters For 196:307–328
Acknowledgments
This study was supported in part by a Grant-in-Aid for a Research Project utilizing Advanced Technologies in Agriculture, Forestry and Fisheries and by a grant from the Ohara Foundation for Agricultural Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izumi, Y., Tian, R., Sonoda, S. et al. Analysis of peach fruit headspace volatiles and response by the fruit-piercing moth Oraesia excavata (Lepidoptera: Noctuidae). Appl Entomol Zool 50, 231–238 (2015). https://doi.org/10.1007/s13355-015-0330-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-015-0330-2