Abstract
Almost like a living being in and of itself, tumors actively interact with and modify their environment to escape immune responses. Owing to the pre-formation of cancer-favorable microenvironment prior to anti-cancer treatment, the numerous attempts that followed propose limited efficacy in oncology. Immunogenicity by activation of immune cells within the tumor microenvironment or recruitment of immune cells from nearby lymph nodes is quickly offset as the immunosuppressive environment, rapidly converting immunogenic cells into immune suppressive cells, overriding the immune system. Tumor cells, as well as regulatory cells, namely M2 macrophages, Treg cells, and MDSCs, derived by the immunosuppressive environment, also cloak from potential anti-tumoral factors by directly or indirectly secreting cytokines, such as IL-10 and TGF-β, related to immune regulation. Enzymes and other metabolic or angiogenetic constituents — VEGF, IDO1, and iNOS — are also employed directed for anti-cancer immune cell malfunctioning. Therefore, the conversion of “cold” immunosuppressive environment into “hot” immune responsive environment is of paramount importance, bestowing the advances in the field of cancer immunotherapy the opportunity to wholly fulfill its intended purpose. This paper reviews the mechanisms by which tumors wield to exercise immune suppression and the nanoengineered delivery strategies being developed to overcome this suppression.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor development involves a chaotic interplay between cancer and immune cells, virtually uncountable complex cross-talks between the two to tip over the intricate balance between immune suppression and activation [1, 2]. Immune cells such as cytotoxic T lymphocytes (CTL), dendritic cells (DC), and natural killer (NK) cells attempt to eliminate cancer cells by various mechanisms such as perforin and granzyme secretion, secretion of proinflammatory cytokines such as interferon (IFN)-γ, or recruitment of more T lymphocytes to the tumor microenvironment (TME) through production of chemokines such as chemokine (CXC motif) ligand (CXCL)12 [3,4,5]. Nevertheless, cancer cells possess their own means of immune evasion. Inhibitory molecules such as programmed death-ligand 1 (PD-L1) deactivate T cells [6,7,8]. Cancer cells too are capable of secreting cytokines such as transforming growth factor β (TGF-β) themselves, thereby recruiting regulatory cells to maintain immunosuppression within the TME [4, 9]. However, the scale favors tumor cells, for immune cells’ activities are limited by time. May it be the case that immune cells fail to assassinate tumors acutely, chronic inflammation lingers behind, fortifying immunosuppression within the microenvironment, fostering tumor development, and advancing to malignant metastasis [10,11,12,13].
Various attempts targeting different suppressive factors that contribute to TME’s immune suppression have shown auspicious results. Repolarization of immunosuppressive M2 macrophages and myeloid-derived suppressor cells (MDSCs) into proinflammatory M1 macrophages proves to be not only effective in hot tumor conversion by itself, but also synergetic when combined with other therapeutic agents [14,15,16,17,18], while depletion of regulatory T cells (Treg cells) has also received spotlight for its potency [19, 20]. Vanquishing suppressive cytokines play a crucial role in deterring the conversion of anti-tumor cells into suppressive cells or hindering the recruitment of regulatory cells [21, 22]. Other factors include angiogenesis prompters for tumor growth, such as vascular endothelial growth factor (VEGF), and metabolic features that tumor cells employ to establish cancer-favorable environments, such as indoleamine 2,3-dioxygenase (IDO)1 and iNOS/arginase 1 [23,24,25,26,27,28,29,30,31].
The use of nanotechnology provides many advantages in designing drugs for such cancer immunotherapy. By engineering molecules to be released slowly, or to be activated only under unique circumstances, nanoengineered drugs allow reduced toxicity, while also maintaining drugs at an effective dose level in targeted sites. Since nanotechnology deals with engineering at molecular levels, it opens doors for enhancements in numerous ways. Typically, functionalization of nanoparticles is performed by modifying particle surface: conjugating various drugs, layering multiple surfaces, or modulating surface charges. Furthermore, formulating particles in nanometer sizes itself is an advantage. Nanoparticle displays much higher delivery efficacy, as it can be more easily taken up by cells; therefore, a lower EC50 value can be obtained owing to facilitated cell internalization, which ultimately means that nanoengineered drugs propose drugs of not only more efficient, but also safer.
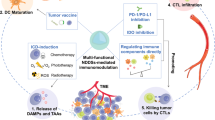
Therefore, switching the immune battlefield by converting “cold” immunosuppressive TME into “hot” immune responsive territory is a promising strategy in overcoming the current limitations of cancer immunotherapy [32,33,34] (Fig. 1). Of the numerous routes immunotherapy can take to enhance anti-tumoral efficacy, this review has focused specifically on nanoengineering-based drug delivery systems that does so by overcoming immunosuppression within the TME.
Schematic illustration of nanoengineered strategies for overcoming immunosuppression within TME. Mainstream methods, as illustrated, include repolarizing or depleting suppressive cells (i.e., M2 macrophage, MDSC, and Treg cell), or inhibiting suppressive factors (e.g., ARG1, IL-10, VEGF). Such mechanisms enhance tumor immunogenicity, expediting successful immunotherapy
Nanoengineered drug delivery for targeting cell-mediated immunosuppression in TME
Macrophages
Macrophages are one of the representative figures when it comes to innate immunity. As the name implies, their main function is to respond to pathogens, dead cells, and various debris by phagocytosis. They also partake in adaptive immunity through cytokine secretion and surface interaction [35]. Cytokines secreted by M1 macrophages, such as interleukin (IL)-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α, differentiate and proliferate various immune cells in a proinflammatory fashion [36]. Major histocompatibility complex (MHC) II and T cell receptor (TCR) interaction [37], accompanied by cluster of differentiation (CD)86-CD28 interaction between macrophages and T cells, results in T cell activation [38]. Nonetheless, their participation can easily be neglected through direct cell–cell contact mechanisms in anti-tumor activity. The interaction between CD47 expressed on tumor cells and signal regulatory protein (SIRP)-α expressed on macrophages delivers the so-called don’t eat me signal, by which tumor cells escape macrophages’ immunosurveillance [39]. In addition, macrophages are double-edged swords when it comes to immune regulation; IL-10 and TGF-β can reshape M1 into M2 macrophages, which in turn can also be released from M2 macrophages themselves, leading to immune suppression and tumor growth. Thus, redirecting M2 macrophages within the TME is salient in subduing its immune suppression [40].
Polarization (naïve macrophages into M1 or M2) and repolarization (M2 macrophages into M1) of macrophages were significantly affected via the introduction of TLR7/8 agonists (TLR7/8a), known to stimulate NF-κB. Wei et al. have harmonized bacterial therapy, M2 repolarization therapy, and immunogenic cell death (ICD) therapy into a single treatment, demonstrating synergy between all three factors. Resiquimod (R848) and doxorubicin (DOX) were separately encapsulated in poly(lactide-co-glycolide) (PLGA) particles, formulated via nanoengineering procedures involving emulsion solvent evaporation method, forming PR848 and PDOX. PR848 attached to the Escherichia coli (E. coli) surface through electrostatic interaction, and the conjugated result was co-treated with PDOX in mice bearing 4T1 tumor cells (Fig. 2A). The combination showed remarkable performance compared to the adjuvant, drug, or bacteria-only groups. An M1/M2 ratio of 1.34 was recorded when cancer cells were treated with R848 conjugated to E. coli, and this was further improved to 1.59 through the involvement of PDOX (Fig. 2B, C). Establishing a proinflammatory environment is crucial for M1 polarization [41]. On that account, pre-constructing such macrophage-exclusive microenvironment through cellular “backpacking” and adoptively transferring modified macrophage to TME has emerged as a novel mechanism of macrophage-mediated suppression reversal. The retarded release of cytokines constantly surrounds engineered macrophages, facilitating M1 retention among tumor cells and consequently promoting M2 repolarization [42]. Shields et al. found that the attachment of IFN-γ onto macrophages retains the M1 phenotype. Phenotypic comparisons were made between IFN-γ “backpacked” M1 macrophages and blank backpacked M1 macrophages that have been incubated under tumor mimicking conditions. After 48 h, MHC II expression in the IFN-γ backpacked group was 6.3 folds higher compared to the control group, whereas it was only 1.4 folds higher in the blank backpacked group. A massive 629.3-fold higher expression was seen, while only a 2.4-fold higher level was seen with inducible nitric oxide synthase (iNOS). When analyzed after 5 days, iNOS levels decreased by 89.1% in macrophages with blank backpacks, while a 59.1% decrease was observed in IFN-γ-backpacked macrophages. MHC II and CD80 expression was decreased by 30.1% and 37.6%, respectively, in the blank backpacked group, while a 95.7% and 248.4% increase was observed in the IFN-γ backpacked group. Decisively, the tendencies exhibited no major difference in standard culture conditions, suggesting compelling M1 retention and repolarization capacity of engineered macrophage adoptive cell transfer [43].
M1-M2 conversion efficacy of nanoparticle/bacteria complex in tumor regression. A Schematic illustration of enhanced immunotherapeutic efficacy via M2 macrophage repolarization into M1. B Macrophage repolarization effect tested in vitro. Decrease of M2 macrophages (F4/80+ CD206+), along with increase of M1 macrophages (F4/80+ CD80+) shows successful repolarization as percentage. C M1, M2, and M1/M2 ratio in tumor microenvironment analyzed show a significant increase in anti-tumor M1 macrophage and decrease in M2 macrophage. The figures were adapted with permission from Wei et al. [41]
Regulatory T cells (Treg cells)
Treg cells are essential in dictating balanced immune reactions, playing a key role in suppressing autoimmune diseases [44]. This very functionality is exploited by tumor cells, overriding Th1 responses through Treg cell interactions, manipulated to greatly contribute to TME’s immunosuppression [45]. Thus, depletion of Treg cells from suppressive environments has been acknowledged as an eloquent mechanism through which cancer can be treated. For target specificity is of utmost importance in Treg cell depletion, monoclonal antibodies (mAbs) targeting CD25 expressed on Treg cells are used.
Antibody–drug conjugate (ADC) is an emerging technique involving high level nanoengineering. Consisting of antibody as the delivery vehicle, molecules to be delivered are grafted onto the carrier through nanoscale engineering, forming a target-specific drug delivery system. For the case of Treg cell depletion, cytotoxic warhead is attached to CD25 antibodies [46]. While the warheads lead to cell death, CD25 binding cripples IL-2 starving immunosuppression mechanism of Treg cells; consequently, not only are suppressive factors removed, an opportunity for improved immune activation solely through Treg cell depletion can also be expected [47]. Zammarchi et al. explored the possibility of conjugating SG3199 warheads to an anti-CD25 mAb (Fig. 3A). The average drug-to-antibody ratio (DAR) of 2.3 ADC brought about rapid anti-tumor response against the MC28 colon cancer model (Fig. 3B). Studies on its mode of action have reported that depletion of CD8+ cells annihilated anti-tumor activity (Fig. 3C), advocating CD8+ effector T (Teff) cell-dependent tumor killing prompted by Treg cell depletion. Conversion from “cold” to “hot” tumors was enhanced when combined with anti-PD-1 immune checkpoint blockade therapy (ICBT) as well (Fig. 3C) [48].
Effect of Treg cell depletion by nanoengineered ADC in cancer immunotherapy. A Schematic illustration of CD25 mAb antibody–drug conjugate (ADC). B Anti-tumor efficacy of Treg cell depletion through CD25-ADC shown by tumor regression volume. C Immune activation represented by elevated CD8+ population level, and immunosuppression within TME overcame as shown by greater CD8+/Treg cell ratio. ADC’s synergy with anti-PD-1 demonstrated as well. The figures were adapted with permission from Zammarchi et al. [48]
Myeloid-derived suppressor cells (MDSCs)
The means applied by MDSCs to prevent immunogenic event occurrence within the TME are applicable to most immune cells; however, as MDSCs primarily target T cells, inhibition of MDSC functioning can be pivotal in empowering Teff cells in suppressive environments [49]. Thus, extinguishing MDSCs from the TME can dispatch cancer cells by simultaneously reducing suppression and enhancing activation. This cornerstone mechanism has fascinated many researchers to deplete MDSCs, achieving a milestone in the history of oncology [50]. A strategy proposed by Zhang et al. obliterates MDSCs through nanoprodrug embodying two different oncolytic agents. Through combination of photosensitizer (PS) indocyanine green (ICG) and ferric ions (Fe3+) in the presence of tadalafil (TAD), ICG and Fe3+ form a nanoparticle through self-assembly mechanism, around which TAD interacts to form a FIT nanoparticle (Fig. 4A). The particle is degraded through photothermal therapy at the tumor site with near-infrared (NIR). Disintegration of FIT nanoparticles liberates ICG, which performs photodynamic therapy (PDT), generating antigens for T cell activation. TAD release would lead to alleviation of MDSCs, resulting in lowered immunosuppression together with enhanced CTL response (Fig. 4A). When administered intravenously, this system has successfully led to tumor regression with minimum side effects as shown by tumor volume and body weight change (Fig. 4B). Significant decrease of MDSCs was observed in tumor site, along with increase of mature DCs (Fig. 4C). ARG1, a crucial factor for T cell exhaustion, was shown to be dramatically decreased (Fig. 4C); thus, it is deduced that with reduced MDSC and Arg1, along with increased mature DC in the tumor, T cell activation must have been increased, proven by anti-tumor efficacy [51].
Enhanced anti-tumor efficacy via MDSC reduction by FIT nanoparticle prodrug. A Schematic illustration of FIT nanoprodrug. B Anti-tumor efficacy and low toxicity demonstrated by tumor volume regression and consistent body weight. C MDSC and mature DC population within tumor site analyzed by flow cytometry show reduced MDSC population, as well as increased DC population. Immunohistochemistry shows significant reduction of Arg-1 level, indicating improved T cell activation via FIT nanoprodrug treatment. The figures were adapted with permission from Zhang et al. [51]
Nanoengineered drug delivery for modulating immunosuppressive cytokines in TME
Interleukin (IL)-10
IL-10, one of the key immunosuppressive cytokines. is involved in numerous tumor-favorable activities [52, 53]. Its activities include polarizing macrophages into M2 types, directing CD4+ cells to Treg cell differentiation, and downregulating antigen presentation by DCs, all of which are important in strengthening the tumor environment [54,55,56].
One mechanism through which IL-10 levels are abated is the introduction of an artificially generated IL-10 receptor (IL-10R) into the TME, which is often called the “IL-10 trap.” Nanoscale modifications at genetic level to cells on the TME circumference are implemented. Silva et al. intentionally mutated genes in muscle cells surrounding the tumor by injecting a plasmid vector encoding IL-10R (pIL-10R). The plasmid vector was co-administered with human papillomavirus (HPV-16) E7 oncoprotein fused with glycoprotein D of a herpes simplex virus (HSV) (pgDE7H)-encoded DNA vaccine. The genetic material was injected intramuscularly and uptaken by tibialis anterior muscle cells near the tumor tissue, leading to secretion of unbound IL-10R in cells neighboring the tumor. The reinforcement of the IL-10 trap from nearby cells significantly reduced free IL-10 within the tumor-friendly environment, mitigating the possibility of its interaction with naïve or immunogenic cells and, thus, empowering them to differentiate and function as anti-tumor effector cells [57]. While administration of pgDE7H induced higher CD8+ T cell levels, as well as IFN-γ levels, leading to tumor regression at early stages of cancer, its effect was dwindled at more advanced stages [58]. However, synergy with pIL-10R exceeded such limitations, displaying increased survival rates and various anti-tumor effects in advanced stages. Similarly, Shen et al. developed a system that synergizes with IL-10 trap introduction into the TME. In their study, IL-10R encoding genes were delivered intravenously within a liposome-protamine-DNA (LPD) nanoparticle (NP) platform formulated through nanoengineered thin-film technique (Fig. 5A). The IL-10 trap formulation’s anti-tumor efficacy was amplified by loading CXCR12 receptor (CXCR12R) encoding genes together with IL-10R encoding genes, for CXCR12 is a well-known chemokine whose function is to hamper T cell infiltration into the TME. Notable curtailment of immunosuppressive cells, which in return surged pro-inflammation through the activation of cytotoxic T lymphocytes (CTLs), activated DCs, and NK cells (Fig. 5B). Additionally, mRNA expression of IL-10 was reduced after treatment of LPD NP in TME of 4T1 tumor-bearing mouse model (Fig. 5C). The results showed higher efficacy when IL-10R encoding genes were delivered along with CXCR12R encoding genes, proving synergetic with CXCR12R encoding genes (Fig. 5D) [59].
Anti-tumor efficacy of pIL-10R as IL-10 blockade. A Scheme of LPD NP encapsulated IL10 trap and CXCL 12 trap. B Increased activated innate immune cells (DC, NK) after treatment of combination blockades. C mRNA expression of IL-10 was reduced after LPD NP treatment at TME in 4T1 model. D Tumor regression and enhanced survival rate after treatment of blockade combination. The figures were adapted with permission from Shen et al. [59]
Transforming growth factor (TGF)-β
TGF-β is a master coordinator of immunity. Despite the common rationale that TGF-β is an immune-inhibiting cytokine, it is also necessary for immune activation [60]. Nevertheless, their drawbacks outweigh these benefits. TGF-β has a general inhibitory effect on the development and function of most other cells, such as DCs, NK cells, and macrophages [60]. For example, TGF-β signaling in NK cells reduces IFN-γ production, as well as T-box expression in T cell (T-bet) regulation, ultimately leading to Th1 activity inhibition.
Frustrating TGF-β production by tumors can be detrimental for tumor growth. Inhibiting the TGF-β receptor has boosted chemodrug’s potency as exhibited by Cai et al. In this research, molecular modifications at nanoscale have been conducted to thiolate TGF-β antibodies in order to transform it into CuS attachable form. The final product used in this research was TGF-β attached CuS particle, which encapsulates ataxia telangiectasia mutated (ATM) inhibitor as anti-tumor drug (Fig. 6A). This particle, when administered intravenously, was accumulated in the TME, resulting in anti-tumor response shown by proinflammatory effector cell populations as the parameter. As shown by Fig. 6B, CD3+CD4+ and CD3+CD8+ population has significantly increased, notably in the group containing TGF-β antibody attached to the NP’s surface, leading to remarkable tumor regression (Fig. 6C) [61]. Zhou et al. have also demonstrated the importance of TGF-β blockade in cancer immunotherapy, as in a hydro-xyethyl starch-polylactide (HES-PLA) nanoparticle containing DOX and TGF-β receptor inhibitor (Fig. 6D). There are limitations to the drug delivery efficiency of LY2157299, TGF-β receptor 1 inhibitor (LY). LY is a hydrophobic small molecule that is administered via organic solvent and self-aggregates after injection. As a result, the hydrophobic LY becomes toxic and its distribution is hindered. For overcoming, they use strategies that deliver both anti-cancer drug (DOX) and LY synchronously to in vivo using polymeric nanoparticles. It has significantly dropped TGF-β level in mouse serum after DOX/LY@HES-PLA NP treatment, which is in contrast with DOX + LY group (Fig. 6E). Eventually, DOX/LY@HES-PLA NP treatment leads up to regression of 4T1 tumor model in mice (Fig. 6F) [62].
Anti-tumor efficacy of nanoparticle encapsulated TGF-β inhibitor, TGF-β antibody, and LY2157299. A Scheme of the preparation of ATM inhibitor-loaded and anti-TGF-β-modified CuS NPs for low-temperature PTT in hepatocellular carcinoma model. B Increasing percentage of CD3+CD4+ and CD3+CD8+ cells of tumor-bearing mice in CuS-ATM@anti-TGF-β-treated groups measured by flow cytometry. C Regression of tumor size in hepatoma model (H22 cell line) after CuS-ATM@anti-TGF-β treatment. D Schematic preparation process of DOX/LY@HES-PLA. E Decreasing of TGF-β expression in serum by DOX/LY@HES-PLA NP treatment. F Regression of 4T1 tumor growth after DOX/LY@HES-PLA NP treatment. The figures were adapted with permission from Cai et al. [61] and Zhou et al. [62]
Nanoengineered drug delivery for modulating VEGF and immunosuppressive metabolic byproducts in TME
Vascular endothelial growth factor (VEGF)
Cancer arises from mutations in genes instructing the cell growth. Mutations in cell cycle checkpoints permit uncontrolled cell growth and division, and immature cells do not mature into differentiated cells with specific roles, thus ever growing and ever dividing. Originating from normal cells, cancer cells have the same requirements as healthy cells: oxygen, minerals, and vital substances. Nonetheless, as cancer grows at an unprecedented rate, the regular supply of such substances is inadequate to keep up with cancer cell growth [63,64,65]. To secure supplies for the unmanageable growth, cancer cells secrete signaling proteins for the construction of new blood vessels [66, 67]. VEGF is a signaling molecule that is secreted to build new blood vessels in a process termed angiogenesis.
Confronting such hurdles placed on effective cancer immunotherapy methods, Zhao et al. used VEGF as the target to be modulated. Poly[bis(ε-Lys- polyethylenimine)Glut-polyethylene glycol] (PLEGP) was nanoengineered at optimized polymer: PEI ratio, and synthesized into siRNA/PLEGP nanocomplex, which was employed as the vehicle for VEGF siRNA delivery to triple negative breast cancer (TNBC) (Fig. 7A). siRNA attached onto the linear polymer PLEGP formed a low-molecular-weight nanocomplex, favorable for delivery under high pressure without inducing cytotoxicity. Delivery of VEGF siRNA into the TME silenced the VEGF gene in VEGF-producing cells and significantly reduced VEGF levels within the TME by 72% (Fig. 7B, C). Ultimately, suffocating the TME through reduced angiogenesis by reducing VEGF secretion was an efficient tool against cancer, resulting in reduced tumor volume (Fig. 7D) [68].
Anti-tumor activity by VEGF siRNA-loaded PLEGP nanoparticle. A Synthesis reaction of PLEGP and production of the VEGF siRNA/PLEGP nanocomplex. B VEGF silencing activity of nanocomplexes in MDA-MB-231 cells (HER2+ human breast cancer cells). C Mean fluorescence intensity of the z-stacked confocal images by the distance from the periphery of the spheroids. D Relative tumor volume of orthotopically implanted MDA-MB-231 cells. VEGF siRNA/PLEGP1800 nanocomplex showed significant inhibition of tumor growth. The figures were adapted with permission from Zhao et al. [68]
Indoleamine 2,3-dioxygenase (IDO)1
Tryptophan is an essential amino acid that is metabolized to melatonin and serotonin [69]. Serotonin serves an immunoregulatory role by activating various immune cells during inflammation via binding to serotonin receptors. This can threaten the survival of cancer cells [70, 71]. To fight against their extinction, cancer cells induce IDO production within the TME. IDO1 catalyzes the breakdown of tryptophan, transforming tryptophan into kynurenines. This starves immune cells, especially T cells, of tryptophan, dramatizing immunosuppression [72,73,74,75].
Ameliorated anti-tumor activity stemming from IDO1 inhibition can be achieved through TLR7/8 agonist and anti-cancer drug combinations [23, 25]. Jin et al. assembled paclitaxel (PTX), R848, and epacadostat (EPT), each functioning as an ICD mediator, adjuvant, and IDO1 inhibitor, into oil-in-water (O/W) nano-emulsions respectively, and combination at optimized ratios of each showed remarkable anti-tumoral effect (Fig. 8A, B). This system, coined AIMS (assemblable immune modulating suspension), forms an in situ depot when administered intratumorally. The activated DCs in the TME migrated to nearby tumor-draining lymph nodes (TDLNs), granting more options for tumor killing by differentiating, proliferating, and recruiting CTLs (Fig. 8A). Consequently, not only was this method effective in local tumor eradication, but also robust in distant tumors, as well as in confining metastases (Fig. 8C, D). AIMS has also been demonstrated to be compatible for combination with ICBT and superior anti-tumor results were obtained when synergized with anti-PD-L1 mAb (Fig. 8E) [76].
Anti-tumor efficacy of the nano emulsion AIMS system. A Schematic representation of the AIMS system and function, incorporating ICD mediator, adjuvant, and IDO1 inhibitor. B IDO1 activity of AIMS (EPT, R848) in TME and TDLN. C Tumor weight in local and distant site. D Number of metastatic lung nodules corresponding to lung metastasis. E Tumor volume and survival rate in AIMS (EPT, R848, PTX) group and effectiveness of combination with ICBT. The figures were adapted with permission from Jin et al. [76]
Arginase (ARG)1/inducible nitric oxide synthase (iNOS)
L-Arginine is a critical regulator of lymphocyte function, essential for T cell survival and proliferation; its absence results in anergy of effector T cells and suppression of NK cells [77]. ARG1 metabolizes l-arginine to l-ornithine and urea. Hence, its abundance within the TME directly correlates with the l-arginine famine, thereby obstructing lymphocyte proliferation and function [13, 28, 78, 79]. Pharmacological inhibition of ARG1 could facilitate the replenishment and recovery of effector immune cells within a tumor-friendly environment, capsizing it into a tumor-eradicating environment [28, 80,81,82,83,84].
To topple the suppressive environment, Grzybowski et al. introduced OATD-02, a boronic acid derivative which functions as ARG1 inhibitor, to the CT26 tumor model. As shown by Fig. 9A, OATD-02 has proven its anti-tumor efficacy at results even better than the reference inhibitor group, showing higher levels of arginine within the TME, leading to greater tumor regression. When compared to other renowned oncologic drugs, such as EPT and anti-PD-L1, OATD-02 proved to be either comparable, or showed superior tumor regression results (Fig. 9B). Furthermore, combination of OATD-02 with EPA and anti-PD-L1 showed synergetic results (Fig. 9B). After testing OATD-02 in Renca model, cellular analysis has shown the drug’s capability of overcoming immunosuppression, for anti-inflammatory cells such as MDSC and Treg cell has decreased in population size, resulting in high CD8+/Treg cell ratio (Fig. 9C). For such promising results, OATD-02 is expected to go on to clinical trials in the short future [85].
Anti-tumor efficacy of boronic acid-based ARG1 inhibitor, OATD-02. A OATD-02 inhibited tumor growth in size, as well as maintained arginine level with the TME. B Tumor regression levels compared with other renowned anti-tumoral drugs have shown OATD-02’s superior capacity, and potential for combinational therapy. C OATD-02’s arginase inhibition led to downsized immunosuppressive cell population, leading to higher CD8+/Treg ratio, creating a cancer-hostile environment. The figures were adapted with permission from Grzybowski et al. [85]
Along with arginase metabolism, l-arginine is involved in NO and NOS. NOS catalyzes the conversion of l-arginine to l-citrulline and NO [13, 86]. NO, a lipophilic gas molecule, mediates several biological functions, among which anti-inflammatory effects are prominent [78]. A specific isoform of NOS, iNOS, is expressed by various inflammatory cells and generates high amounts of NO for a longer period than neuronal NOS or endothelial NOS [29]. The relationship between NOS, NO, and tumor progression is rather complicated, for the NOS and NO level is a tipped balance which can determine pro-tumor or anti-tumor character of TME [31]. Therefore, the concentration and duration of residual NO, differences in types of tumors, and balance between NOS and ARG1 all mediate tumorigenesis [30, 78].
Conclusion
Conventional chemotherapies are performed with the hope that each session would kill more tumor cells than the host’s red or white blood cells, and cumulatively would free the host from the cancer’s grasp [87]. Although it is a gamble the patients take to overcome the disease, severe side effects are a certainty [88]. Cancer immunotherapy aims to rescue patients from the losing game that they are forced to play. It stimulates the host’s own immunity so that anti-tumor activities occur in a much more specific, efficient, and safe manner [89].
A major hurdle in the efficacy of cancer immunotherapy is that cancer cells actively construct a tumor-friendly environment for their survival [90]. Therefore, reverting such suppressive factors is of chief eminence in escalating oncological immunotherapeutic potency. M2 macrophages, Treg cells, and MDSCs are momentous figures in discussing immunosuppressive cells in the TME. Anti-inflammatory cytokine secretion by these cells saturates the TME with suppressive proteins; therefore, a reduction in the population of these cells is a necessity in overcoming TME immune suppression [91]. Depleting these cells through nanoengineered antibodies and surface-modified nanoparticles has been proven to be efficient, as shown in various studies, including those mentioned above. Not only removal can be done, but conversion of M2 macrophages into M1 types by hydrogel containing TLR7/8a with nanoscale modulation for controlled release, or cell surface engineering via backpacking IFN-γ onto M1 macrophages has been shown to further enhance therapeutic efficacy, as reduction of M2 and expansion of M1 occurs simultaneously [92].
The secreted products themselves can be preyed upon to deny them from exerting their effects. Research on the inhibition of IL-10 and TGF-β has shown promising results. Artificial “IL-10 traps” created by IL-10 receptor encoding genes delivered via nanoparticles to function as nanoengineered “IL-10 sink” are interposed within the TME, pocketing IL-10 instead of effector cells and allowing effector cells to retain their purpose [57]. TGF-β can be blocked using TGF-β inhibitor or monoclonal antibody within immunosuppressive or tumor cells directly, and their extermination results in more vigorous activation of CD8+ T cells, leading to shrinkage in tumor volume [61, 62].
Other angiogenic and metabolic factors such as VEGF, IDO1, and ARG1 are potential candidates for abatement to decorate anti-tumor efficacy through immune stimulation [68, 76, 85]. Downregulation of VEGF using nanoparticles encapsulating VEGF silencing siRNA shackles angiogenesis, which is indispensable for tumor growth. Without the support of vascular generation and growth, tumors are deprived of essential nutrients and eventually starve to death [68]. Blocking IDO1 enzymes with nano-emulsions containing epacadostat (EPT) prior to their interaction with tryptophan is another means of targeting metabolic byproducts. This paves way for production of serotonin, which in turn sets the ground for more fervent immune activation to take place [76].
As mentioned, immunotherapy has made its entrance to oncology with the promise of not simply relieving patients from conventional chemotherapy’s excruciating side effects, but also of improved efficacy in both therapy and relapse prevention [87,88,89]. However, current immunotherapy applied in oncology is dulled by the TME’s suppressive mechanisms through cells, cytokines, angiogenetic factors, or other metabolic factors; therefore, it is not sufficiently potent to substitute current chemotherapy [93]. Nevertheless, it is not entirely disappointed. Progressions are being made with advancements in nanotechnology, as nanoengineered drug delivery systems are showing promising results in terms of both efficiency and safety, earning its deserved attention as a necessity in taking oncology to the next level. Although current nanoengineered drugs remain to be used in combination with conventional chemotherapy, promising enough results that nanoengineered drugs will completely replace current chemotherapy in near future. To achieve so, combining the means by which tumor immunosuppressive activities are inhibited by other immune activation or tumor eradication methods lies at the center of importance for improving the anti-tumor performance of the current clinical state of the art.
Availability of data and materials
On request.
References
Turan T, Kongpachith S, Halliwill K, Roelands J, Hendrickx W, Marincola FM, et al. A balance score between immune stimulatory and suppressive microenvironments identifies mediators of tumour immunity and predicts pan-cancer survival. Br J Cancer. Springer US; 2021;124:760–9. Available from: https://doi.org/10.1038/s41416-020-01145-4.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–84.
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. Springer US 2021;6. Available from: https://doi.org/10.1038/s41392-021-00658-5.
Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. Springer US 2019;120:6–15. Available from: https://doi.org/10.1038/s41416-018-0328-y.
Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res Biomarker Research. 2020;8:1–16.
Schnell A, Bod L, Madi A, Kuchroo VK. The yin and yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res. Springer US 2020;30:285–99. Available from: https://doi.org/10.1038/s41422-020-0277-x.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. Springer US 2021;21:345–59. Available from: https://doi.org/10.1038/s41568-021-00347-z.
Cao Y, Wang X, Jin T, Tian Y, Dai C, Widarma C, et al. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct Target Ther. Springer US 2020;5. Available from: https://doi.org/10.1038/s41392-020-00348-8.
Kim R, Emi M, Tanabe K, Arihiro K. Tumor-Driven Evolution of Immunosuppressive Networks during Malignant Progression. Cancer Res. 2006;66:5527–36.
Rogovskii V. Modulation of Inflammation-Induced Tolerance in Cancer. Front Immunol. 2020;11:1–5.
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. Elsevier Ltd 2015;35:S185–98. Available from: https://doi.org/10.1016/j.semcancer.2015.03.004.
Welton RS, Blackman LR. Suicide and the air force mental health provider: Frequency and impact. Mil Med. 2006;171:844–8.
Guerrouahen BS, Maccalli C, Cugno C, Rutella S, Akporiaye ET. Reverting Immune Suppression to Enhance Cancer Immunotherapy. Front Oncol. 2020;9.
Zheng Y, Han Y, Sun Q, Li Z. Harnessing anti-tumor and tumor-tropism functions of macrophages via nanotechnology for tumor immunotherapy. Exploration. 2022;2:20210166.
Kimm MA, Klenk C, Alunni-Fabbroni M, Kästle S, Stechele M, Ricke J, et al. Tumor-Associated Macrophages—Implications for Molecular Oncology and Imaging. Biomedicines. 2021;9:1–20.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11.
Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. BioMed Central 2022;41:1–39. Available from: https://doi.org/10.1186/s13046-022-02272-x.
Luo W, Napoleon JV, Zhang F, Lee YG, Wang B, Putt KS, et al. Repolarization of Tumor-Infiltrating Myeloid Cells for Augmentation of CAR T Cell Therapies. Front Immunol. 2022;13:1–13.
Zhou X, Tang J, Cao H, Fan H, Li B. Tissue resident regulatory T cells: Novel therapeutic targets for human disease. Cell Mol Immunol. 2015;12:543–52.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res Nature Publishing Group. 2017;27:109–18.
Mirlekar B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022;10:205031212110690.
Qiao J, Fu YX. Cytokines that target immune killer cells against tumors. Cell Mol Immunol. Springer US 2020;17:722–7. Available from: https://doi.org/10.1038/s41423-020-0481-0.
Ito H, Ando T, Arioka Y, Saito K, Seishima M. Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumour effects of a Toll-like receptor 7 agonist in an established cancer model. Immunology. 2015;144:621–30.
Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Sosman JA, et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front Immunol. 2020;11:1–15.
Huang L, Xu H, Peng G. TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol Immunol. Nature Publishing Group 2018;15:428–37. Available from: https://doi.org/10.1038/cmi.2018.4.
Dai L, Yao M, Fu Z, Li X, Zheng X, Meng S, et al. Multifunctional metal-organic framework-based nanoreactor for starvation/oxidation improved indoleamine 2,3-dioxygenase-blockade tumor immunotherapy. Nat Commun. Springer US 2022;13:1–17.
Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. Springer US 2020;52:1475–85. Available from: https://doi.org/10.1038/s12276-020-00500-y.
Czystowska-Kuzmicz M, Sosnowska A, Nowis D, Ramji K, Szajnik M, Chlebowska-Tuz J, et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat Commun. 2019;10:1–16.
Xue Q, Yan Y, Zhang R, Xiong H. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci. 2018;19.
Clemente GS, van Waarde A, Antunes IF, Dömling A, Elsinga PH. Arginase as a potential biomarker of disease progression: A molecular imaging perspective. Int J Mol Sci. 2020;21:1–36.
Basudhar D, Bharadwaj G, Somasundaram V, Cheng RYS, Ridnour LA, Fujita M, et al. Understanding the tumour micro-environment communication network from an NOS2/COX2 perspective. Br J Pharmacol. 2019;176:155–76.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. Springer US 2019;18:197–218. Available from: https://doi.org/10.1038/s41573-018-0007-y.
Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5265–86.
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: A therapeutic challenge for immunotherapy. Front Immunol. 2019;10:1–10.
Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2018;19.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol Nature Publishing Group. 2010;11:889–96.
Underhill DM, Bassetti M, Rudensky A, Aderem A. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999;190:1909–14.
Long KB, Beatty GL. Harnessing the antitumor potential of macrophages for cancer immunotherapy. Oncoimmunology. 2013;2:1–9.
Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6.
Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. Springer US 2021;6:1–21.
Wei B, Pan J, Yuan R, Shao B, Wang Y, Guo X, et al. Polarization of Tumor-Associated Macrophages by Nanoparticle-Loaded Escherichia coli Combined with Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2021;21:4231–40.
Aminin D, Wang YM. Macrophages as a “weapon” in anticancer cellular immunotherapy. Kaohsiung J Med Sci. 2021;37:749–58.
Wyatt Shields C, Evans MA, Wang LLW, Baugh N, Iyer S, Wu D, et al. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6:1–12.
Mikami N, Kawakami R, Sakaguchi S. New Treg cell-based therapies of autoimmune diseases: towards antigen-specific immune suppression. Curr Opin Immunol Elsevier Ltd. 2020;67:36–41.
Kim JH, Kim BS, Lee SK. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. 2020;20:1–17.
Dees S, Ganesan R, Singh S, Grewal IS. Regulatory T cell targeting in cancer: Emerging strategies in immunotherapy. Eur J Immunol. 2021;51:280–91.
Solomon I, Amann M, Goubier A, Arce Vargas F, Zervas D, Qing C, et al. CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. Springer US 2020;1:1153–66. Available from: https://doi.org/10.1038/s43018-020-00133-0.
Zammarchi F, Havenith K, Bertelli F, Vijayakrishnan B, Chivers S, van Berkel PH. CD25-targeted antibody-drug conjugate depletes regulatory T cells and eliminates established syngeneic tumors via antitumor immunity. J Immunother cancer. 2020;8:1–13.
Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front Immunol. 2020;11:1–22.
Raskov H, Orhan A, Gaggar S, Gögenur I. Neutrophils and polymorphonuclear myeloid-derived suppressor cells: an emerging battleground in cancer therapy. Oncogenesis. Springer US 2022;11:1–16.
Zhang T, Xiong H, Ma X, Gao Y, Xue P, Kang Y, et al. Supramolecular Tadalafil Nanovaccine for Cancer Immunotherapy by Alleviating Myeloid-Derived Suppressor Cells and Heightening Immunogenicity. Small Methods. 2021;5:1–14.
Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. Elsevier Inc 2010;140:883–99.
Stanilov N, Miteva L, Deliysky T, Jovchev J, Stanilova S. Advanced Colorectal Cancer Is Associated With Enhanced IL-23 and IL-10 Serum Levels. Lab Med. 2010;41:159–63.
Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, et al. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J Immunol. 2015;195:3665–74.
Kim R, Emi M, Tanabe K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol Ther. 2005;4:924–33.
De Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med Informa Healthcare. 1995;27:537–41.
Marchi LHL, Paschoalin T, Travassos LR, Rodrigues EG. Gene therapy with interleukin-10 receptor and interleukin-12 induces a protective interferon-γ-dependent response against B16F10-Nex2 melanoma. Cancer Gene Ther Nature Publishing Group. 2010;18:110–22.
Silva JR, Sales NS, Silva MO, Aps LRMM, Moreno ACR, Rodrigues EG, et al. Expression of a soluble IL-10 receptor enhances the therapeutic effects of a papillomavirus-associated antitumor vaccine in a murine model. Cancer Immunol Immunother. Springer Berlin Heidelberg 2019;68:753–63.
Shen L, Li J, Liu Q, Song W, Zhang X, Tiruthani K, et al. Local Blockade of Interleukin 10 and C-X-C Motif Chemokine Ligand 12 with Nano-Delivery Promotes Antitumor Response in Murine Cancers. ACS Nano. 2018;12:9830–41.
Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFβ. Nat Rev Immunol Nature Publishing Group. 2010;10:554–67.
Cai H, Dai X, Guo X, Zhang L, Cao K, Yan F, et al. Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. Acta Biomater. Elsevier Ltd 2021;127:276–86. Available from: https://doi.org/10.1016/j.actbio.2021.03.051.
Zhou Q, Li Y, Zhu Y, Yu C, Jia H, Bao B, et al. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J Control Release. Elsevier 2018;275:67–77. Available from: https://doi.org/10.1016/j.jconrel.2018.02.026.
Brücher BLDM, Jamall IS. Epistemology of the origin of cancer: a new paradigm. BMC Cancer. 2014;14:1–15.
Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22.
Collins K, Jacks T, Pavletich NP. The cell cycle and cancer. Proc Natl Acad Sci USA. 1997;94:2776–8.
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–9.
Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–56.
Zhao Z, Li Y, Shukla R, Liu H, Jain A, Barve A, et al. Development of a Biocompatible Copolymer Nanocomplex to Deliver VEGF siRNA for Triple Negative Breast Cancer. Theranostics. 2019;9:4508–24.
Friedman M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int J Tryptophan Res. 2018;11.
Szabo A, Gogolak P, Koncz G, Foldvari Z, Pazmandi K, Miltner N, et al. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci Rep. Springer US 2018;8:1–12. Available from: https://doi.org/10.1038/s41598-018-20173-y.
Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med. 2017;4:1–11.
Sun B, Hyun H, Li L tao, Wang AZ. Harnessing nanomedicine to overcome the immunosuppressive tumor microenvironment. Acta Pharmacol Sin. Springer US 2020;41:970–85. Available from: https://doi.org/10.1038/s41401-020-0424-4.
Holmgaard RB, Zamarin D, Munn DH, Merghoub T, Wolchok JD. Expression of indoleamine 2.3-dioxygenase by tumors induces local and systemic immunosuppressive effects in a murine melanoma model. J Immunother Cancer. 2014;2.
Meireson A, Devos M, Brochez L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front Immunol. 2020;11:1–17.
Silk JD, Lakhal S, Laynes R, Vallius L, Karydis I, Marcea C, et al. IDO Induces Expression of a Novel Tryptophan Transporter in Mouse and Human Tumor Cells. J Immunol. 2011;187:1617–25.
Jin SM, Lee SN, Kim JE, Yoo YJ, Song C, Shin HS, et al. Overcoming Chemoimmunotherapy-Induced Immunosuppression by Assemblable and Depot Forming Immune Modulating Nanosuspension. Adv Sci. 2021;8:1–15.
Grobben Y, Uitdehaag JCM, Willemsen-Seegers N, Tabak WWA, de Man J, Buijsman RC, et al. Structural insights into human Arginase-1 pH dependence and its inhibition by the small molecule inhibitor CB-1158. J Struct Biol X. Elsevier 2020;4:100014. Available from: https://doi.org/10.1016/j.yjsbx.2019.100014.
Niu F, Yu Y, Li Z, Ren Y, Li Z, Ye Q, et al. Arginase: An emerging and promising therapeutic target for cancer treatment. Biomed Pharmacother. 2022;149.
Munder M. Arginase : an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–51.
Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5:1–18.
Lu M, Zhang H, Li D, Childers M, Pu Q, Palte RL, et al. Structure-Based Discovery of Proline-Derived Arginase Inhibitors with Improved Oral Bioavailability for Immuno-Oncology. ACS Med Chem Lett. 2021;12:1380–8.
Li YN, Wang ZW, Li F, Zhou LH, Jiang YS, Yu Y, et al. Inhibition of myeloid-derived suppressor cell arginase-1 production enhances T-cell-based immunotherapy against Cryptococcus neoformans infection. Nat Commun. Springer US 2022;13:4074. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35835754%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC9283461.
Sosnowska A, Chlebowska-Tuz J, Matryba P, Pilch Z, Greig A, Wolny A, et al. Inhibition of arginase modulates T-cell response in the tumor microenvironment of lung carcinoma. Oncoimmunology. Taylor & Francis 2021;10:1–15. Available from: https://doi.org/10.1080/2162402X.2021.1956143.
Vonwirth V, Bülbül Y, Werner A, Echchannaoui H, Windschmitt J, Habermeier A, et al. Inhibition of Arginase 1 Liberates Potent T Cell Immunostimulatory Activity of Human Neutrophil Granulocytes. Front Immunol. 2021;11:1–16.
Grzybowski MM, Stańczak PS, Pomper P, Błaszczyk R, Borek B, Gzik A, et al. OATD-02 Validates the Benefits of Pharmacological Inhibition of Arginase 1 and 2 in Cancer. Cancers (Basel). 2022;14:1–17.
Vanini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–43.
Moorthi C, Manavalan R, Kathiresan K. Nanotherapeutics to Overcome Conventional Cancer Chemotherapy Limitations. J Pharm Pharm Sci. 2011;14:67–77.
Nurgali K, Jagoe RT, Abalo R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front Pharmacol. 2018;9:1–3.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. Springer US 2019;18:175–96.
Sadeghi Rad H, Monkman J, Warkiani ME, Ladwa R, O’Byrne K, Rezaei N, et al. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev. 2021;41:1474–98.
Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–15.
Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22.
Lee S, Margolin K. Cytokines in Cancer Immunotherapy. 2011;3856–93.
Acknowledgements
The authors are thankful to National Research Foundation (NRF) of Korea government for the financial support (grant numbers 2020R1A2C3006888 and SRC-2017R1A5A1014560).
Funding
This work was supported by National Research Foundation (NRF) grants funded by the Korean government (grant numbers 2020R1A2C3006888 and SRC-2017R1A5A1014560).
Author information
Authors and Affiliations
Contributions
All authors contributed to conceptualization of the work. Sei Hyun Park contributed to idea design, Ryounho Eun contributed to figure design, Janghun Heo wrote the draft of the manuscript, and Yong Taik Lim critically revised the draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is a review article and does not involve a research protocol or animal and human studies requiring approval by ethics committee or the relevant institutional review board.
Consent for publication
All the authors reviewed and approved the manuscript of this review paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, S.H., Eun, R., Heo, J. et al. Nanoengineered drug delivery in cancer immunotherapy for overcoming immunosuppressive tumor microenvironment. Drug Deliv. and Transl. Res. 13, 2015–2031 (2023). https://doi.org/10.1007/s13346-022-01282-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01282-8