Abstract
To date, promising therapy for triple negative breast cancer (TNBC) remains a serious concern clinically because of poor prognosis, resistance, and recurrence. Herein, anti-cancer potential of synthetic cannabidiol (CBD; Purisys, GA; GMP grade) was explored either alone or as a chemosensitizer followed by post-treatment with doxorubicin (DOX) in TNBC (i.e., MDA-MB-231 and MDA-MB-468) cells. In comparison to 2D cultures, CBD showed greater IC50 values in 3D (LDP2 hydrogel based) cultures of MDA-MB-231 (6.26-fold higher) and MDA-MB-468 (10.22-fold higher) cells. Next-generation RNA sequencing revealed GADD45A, GADD45G, FASN, LOX, and integrin (i.e., -α5, -β5) genes to be novelly altered by CBD in MDA-MB-231 cells. CIM-16 plate-based migration assay and western blotting disclosed that CBD induces anti-migratory effects in TNBC cells by decreasing fibronectin, vimentin, and integrins-α5, -β5, and -β1. Western blotting, RT-qPCR, and immunocytochemistry revealed that CBD inhibited autophagy (decreased Beclin1, and ATG-5, -7, and -16) of TNBC cells. CBD pre-treatment increased DOX sensitivity in TNBC cells. CBD pre-treatment accompanied by DOX treatment decreased LOX and integrin-α5, and increased caspase 9 protein respectively in MDA-MB-468 cells.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) predominantly contributes to mortality in women worldwide [1]. Approximately 15–20% of all diagnosed BC cases are of triple-negative breast cancer (TNBC) subtype [2]. Neoadjuvant therapy followed by surgery is considered the preferred approach for the therapy of early stage TNBC [3]. Nevertheless, therapy of TNBC still remains as a concern due to its intractable qualities, unreliable molecular targets, and development of multidrug resistance [4].
Medical marijuana is used as a supplement for the symptomatic treatment of cancers [5]. The majority of the varieties of marijuana contains 60–66 cannabinoids, of which tetrahydrocannabinol (THC) and cannabidiol (CBD) are responsible for its pharmacological effects in various conditions [6, 7]. Cannabinoids (Δ9-THC and CBD) mediate chemotherapeutic effects by modulating multiple cancer signalling pathways [8, 9]. In glioma and breast cancer models, Id1 inhibition attributes to anti-metastatic effects of CBD [10].
Doxorubicin (DOX), a potent anthracycline derivative, is effective in TNBC by inhibiting the topoisomerase II enzyme and causing DNA damage [11]. However, altered levels/function/expression of ATP-binding cassette transporters, EMT, topoisomerase II enzyme, glutathione S-transferase, and autophagy regulate cancer cells’ resistance to DOX [12,13,14].
Chemosensitization by using natural products improve the sensitivity, decrease the side effects and also delay the resistance of chemotherapeutics in cancer cells [15]. In TNBC, noscapine increased docetaxel sensitivity [16]. In cancer, CBD induces cytotoxic effects at higher doses but it acts as a chemosensitizer at lower doses [17]. CBD showed synergistic chemotherapeutic effects with temozolomide/doxorubicin/carmustine in glioblastoma [18]. Recently, CBD microparticles in combination with paclitaxel/DOX showed synergistic effects in breast cancer and TNBC cells [19].
To date, this is the first study which evaluates the pharmacological effects of CBD in 3D spheroid cultures of TNBC cells and organoid cultures, identifies the novel potential targets affected by CBD treatment in TNBC through performing next-generation RNA sequencing and molecular biology studies, and also depicts the possible molecular mechanisms responsible for CBD-induced improved sensitization of DOX.
Materials and methods
In vitro culture
TNBC and immortalized non-tumorigenic MCF-10A cells were procured from ATCC (Rockville, MD, USA). Culturing and maintenance of these cells were carried out as mentioned [20]. TheWell Biosciences (NJ, USA) company has generously provided us the supplies (i.e., Type 1 dilution and VitroGel LDP2 hydrogel) required for performing 3D culture studies. FBS was received from the Thomas Scientific Company. DMEM/Ham’s F12 medium supplemented with antibiotics (i.e., penicillin (5000 units), streptomycin (5 mg), and neomycin (10 mg/mL); GIBCO) and FBS (10%) was used for culturing MDA-MB-468 cells. CBD (GLP and GMP grade) and DOX were procured from Purisys (Athens, GA) and AK Scientific (Union City, CA) respectively.
Cell viability assays
Cells (TNBC and MCF-10A) at a density of 5000 cells were added into each well of a 96-well tissue culture-treated plates and cytotoxic effects were evaluated for CBD and DOX (i.e., 100 nM to 10 µM) for 48 h. For chemosensitivity studies in 2D cultures, we have pre-treated TNBC cells with CBD (1 and 2.5 µM) for 24 h. After the removal of drug-containing media, the respective CBD pre-treated cells were treated with DOX in various concentrations (i.e., 0.39 to 25 µM) for 48 h. The same procedure was followed for performing chemosensitivity studies in 3D cultures, wherein we have pre-treated TNBC cells with CBD (5 µM; 24 h) and then treated with multiple concentrations of DOX (i.e., 5 to 100 µM; 48 h). For combination studies, fixed concentrations of CBD (1 µM and 2.5 µM) and different concentrations of doxorubicin (i.e., 0.39 to 25 µM; 48 h) together are used for treating TNBC cells. CompuSyn software (version 1.0, ComboSyn, Inc., www.combosyn.com) is used for determining the combination index (CI) values as per the earlier reports [21, 22]. CBD and DOX cytotoxic effects in 3D cultured TNBC cells were investigated as earlier reported by using VitroGel LDP2 hydrogel [23]. MTT assay was used for assessing cell viability [24].
Extraction of cells from 3D culture
Cell-embedded 3D hydrogels were washed thrice with DPBS solution and transferred into individual 15-mL centrifuge tubes according to the treatment and control groups. The cell-embedded hydrogels were dissolved thoroughly by using warm (37 °C) VitroGel cell recovery solution (The Well Bioscience, USA), filtered by 70-µm filter and then centrifugation (i.e., 2000 rpm for 10 min) was performed. Supernatant was removed and proteins were isolated from the pellet.
Immunoblotting
Isolation of whole cell lysates followed by western blotting was done through the previously published procedures [23, 25]. All the western blots were cut before probing the membrane with primary antibodies diluted in PBST (1:1000). Antibodies used were GADD45-α (Catalog no: 4632S), β-actin (Catalog no: 4970S), Phospho-p38 (Catalog no: 9211S), p38 (Catalog no: 9212S), p53 (Catalog no: 9282S), ATG-5,7,16 (Catalog no: 12994 T, 8558 T, 8089 T), Beclin-1 (Catalog no: 3495S), LC3AB-1/2 (Catalog no: 4599S), Integrin β-1,5 (Catalog no: 34971S, 3629 T), Integrin α-5 (Catalog no: 4705 T), and LOX (Catalog no: 58135S). Blots were washed thrice (5 min each) with PBST, followed by probing with respective secondary antibodies (HRP-conjugated) as mentioned elsewhere [23]. Antibodies were obtained from Cell Signalling Technology, USA. Chemidoc Instrument (Bio-Rad) was used for capturing images. ImageJ software (1.43u) was utilized for performing densitometric analyses of western blots. Band intensities of the respective protein in untreated and treated groups were initially measured and were compared to β-actin. Protein expression was represented as a percent increase/decrease relative to the control group values. Original uncropped blot images have been included in supplementary data.
Flow cytometry
CBD effect on cell cycle was evaluated as described earlier [13]. Quantification was carried out using Flow Jo 7.6.1 software (Ashland, OR) [26, 27].
Isolation of RNA, cDNA synthesis, and RT-qPCR
RNA is extracted through TRIzol reagent (Invitrogen, CA, USA) and purified by RNeasy Mini kit. For synthesizing cDNA, we used first-strand cDNA Synthesis Kit (Catalog no: K1651). BECN1 (qHsaCID0016032), GAPDH (qHsaCED0038674), CCND1 (qHsaCID0013833), GADD45G (qHsaCED0044300), GADD45A (qHsaCED0036441), FASN (qHsaCID0008460), and LOX (qHsaCED0043469) primers were procured from Bio-Rad. We used Bio-Rad’s SYBR green master mix (Catalog no: 1725270) and PCR Detection System (i.e., CFX96 touch; real-time) and SsoAdvanced Universal SYBR Green Supermix for performing quantitative PCR (i.e., estimating gene expression). Comparative Ct (ΔCt) method was used for determining relative mRNA expression [28]. 18S rRNA and GAPDH were considered reference genes. Fold change in the mRNA expression of respective genes was depicted in comparison to control group values.
TNBC tissue processing and organoid culture
Florida Agricultural and Mechanical University’s Animal Care and Use Committee had reviewed and approved our experiments on female athymic nude mice (Animal protocol no: 020–06; Foxn1nu; age: 3–5 weeks, body weight: 20–25 g, n = 3). Mice (Envigo) were acclimatized for 7–10 days with light and dark cycle (1:1 ratio). ARRIVE guidelines [29], NIH guidelines (Guide for the Care and Use of Laboratory Animals), and applicable national laws had been followed for performing experiments. MDA-MB-231 TNBC tumor model was developed as mentioned earlier [13]. After observing a tumor volume of 2000 mm3, tumors were collected from the mice. Tumor tissue was crushed and washed with DMEM: F12 medium (supplemented with antibiotics, Glutamax and HEPES), followed by digestion with breast cancer (BC) organoid medium containing collagenase (1–2 mg/mL) as mentioned [30]. In brief, cell pellet was dispersed in cold Cultrex growth factor reduced BME, Pathclear (10 mg/mL; R&D Systems; catalog no: 3433–005-01). After prewarming 24-well suspension culture plate, the cell suspended mixture (40 µL) was added in each well and allowed to solidify for 20–30 min at 37 °C. Later, BC organoid medium (400 µL) was supplemented in every well. Replenishing of the media was performed for every 3–4 days. Organoids were dissociated by using ice-cold DMEM:F12 medium through mechanical shearing or using TryPLE express (Invitrogen).
Next-generation sequencing and data analysis
Extraction of RNA was performed as described before for untreated and CBD treated MDA-MB-231 cells. DNase I was used for removing traces of genomic DNA. Preparation of mRNA library, determination of quality control (i.e., HS DNA chip; Agilent), and quantification of the libraries were carried out as mentioned [31]. Equimolar concentrations of the libraries were combined and proceeded for RNA sequencing (Illumina NovaSeq 6000 system; College of Medicine, Florida State University, Translational Laboratory). In our study, we did not use any positive controls for the RNA sequencing work. We had considered untreated MDA-MB-231 cells as control during comparison of gene changes in CBD-treated MDA-MB-231 cells.
NetworkAnalyst 3.0 was used for analyzing RNA-seq data as described [31, 32]. Genes having counts (< 10) and variance (< 10%) were only considered for analysis. DEseq2 identifies differentially expressed genes. NetworkAnalyst 3.0 also determines heat maps of globally differentially expressed genes and gene enrichment pathways. Volcano plot was generated from DESeq2 data as described [33].
Migration assay
Migration assay was carried out in MDA-MB-231 cells as mentioned [13, 34] by using CELLigence label-free RTCA (real-time cell analysis) DP instrument. Readings were taken every 10 min for a period of 40 h.
Immunocytochemistry
Immunocytochemistry was performed as per the earlier reports [35,36,37]. Briefly, glass cover slips were used for growing cells. TNBC cells were exposed to CBD for 48 h. Afterwards, 4% paraformaldehyde dispersed in PBS was used for fixing cells, and cells were washed thrice with PBST, permeabilized, and blocked. After washing thrice with PBST, probing with primary antibodies such as anti-rabbit GADD45α (1:100) and anti-rabbit Beclin1 (1:100) in 3% BSA is carried out overnight at 4 °C, which is further followed by probing under dark conditions with red conjugate or Alexa488 conjugated anti-rabbit secondary antibodies (1:200 dilutions in PBST; Santa Cruz Biotechnology Inc., CA, USA). Afterwards, mounting of coverslips with NucBlue reagent and capturing of the images were performed as described [13] by confocal microscopy (Leica TCS SP8 Laser Scanning Spectral Confocal microscope, Germ), which allows us to capture fluorescent images of a fluorochrome in a confocal mode but not in bright field or phase contrast mode. Relative florescence intensities of the respective images were analyzed by using the LASX software module.
Statistical analysis
Data shown were represented as mean ± standard error of the mean and indicative of 3 independent experiments. GraphPad Prism software of 5.0 version was employed for determining statistical analysis. Based on the number of groups, T-test (i.e., between 2 groups) and analysis of one-way variance (> two groups) were used to assess statistical significance (P < 0.05). Post hoc analysis was done using Tukey’s test.
Results
Cytotoxic effects of CBD in various cells and organoid cultures
Cytotoxic effects of CBD and DOX were assessed through viability assays wherein TNBC and MCF-10A cells are exposed to different concentrations of CBD and DOX (i.e., 100 nM to 10 µM) for 48 h. Herein, anti-cancer effects of CBD were compared to those of DOX. CBD showed cytotoxic effects in MDA-MB-231 (IC50: 3.22 µM) and MDA-MB-468 (IC50: 3.31 µM) cells (Fig. 1A, B). However, IC50 values of DOX were found as 2.26 µM and 2.56 µM in MDA-MB-231 and MDA-MB-468 cells respectively (Fig. 1A, B). CBD and DOX demonstrated cytotoxic effects with IC50 values of 6.5 µM and 400.73 nM respectively in MCF-10A cells (Fig. 1C). This indicates that CBD differentially kills TNBC cells at lower concentrations in comparison to its effect in MCF-10A cells. In addition, CBD showed cytotoxic effects in 3D cultures (i.e., LDP2 hydrogel based) of MDA-MB-231 (IC50: 20.18 µM) and MDA-MB-468 (IC50: 33.85 µM) cells (Fig. 1D). In addition, we evaluated the efficacy of CBD in 3D organoids of MDA-MB-231 xenografts. After the 8th day of organoid formation, CBD treatment was given in various concentrations ranging from 2.5 to 100 µM. IC50 value of CBD was found to be 75.36 µM (Fig. 1D).
CBD induced differential regulation of various genes in MDA-MB-231 cells
RNA sequencing was performed in MDA-MB-231 cells to determine the novel targets/genes altered by CBD (i.e., 1, 2.5, and 5 µM). RNA sequencing revealed differential regulation of various genes upon CBD treatment. Figure 2A depicts a heat map image, which shows the expression of various genes upon CBD treatments in comparison to untreated MDA-MB-231 cells (control). Supplementary Fig. 1 displays the high-resolution heat map image of various genes which were differentially regulated upon CBD treatment in MDA-MB-231 cells. Volcano plot (P value < 0.05 and FC > 1.0) revealed that CBD (2.5 µM) upregulates 169 genes and downregulates 311 genes (Fig. 2B). KEGG pathway analysis revealed that CBD (2.5 µM) upregulated genes were involved in transcriptional misregulation in cancer, ferroptosis, apoptosis, p53 signalling pathway, regulation of various signalling pathways in cancer (i.e., HMOX1, HES1, GADD45G, IL23A, GADD45A, RASSF1, GADD45B, GNG5, NFKBIA, FOS, CKS2, RALA, GSK 3β) (Fig. 2C). KEGG pathway analysis revealed that CBD (2.5 µM) downregulated genes are involved in steroid biosynthesis, metabolism of cancer cells, ECM-receptor interaction, and progression and arrest of BC (Fig. 2D). Figure 2E shows that CBD induces upregulation of GADD45A, GADD45G, and ID2 genes, and downregulation of FASN, LOXL4, and ITGB4 genes in MDA-MB-231 cells. Validation of RNA sequencing data (i.e., GADD45A, GADD45G, CCND1, LOX, and FASN) is carried out by performing RT-qPCR. RNA sequencing data revealed that CBD (2.5 µM and 5 µM) treatment in MDA-MB-231 cells induced upregulation of GADD45 alpha (i.e., sevenfold and 13-fold higher respectively) and GADD45 gamma (i.e., 20-fold and 42-fold higher respectively), and downregulation of LOX (1.8-fold and twofold lower respectively) and FASN (8.66-fold and 9.92-fold lower respectively) genes in comparison to control. It also revealed that CBD (5 µM) downregulated CCND1 expression (fivefold lower) in comparison to control. We observed that CBD (2.5 µM and 5 µM) treatment increased the mRNA expression of GADD45A (2.5-fold and 4.3-fold higher respectively) and GADD45G (1.67-fold and 2.38-fold higher respectively), and decreased the mRNA expression of LOXL4 (twofold and 5.95-fold lower respectively) and FASN (1.85-fold and 4.76-fold lower respectively) genes through performing RT-qPCR (Fig. 2F). CBD also decreased CCND1 mRNA expression (fivefold lower) in comparison to control. KEGG pathway analysis and RT-qPCR revealed that GADD45A in the p53 signalling pathway was significantly upregulated by CBD treatment.
Next-generation RNA sequencing and RT-qPCR analysis in MDA-MB-231 cells. A Heat map image of RNA sequencing data (n = 3), where “n” represents the number of biological replicates per group. B Volcano plot showing that CBD (2.5 µM) treatment induced upregulated (in orange) and downregulated (in blue) genes. C Bar graphs showing KEGG pathway analysis of CBD (2.5 µM) upregulated genes. D Bar graphs showing KEGG pathway analysis of CBD (2.5 µM) downregulated genes. E Illustrative images of CBD-induced up- (GADD45A, GADD45G, and CCNB2) and downregulated (LOXL4, FASN, and ITGB4) genes. F RT-qPCR analysis of GADD45A, GADD45G, LOX, FASN, and CCND1 genes. Control group represents untreated MDA-MB-231 cells. ***P < 0.001, **P < 0.01, *P < 0.05 were considered to be statistically significant
CBD mediates apoptosis by upregulating GADD45 alpha, p-p38, and p53 proteins in TNBC cells
RNA sequencing and RT-qPCR data revealed that CBD (2.5 µM and 5 µM) induced upregulation of GADD45 alpha in MDA-MB-231 cells. We also confirmed through western blotting that CBD treatment significantly increased GADD45 alpha protein expression in TNBC cells (Fig. 3A, B). In order to investigate the downstream effectors responsible for CBD-induced cytotoxicity in these cells, we also checked phosphorylated p38 and p53 proteins. CBD significantly increased the expression of these proteins in TNBC cells (Fig. 3A, B). Immunocytochemistry staining also revealed that CBD increased GADD45 alpha expression in TNBC cells (Fig. 3C and Supplementary Fig. 2). This suggests that CBD induces apoptosis by upregulating the expression of GADD45 alpha, p-p38, and p53 proteins in TNBC cells.
Effect of CBD on GADD45-alpha, p-p38, and p53 proteins in TNBC cells. A Immunoblots and densitometry data of GADD45-alpha, p-p38, and p53 proteins upon CBD treatment in MDA-MB-231 cells. B Immunoblots and densitometry data of GADD45-alpha, p-p38, and p53 proteins upon CBD treatment in MDA-MB-468 cells. After ascertaining p-p38 expression, we performed stripping and reprobed the same blot with β-actin antibody. C Immunostaining with GADD45α antibody. ***P < 0.001, **P < 0.01, *P < 0.05 were considered to be statistically significant
CBD induced G1 arrest in TNBC cells
Propidium iodide staining of TNBC cells (i.e., flow cytometry for cell cycle analysis) revealed that CBD (2.5 µM) treatment significantly increases the accumulation of cell population at sub G1 phase in comparison to control. MDA-MB-231 control and CBD (2.5 µM) showed 66.23% and 78.10% of cell accumulation respectively in sub G1 phase (Supplementary Fig. 3). MDA-MB-468 control and CBD (2.5 µM) showed 56.17% and 71.94% of cell accumulation respectively in sub G1 phase (Supplementary Fig. 3). These results imply that CBD induces G1 arrest and also support the cytotoxic effects of CBD in TNBC cells.
Anti-migratory effects of CBD in TNBC cells
Integrins, fibronectin, and vimentin regulate multiple signalling pathways of cancer, including migration. We observed that CBD significantly decreased integrin- alpha 5, -beta 1, and -beta 5 protein expression in TNBC cells (Fig. 4A, D). Moreover, CBD significantly decreased these proteins in 3D cultures of TNBC cells (Fig. 4C and Supplementary Fig. 4). In addition, xCELLigence cell migration analysis in MDA-MB-231 cells further confirmed anti-migratory effects of CBD (Fig. 5A). CBD downregulated vimentin and fibronectin proteins in TNBC cells (Fig. 5B, C). All these suggest the anti-migratory properties of CBD in TNBC cells.
Effect of CBD on integrin-α5, integrin-β5, and integrin-β1 proteins in TNBC cells. A, B Immunoblots and densitometry data of integrin-α5, integrin-β5, and integrin-β1 proteins in TNBC cells. After ascertaining the Integrin β1 expression, we performed stripping and reprobed the same blot with Integrin α5 and Integrin β5 antibodies. C Immunoblots and densitometry data of integrin-α5, -β5, and -β1 proteins in MDA-MB-468 cells (3D). After investigating the Integrin β5 expression, we performed stripping and the same blot was reprobed with Integrin β1 antibody. ***P < 0.001, **P < 0.01, *P < 0.05 were considered to be statistically significant
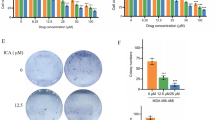
CBD decreased the migration of TNBC cells. A xCELLigence CIM-16 plate migration-based assay in MDA-MB-231 cells exposed to CBD. B, C Immunoblots and densitometry data of fibronectin and vimentin proteins in TNBC cells. ***P < 0.001, **P < 0.01, *P < 0.05 were considered to be statistically significant
CBD decreased the autophagy of TNBC cells
Since autophagy plays a pivotal role in regulating anti-cancer effects of various chemotherapeutics, we ascertained the protein expression of autophagy markers upon CBD treatment. It was observed that CBD induces inhibition of autophagy by significantly decreasing the protein expression of Beclin 1, ATG5, ATG7, and ATG16 in 2D monolayers of TNBC cells (Supplementary Figs. 5A and 6A). RT-qPCR analysis in MDA-MB-231 cells revealed dose-dependent amelioration of Beclin 1 mRNA expression by CBD (Supplementary Fig. 5B). Immunocytochemistry further validates and confirms that CBD decreased beclin1 in TNBC cells (Supplementary Figs. 5C and 6C). We have checked the CBD effect on autophagy markers (Beclin1 and LC3A/B) in 3D cultures of TNBC cells and noticed that CBD significantly decreased the expression of beclin1 and LC3A/B (Supplementary Figs. 5D and 6B). Western blotting and RT-qPCR data support RNA sequencing data, which also showed that CBD induces decrease in autophagy.
CBD increased DOX sensitivity in TNBC cells
We have evaluated the chemo-sensitization potential of CBD in TNBC cells by pre-treating them with CBD (1 and 2.5 µM) for 24 h and later exposed to multiple doxorubicin concentrations (i.e., 0.39 µM to 25 µM) for 48 h. IC50 values of DOX in MDA-MB-231 cells pre-treated with CBD (1 µM and 2.5 µM) were observed as 952 nM and 300 nM, respectively, suggesting that CBD (1 µM and 2.5 µM) can effectively improve the DOX sensitivity by 2.5-fold and 7.5-fold respectively (Fig. 6A). DOX IC50 values in MDA-MB-468 cells pre-treated with CBD (1 µM and 2.5 µM) were observed as 1.72 µM and 1.02 µM, respectively, indicating that CBD (1 µM and 2.5 µM) significantly increased DOX sensitivity by 1.48-fold and 2.5-fold respectively (Fig. 6B). The combinatorial anti-cancer effects of CBD and DOX were investigated by exposing TNBC cells to constant concentrations of CBD (i.e., 1 µM and 2.5 µM) and varying concentrations of DOX (i.e., 0.39 to 25 µM) for 2 days. Cannabidiol (1 µM) in combination with doxorubicin (i.e., 0.39, 0.78, 1.56, and 3.12 µM) showed synergistic effects (i.e., CI < 1) in MDA-MB-231 cells (Supplementary table 1). However, synergistic effects (i.e., CI < 1) were observed with CBD (1 µM) and DOX (0.39 µM) combination in MDA-MB-468 cells (Supplementary table 2). CBD (2.5 µM) in combination with all DOX concentrations (i.e., 0.39 to 25 µM) had shown antagonistic effects (i.e., CI > 1) in TNBC cells (Supplementary tables 1 and 2). This suggests that a low dose of CBD (1 µM) was more effective in producing synergistic effects with DOX. Dose reduction index (DRI) values with various CBD and DOX concentrations were also shown in supplementary tables 1 and 2. Moreover, CBD chemo-sensitization effects in improving DOX sensitivity were investigated in 3D cultures of TNBC cells. Upon CBD (5 µM) pre-treatment, DOX showed cytotoxic effects in 3D cultures of MDA-MB-231 (IC50: 17.42 µM) and MDA-MB-468 (IC50: 31.72 µM) cells, suggesting that CBD (5 µM) pre-treatment facilitates increased DOX sensitivity in TNBC cells (Fig. 6C, D). Cannabidiol and doxorubicin combination also showed anti-migratory effects as compared to control, DOX, and CBD groups (Fig. 6E). CBD decreased LOX protein in TNBC cells (Supplementary Fig. 7). Moreover, we investigated the CBD pre-sensitization effects in facilitating DOX sensitivity by checking caspase 9, integrin-α5, and LOX proteins through western blotting. It was observed that pre-treatment with CBD followed by DOX post-treatment ameliorated integrin-α5 and LOX proteins, and increased caspase 9 protein expression in MDA-MB-468 cells (Fig. 6F).
Chemo-sensitization effects of CBD in TNBC cells. A, B Anti-cancer activity of Synthetic CBD (1 µM and 2.5 µM) and DOX combination in 2D cultures of TNBC cells. C, D Anti-cancer activity of Synthetic CBD (5 µM) and DOX combination in 3D cultures of TNBC cells. E xCelligence CIM-16 plate migration-based assay in MDA-MB-231 cells with CBD and DOX combination in MDA-MB-231 cells. F Immunoblots and densitometry data of caspase 9, LOX, and integrin-α5 proteins in CBD pre-treated MDA-MB-468 cells (24 h)and further treated with DOX treatment (2 days). After ascertaining LOX expression, we performed stripping and reprobed the same blot with caspase 9 and β-actin antibodies. Statistical significance considerations: ***P < 0.001, **P < 0.01, and *P < 0.05 vs control; ##P < 0.01 significant vs DOX (1 µM); $$$P < 0.001, $P < 0.05 vs CBD (1 µM)
Discussion
To date, effective therapeutic options readily available for TNBC are limited [20]. Due to poor diagnosis and less efficacy of conventional chemotherapeutics, identification of new chemotherapeutics is an urgent clinical need for TNBC. Herein, we investigate the anti-cancer effects of CBD in unexplored 3D cultures of TNBC cells, identify novel targets responsible for chemotherapeutic effects of CBD in TNBC, and also depict the possible molecular mechanisms responsible for improved sensitization of DOX in TNBC cells.
Cannabinoids are gaining enormous interest recently because of their therapeutic applications in various diseases including cancer. CBD mediates anti-cancer effects through reactive oxygen species (ROS) generation and ERK phosphorylation, which all are involved in the downregulation of Id-1, a major molecule that regulates cancer cell proliferation [38].
To date, CBD effects are well demonstrated in 2D monolayers of cancer cells but not in 3D cultures, which usually mimic the tumor microenvironment in vivo. 3D systems closely mimic the in vivo settings of tissues regarding interactions between cell–cell and also differentiation patterns [39]. 2D culture platforms are unable to mimic in vivo tumor microenvironment (i.e., complexity and heterogeneity) when compared to 3D cultures [40,41,42,43]. 3D cultures were developed by using hydrogels. Cell density is an important aspect to be considered during the evaluation of the cytotoxicity of chemotherapeutics both in 2D and 3D culture models. Usage of the right number of cells is critical and we used different numbers of cells to evaluate the right number for getting reproducible spheroids in 3D cultures. Our observations indicated that a majority of the spheroids formed with 5 × 104 cells/well were virtually consistent in size, suggesting that the data generated from them is reliable for the agents tested in 3D cultures. CBD IC50 values were found to be higher in 3D cultures of TNBC cells in comparison to 2D cultures. Higher IC50 values in 3D cultures were majorly attributed due to the development/creation of tumor microenvironment (i.e., by using VitroGel LDP2 hydrogel), which can closely mimic/recapitulate in vivo or natural 3D tumor microenvironment. We also observed that CBD differentially kills TNBC cells at lower doses (IC50 value ≈ 3–3.5 µM) in comparison to MCF-10A cells (IC50: 6.5 µM). Organoid cultures are developed from the respective tissue of origin. Organoid cultures (i.e., three-dimensional tissue cultures) are considered as promising preclinical in vitro disease models for investigating the efficacy of various chemotherapeutics and determining various genomic modifications responsible for the pathogenesis of various cancers because of their ability to self-organize into mini-organs showing similarity to the respective tissue of origin and bridge the gap between 2D cultures and patient-derived xenografts by recapitulating tumor environment [44]. We used Cultrex growth factor reduced BME for the development of breast cancer organoids model, whose potential for screening of various chemotherapeutics is well demonstrated [30]. As we had seen good cytotoxic effects with CBD in MDA-MB-231 3D cultures, we ascertained CBD effects in only 3D organoid cultures derived from MDA-MB-231 xenografts. It was observed that CBD treatment decreased the growth of 3D organoids (IC50: 75.36 µM) in comparison to untreated organoids. Previous evidences in cancer studies also suggest higher IC50 values in 3D cultures with chemotherapeutics [45, 46]. We also investigated the stability of CBD in both PBS and in medium at pH 7.2. Degradation of CBD was not observed after 45 days as ascertained by HPLC.
Since we observed good cytotoxic effects with CBD in 3D organoids (from MDA-MB-231 xenografts), we would speculate to some extent that CBD might also show similar anti-cancer effects in TNBC xenografts. Accumulating evidence has demonstrated that administration of CBD for a period of 1–4 weeks at different doses (i.e., 1–20 mg/kg daily) caused significant tumor regression [8, 13, 47,48,49]. It was recently reported that CBD (i.e., 5 mg/kg, i.p. 3–4 times a week) not only showed cytotoxic effects in head and neck cancers but also demonstrated less toxicity to normal oral keratinocytes [50].
Molecular mechanisms responsible for anti-cancer effect of CBD in TNBC cells are unexplored. To determine the novel targets/genes altered by CBD treatment in MDA-MB-231 cells, we performed next-generation RNA sequencing on Illumina NovaSeq 6000 system, which revealed GADD45A, integrin α5, and integrin β5 genes as novelly altered. Next-generation RNA sequencing technologies are free from probe-specific microarray-hybridization, can detect even less-abundance transcripts along with coding and noncoding novel transcripts, and also determine the differentially expressed genes upon treatment with chemotherapeutics [51, 52]. RT-qPCR validation revealed a similar trend (i.e., upregulation and downregulation) in the experimentally validated genes (GADD45A, GADD45G, LOX, FASN, and CCND1) but their fold-change values varied when compared to RNA-seq analysis.
RNA sequencing and RT-qPCR data had revealed and confirmed that CBD (5 µM) treatment decreased (i.e., fivefold lower) CCND1 (i.e., a gene coding for cyclin D1) mRNA expression. These results corroborate well with previous evidence in breast cancer that CBD mediates apoptosis by altering PPARγ, mTOR, and cyclin D1 [53]. Cyclin D1 inhibition in cancer cells is demonstrated to induce G1 arrest [54]. Moreover, flow cytometry observations revealed that CBD induces G1 arrest in TNBC cells.
GADD45A functions as a stress sensor upon exposure to physiological and environmental stresses and mediates various cell signalling pathways [55, 56]. Expression levels of GADD45A are helpful for determining the prognosis of breast cancer, where TNBC contains either low or no GADD45A levels [57]. KEGG pathway analysis of MDA-MB-231 cells RNA sequencing data has showed that CBD upregulates GADD45A, which was further validated and confirmed through RT-qPCR and western blotting in TNBC cells. Moreover, CBD increased the phosphorylation of p38 MAP kinase, which is a downstream effector of GADD45A. GADD45A and p53 (i.e., a tumor suppressor gene) can regulate each other and mediate the proliferation of various tumor cells [58, 59]. It was noticed that CBD increases p53 expression in TNBC cells. This suggests that CBD mediates growth arrest and apoptosis of TNBC cells through upregulation of GADD45A, and phosphorylated p38 and p53.
In breast cancer, integrins (i.e., integrin-α5, -β5, and -β1), fibronectin, and vimentin mediate the proliferation, migration, invasion, adhesion, differentiation, angiogenesis, and apoptosis evasion [60,61,62,63,64]. RNA sequencing data revealed that CBD induces downregulation of integrin-α5 and -β5 in MDA-MB-231 cells. Moreover, CBD decreased ITGA5, ITGB5, ITGB1, fibronectin, and vimentin proteins in TNBC cells. Additionally, xCELLigence cell analysis platform revealed that CBD significantly decreases MDA-MB-231 cell migration. RNA sequencing also revealed that CBD treatment upregulates the pro-differentiation factor, ID2 (inhibitor of DNA binding 2) around 2–threefold higher in comparison to untreated MDA-MB-231 cells. ID2 overexpression contributes to decreased proliferation, invasion, and increased differentiation in TNBC [65]. Fatty acid synthase (FASN) and lysyl oxidase (LOX) mediate metastasis, resistance of cancer cells to chemotherapeutics, proliferation, and metabolism [66, 67]. RNA sequencing and RT-qPCR have revealed and confirmed that CBD decreased FASN and LOX levels, implying CBD effect on cancer cell metabolism and metastasis.
Autophagy is also involved for chemotherapeutics resistance in various cancers. Beclin1, ATG5, ATG7, and ATG16 proteins play a vital role during various stages of autophagy in various cancers [68]. ATG5 upregulation is responsible for gall bladder cancer cells resistance to DOX [69]. CBD decreased beclin 1, and ATG-5, -7, and -16 in TNBC cells, suggesting CBD role on inhibition of autophagy. These observations also suggest us the therapeutic benefits of CBD when combined with different anti-cancer drugs (i.e., which generally increase autophagy and mediate resistance) in various cancer cells. RNA sequencing data showed that CBD decreases autophagy-related 2A, a protein involved in early stages of autophagosome formation. RT-qPCR data in MDA-MB-231 cells revealed decreased beclin1 mRNA by CBD. CBD induces G1 arrest in TNBC cells (flow cytometry data), which coincides well with earlier evidence demonstrating that autophagy inhibition induces significant cell cycle changes such as G1 arrest [70]. We also observed increased p53 expression upon CBD treatment in TNBC cells, which might be also due to decreased AT7G levels by CBD treatment. Accumulating evidence suggests that autophagy escalates cancer cell migration and invasion [71,72,73]. Decreased migration of TNBC cells might be also due to decreased autophagy by CBD in these cells.
DOX is used either alone or in combination with different chemotherapeutic regimens for multiple types of cancers including breast cancer. Despite being used clinically, decreased sensitivity and resistance of cancer cells to DOX remain a major clinical concern. Autophagy inhibition increases DOX sensitivity in TNBC cells [74]. Recent evidence in breast cancer suggests that CBD microparticles showed synergistic effects with paclitaxel/DOX [19]. In comparison to CBD-loaded microparticles (IC70: 18 µM for 48 h), synthetic CBD (GLP and GMP grade; Purisys, Athens, GA) used in our study showed anti-cancer effects in TNBC cells at very low doses. Moreover, CBD pre-treatment (i.e., as a chemosensitizer) also increased DOX sensitivity in TNBC cells, where the effects are more in MDA-MB-231 cells when compared to MDA-MB-468 cells. CBD (1 µM) in combination with DOX (i.e., at various doses until 3.12 µM) induced synergistic effects in MDA-MB-231 cells. However, CBD (1 µM) when combined with DOX (0.39 µM) induced synergistic effects in MDA-MB-468 cells. Our results suggest that CBD (2.5 µM) in combination with DOX induced antagonistic effects in both MDA-MB-231 and MDA-MB-468 cells. This suggests that CBD at low doses may be combined with DOX to induce synergistic effects in TNBC cells, where the effects can vary depending upon the cell type. Anti-migratory effects were observed with CBD and DOX combination in MDA-MB-231 cells. Recently, it was reported that targeting LOX could mitigate TNBC cell resistance to chemotherapeutics [75]. Our study also demonstrates that anti-cancer effects of CBD also involve downregulation of integrin and LOX proteins. We also speculate that CBD induced improvement of DOX sensitivity in triple negative breast cells might be due to CBD-induced downregulation of LOX protein, which needs further investigation. CBD pre-sensitization accompanied by DOX (1 µM) treatment decreased LOX, integrin-α5, and increased caspase 9 proteins in MDA-MB-468 cells. Integrin beta-1 upregulation mediates DOX resistance in MDA-MB-231 cells [76]. Moreover, RNA sequencing data in MDA-MB-231 cells revealed that CBD treatment decreases ATP binding cassette subfamily A member 2 levels. Chemotherapeutic drug resistance of tumors is mediated by ABCA2 [77, 78]. CBD induced decrease in ABCA2, autophagy, ATG5, and integrin beta-1 proteins might be also responsible for improving DOX sensitivity in TNBC.
Conclusions
CBD induces activation of GADD45alpha/p38/p53 signalling pathway mediated apoptosis, G1 arrest, and downregulation of integrins-α5, -β5, and -β1, fibronectin, and vimentin, and inhibits autophagy in TNBC cells. CBD increased DOX sensitivity in TNBC cells, which might be due to CBD-induced decrease in ABCA2, autophagy, and integrin beta-1 proteins. Our studies suggest that pre-sensitization of TNBC cells with CBD followed by DOX treatment might provide better clinical chemotherapeutic benefits besides decreasing the toxicity of DOX. One of the future goals of our laboratory is to use delivery systems like lipid nanoparticles and exosomes for more efficient delivery of CBD by oral and parenteral routes respectively. It is expected that based on our current results, a delivery system approach will lead to more efficient anti-cancer therapeutic effects of CBD in vivo.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary Information files).
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Prev Biomark. 2016;25:16–27.
Pérez-García J, Soberino J, Racca F, Gion M, Stradella A, Cortés J. Atezolizumab in the treatment of metastatic triple-negative breast cancer. Expert Opin Biol Ther. 2020;20:981–9.
Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2:751–60.
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8.
LeClair JN, Chamberlin KW, Clement J, Holle LM. Documentation of medical marijuana use in cancer patients. J Oncol Pharm Pract. 2020;26:1117–27.
Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–14.
Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2:241–54.
McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129:37–47.
Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett. 2009;285:6–12.
Kovalchuk O, Kovalchuk I. Cannabinoids as anticancer therapeutic agents. Cell Cycle. 2020;19:961–89.
Ghanem A, Emara HA, Muawia S, Abd El Maksoud AI, Al-Karmalawy AA, Elshal MF. Tanshinone IIA synergistically enhances the antitumor activity of doxorubicin by interfering with the PI3K/AKT/mTOR pathway and inhibition of topoisomerase II: in vitro and molecular docking studies. New J Chem. 2020; 44: 17374–17381.
Chen C, Lu L, Yan S, Yi H, Yao H, Wu D, et al. Autophagy and doxorubicin resistance in cancer. Anticancer Drugs. 2018;29:1–9.
Patel N, Kommineni N, Surapaneni SK, Kalvala A, Yaun X, Gebeyehu A et al. Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models. Int J Pharm. 2021; 607: 120943.
Pan YZ, Wang X, Bai H, Wang CB, Zhang Q, Xi R. Autophagy in drug resistance of the multiple myeloma cell line RPMI8226 to doxorubicin. Genet Mol Res. 2015;14:5621–9.
de Oliveira Júnior RG, Adrielly AFC, da Silva Almeida JRG, Grougnet R, Thiery V, Picot L. Sensitization of tumor cells to chemotherapy by natural products: a systematic review of preclinical data and molecular mechanisms. Fitoterapia. 2018;129:383–400.
Doddapaneni R, Patel K, Chowdhury N, Singh M. Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Sci Rep. 2017;7:1–11.
Scott KA, Dalgleish AG, Liu WM. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther. 2014;13:2955–67.
Nabissi M, Morelli MB, Santoni M, Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48–57.
Fraguas-Sánchez A, Fernández-Carballido A, Simancas-Herbada R, Martin-Sabroso C, Torres-Suárez A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int J Pharm. 2020; 574: 118916.
Surapaneni SK, Bhat ZR, Tikoo K. MicroRNA-941 regulates the proliferation of breast cancer cells by altering histone H3 Ser 10 phosphorylation. Sci Rep. 2020;10:17954.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81.
Zhang N, Fu JN, Chou TC. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: experimental design and data analysis using the combination index method. Am J Cancer Res. 2016;6:97–104.
Arthur P, Patel N, Surapaneni SK, Mondal A, Gebeyehu A, Bagde A et al. Targeting lung cancer stem cells using combination of Tel and Docetaxel liposomes in 3D cultures and tumor xenografts. Toxicol Appl Pharmacol. 2020; 401: 115112.
Surapaneni SK, Bashir S, Tikoo K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci Rep. 2018;8:12295.
Patel AR, Chougule MB, Patlolla R, Wang G, Singh M. Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel. Pharm Res. 2013;30:1435–46.
Cui J, Zhao H, Wang C, Sun JJ, Lu K, Ma D. Dexmedetomidine attenuates oxidative stress induced lung alveolar epithelial cell apoptosis in vitro. Oxid Med Cell Longev. 2015; 2015: 358396.
Kalvala AK, Yerra VG, Sherkhane B, Gundu C, Arruri V, Kumar R, et al. Chronic hyperglycemia impairs mitochondrial unfolded protein response and precipitates proteotoxicity in experimental diabetic neuropathy: focus on LonP1 mediated mitochondrial regulation. Pharmacol Rep. 2020;72:1627–44.
Thummuri D, Kumar S, Surapaneni SK, Tikoo K. Epigenetic regulation of protein tyrosine phosphatase PTPN12 in triple-negative breast cancer. Life Sci. 2015;130:73–80.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–9.
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373-386.e310.
Nkosi D, Sun L, Duke LC, Meckes DG, Jr. Epstein-Barr virus LMP1 manipulates the content and functions of extracellular vesicles to enhance metastatic potential of recipient cells. PLoS Pathog. 2020; 16: e1009023.
Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019; 47: W234-w241.
McDermaid A, Monier B, Zhao J, Liu B, Ma Q. Interpretation of differential gene expression results of RNA-seq data: review and integration. Brief Bioinform. 2019;20:2044–54.
Hedrick E, Lee S-O, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Mol Cell Biol. 2016;36:1383–94.
Liu XF, Yang WT, Xu R, Liu JT, Zheng PS. Cervical cancer cells with positive Sox2 expression exhibit the properties of cancer stem cells. PLoS One. 2014; 9: e87092.
Liu X, Sun Y, Guo J, Ma H, Li J, Dong B, et al. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int J Cancer. 2006;118:1922–9.
Kalvala AK, Yerra VG, Kumar A. LONP1 induction by SRT1720 attenuates mitochondrial dysfunction against high glucose induced neurotoxicity in PC12 cells. Toxicol In Vitro. 2020; 62: 104695.
McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–7.
Bissell MJ. Architecture is the message: the role of extracellular matrix and 3-D structure in tissue-specific gene expression and breast cancer. The Pezcoller Foundation journal: news from the Pezcoller Foundation world. 2007;16:2.
Lv D, Hu Z, Lu L, Lu H, Xu X. Three-dimensional cell culture: a powerful tool in tumor research and drug discovery. Oncol Lett. 2017;14:6999–7010.
Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207–18.
Theodoraki MA, Rezende CO Jr, Chantarasriwong O, Corben AD, Theodorakis EA, Alpaugh ML. Spontaneously-forming spheroids as an in vitro cancer cell model for anticancer drug screening. Oncotarget. 2015;6:21255–67.
Michor F, Weaver VM. Understanding tissue context influences on intratumour heterogeneity. Nat Cell Biol. 2014;16:301–2.
Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73.
Godugu C, Patel AR, Desai U, Andey T, Sams A, Singh M. AlgiMatrix™ based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS One. 2013; 8: e53708.
Baek N, Seo OW, Kim M, Hulme J, An SSA. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. Onco Targets Ther. 2016;9:7207.
Elbaz M, Nasser MW, Ravi J, Wani NA, Ahirwar DK, Zhao H, et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol Oncol. 2015;9:906–19.
Fisher T, Golan H, Schiby G, PriChen S, Smoum R, Moshe I, et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr Oncol. 2016;23:S15-22.
Romano B, Borrelli F, Pagano E, Cascio MG, Pertwee RG, Izzo AA. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine. 2014;21:631–9.
Go YY, Kim SR, Kim DY, Chae SW, Song JJ. Cannabidiol enhances cytotoxicity of anti-cancer drugs in human head and neck squamous cell carcinoma. Sci Rep. 2020;10:20622.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY, Zhang X, et al. Expression profile analysis of long non-coding RNA associated with vincristine resistance in colon cancer cells by next-generation sequencing. Gene. 2015;572:79–86.
Sultan AS, Marie MA, Sheweita SA. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast. 2018;41:34–41.
Hanai J, Dhanabal M, Karumanchi SA, Albanese C, Waterman M, Chan B, et al. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. J Biol Chem. 2002;277:16464–9.
Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–54.
Cretu A, Sha X, Tront J, Hoffman B, Liebermann DA. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009;7:268–76.
Tront JS, Willis A, Huang Y, Hoffman B, Liebermann DA. Gadd45a levels in human breast cancer are hormone receptor dependent. J Transl Med. 2013;11:131.
Zhan Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res. 2005;569:133–43.
Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–84.
Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, et al. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–64.
Messica Y, Laser-Azogui A, Volberg T, Elisha Y, Lysakovskaia K, Eils R, et al. The role of vimentin in regulating cell invasive migration in dense cultures of breast carcinoma cells. Nano Lett. 2017;17:6941–8.
Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin β5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene. 2013;32:3049–58.
Qin L, Chen X, Wu Y, Feng Z, He T, Wang L, et al. Steroid receptor coactivator-1 upregulates integrin α5 expression to promote breast cancer cell adhesion and migration. Cancer Res. 2011;71:1742 1751
Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, et al. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70.
Stighall M, Manetopoulos C, Axelson H, Landberg G. High ID2 protein expression correlates with a favourable prognosis in patients with primary breast cancer and reduces cellular invasiveness of breast cancer cells. Int J Cancer. 2005;115:403–11.
Menendez JA, Lupu R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin Ther Targets. 2017;21:1001–16.
Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 2016;76:188–92.
Chen X, Sun Y, Wang B, Wang H. Prognostic significance of autophagy-related genes Beclin1 and LC3 in ovarian cancer: a meta-analysis. J Int Med Res. 2020;48:300060520968299.
Cai Q, Wang S, Jin L, Weng M, Zhou D, Wang J, et al. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18:82.
Zhang J, Mao W, Liu Y, Ding J, Wang J, Yu Z et al. 3-MA enhanced chemosensitivity in cisplatin resistant hypopharyngeal squamous carcinoma cells via inhibiting Beclin -1 mediated autophagy. Curr Pharm Des. 2020.
Hou HH, Pan HJ, Liao WY, Lee CH, Yu CJ. Autophagy in fibroblasts induced by cigarette smoke extract promotes invasion in lung cancer cells. Int J Cancer. 2020;147:2587–96.
Zhu H, Wang D, Zhang L, Xie X, Wu Y, Liu Y, et al. Upregulation of autophagy by hypoxia-inducible factor-1α promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep. 2014;32:935–42.
Singh M, Ghose T, Faulkner G, Kralovec J, Mezei M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Can Res. 1989;49:3976–84.
Aydinlik S, Erkisa M, Cevatemre B, Sarimahmut M, Dere E, Ari F, et al. Enhanced cytotoxic activity of doxorubicin through the inhibition of autophagy in triple negative breast cancer cell line. Biochim Biophys Acta Gen Subj. 2017;1861:49–57.
Saatci O, Kaymak A, Raza U, Ersan PG, Akbulut O, Banister CE, et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11:2416.
Lovitt CJ, Shelper TB, Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. 2018;18:41.
Laing NM, Belinsky MG, Kruh GD, Bell DW, Boyd JT, Barone L, et al. Amplification of the ATP-binding cassette 2 transporter gene is functionally linked with enhanced efflux of estramustine in ovarian carcinoma cells. Cancer Res. 1998;58:1332–7.
Boonstra R, Timmer-Bosscha H, van Echten-Arends J, van der Kolk DM, van den Berg A, de Jong B, et al. Mitoxantrone resistance in a small cell lung cancer cell line is associated with ABCA2 upregulation. Br J Cancer. 2004;90:2411–7.
Funding
The National Institute on Minority Health and Health Disparities of National Institutes of Health, Grant/Award Number: U54 MD007582 and Consortium for Medical Marijuana Clinical Outcomes Research (Reference Award number: SUB00002097) provided the funding for carrying out this study.
Author information
Authors and Affiliations
Contributions
S.K.S. designed and conducted all cell culture studies, flow cytometry, RT-qPCR, western blotting, and RNA seq data analysis. N.P. performed all the western blot studies. L.S. performed the RNA seq studies and also data analysis. N.K. conducted in-vitro cytotoxicity studies. A.K.K. performed confocal microscopy and flow cytometry studies. A.G. conducted the 3D culture cytotoxicity studies. L.C.D. conducted the cell migration studies. P.A. conducted the in-vitro cytotoxicity studies. R.N. performed western blotting. D.G.M provided lab facilities for conducting cell migration and RNA seq studies. S.K.S. and N.P. wrote the manuscript. M.S. majorly contributed in designing and supervision of experiments (i.e., in-vitro and in vivo), and also approved the manuscript final draft. Manuscript had been reviewed by all the authors.
Corresponding author
Ethics declarations
Ethics approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent to participate
Not applicable.
Consent for publication
All the authors have reviewed and approved the manuscript of this research work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Surapaneni, S.K., Patel, N., Sun, L. et al. Anticancer and chemosensitization effects of cannabidiol in 2D and 3D cultures of TNBC: involvement of GADD45α, integrin-α5, -β5, -β1, and autophagy. Drug Deliv. and Transl. Res. 12, 2762–2777 (2022). https://doi.org/10.1007/s13346-022-01137-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01137-2