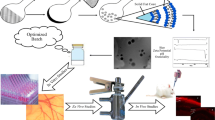
Abstract
The research work was driven to develop, optimize, and characterize novel nanostructured liquid crystalline particles as carriers for the ocular delivery of vancomycin. The formulations were developed by fragmenting the cubic crystalline phase of glycerol monooleate, water, and poloxamer 407. A four-factor, three-level Taguchi statistical experimental design was constructed to optimize the formulation. Formulations exhibited internal-cubic structure of the vesicles with particle size in the range of 51.11 ± 0.96 nm to 158.73 ± 0.46 nm and negative zeta potential. Ex vivo transcorneal permeation studies demonstrated that the optimized cubosomes had a 2.4-fold increase in apparent permeability co-efficient as compared to vancomycin solution, whereas in vivo studies in rabbits demonstrated that the severity of keratitis was considerably lowered on day 3 with optimized cubosomes. Ocular pharmacokinetic studies evaluated the level of drug in aqueous humor, and results revealed that the time to peak concentration (Tmax) of vancomycin-loaded cubosomal formulation was about 1.9-fold higher and mean residence time was 2.2-fold greater than vancomycin solution. Furthermore, histological examination revealed that the corneal layers displayed well-maintained morphology without any stromal swelling, consequently indicating the safety of formulation. It could be concluded that the developed nanostructured liquid crystalline particles of vancomycin demonstrated improved pre-ocular residence time, increased permeability, reduced dosing frequency, controlled drug release, and reduced systemic side-effects. Results manifested that the developed vancomycin-loaded cubosomes could be a promising novel ocular carrier and an ideal substitute for conventional eye drops for the management of bacterial-keratitis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycopeptide antibiotic, vancomycin, is a tricyclic-glycosylated non-ribosomal peptide. It is used to treat diverse bacterial infections, specifically gram positive infections such as species of Staphylococcus. Vancomycin has minimal oral absorption and is delivered by intravenous infusion. It acts by inhibiting polymerization of peptidoglycan layer in cell-wall of bacteria. Specifically, vancomycin averts incorporation of N-acetylmuramic acid-(NAM) and N-acetylglucosamine-(NAG)-peptide sub-units from being incorporated into the peptidoglycan matrix. Vancomycin forms hydrogen bonds with the terminal D-alanyl-D-alanine moieties of the NAM/NAG-peptides, which impedes glucosyltransferase and the P-phospholipid carrier, therefore averting synthesis and polymerization of NAM/NAG-peptide subunits into the peptidoglycan layer. This inhibition weakens the cell-wall of bacteria and causes intracellular contents to leak-out, eventually causing bacterial cell-death [1].
Bacterial keratitis can be described as a vision-threatening ocular infection predominantly caused by Staphylococcus aureus, Streptococcus pneumoniae, and Pseudomonas aeruginosa. It presents clinically with corneal inflammation, pain, excessive tearing, corneal edema, ulcers, reduced vision, redness, and photophobia. Pre-disposing factors, for instance, extended use of contact lenses, eye-trauma, ocular surface-disorders, and dry-eyes, are correlated with bacterial keratitis. These factors might alter the defense mechanism of ocular-surface and allow bacteria to invade the membrane of the cornea. There is a break-down of corneal-epithelium due to which there is a penetration of bacteria. Following the penetration, there is an activation of plasminogen to plasmin, and bacteria secrets protease, chymase, and tryptase that causes corneal necrosis leading to rapid corneal weakening and perforation [2].
Management of bacterial keratitis remains a challenge due to poor corneal penetration of conventional eye drops and unique anatomical eye structure. The entry of drug molecules at the site of action is sometimes restricted owing to the unique structure of the eye. Numerous anatomical and physiological constraints such as tear turnover, nasolachrymal drainage, reflex blinking, ocular static, and dynamic barriers pose a challenge and impede deeper ocular drug permeation. Corneal epithelium’s tight junction acts as a selective barrier for entry of small molecules, whereas diffusion of macromolecules is prevented by paracellular routes [3]. Hence, less than 5% of the topically applied dose reaches deeper ocular tissues. Conventional eye drops exhibit poor corneal permeability, rapid nasolacrimal drainage, low pre-ocular retention time, rapid precorneal clearance, low ocular bioavailability, frequent administration, and rapid drainage due to constant tear flow. To overcome these drawbacks, lipid-based vesicular drug delivery systems such as liposomes, transfersomes, transethosomes, and niosomes are being developed as novel prospective in ocular drug delivery. But with liposomes, there are issues related to physical and chemical stability, oxidative degradation of phospholipids, and poor encapsulation of drugs. They are prone to drug leakage and aggregation of vesicles into larger particles. Niosomes and transferosomes have also reported physical stability issues such as drug-leakage and vesicle fusion. Therefore, nanostructured liquid crystalline particles have been selected as the prospective ocular drug-delivery vehicle [4].
Nanostructured liquid crystalline particles also called cubosomes are discrete, sub-micron cubic nanoparticles consisting of two congruent non-intersecting hydrophilic channels divided by lipid bi-layer. Cubosomes are mainly composed of a binary system of glyceryl monooleate (GMO) and water, where monooleate acts as a precursor for lipid-bilayer. Surfactant provides electrostatic-barrier among particles to avert contact between them, therefore providing steric stabilization to the formulation. Chemically GMO is a mono-easter of glycerol and oleic acid. When assembled into liquid crystalline particles, they mimic the structure of biological-membrane and allow them to amalgamate with highly lipophilic corneal-epithelium. They are assembled in three dimensions as honeycomb (cavernous) structure which is structurally similar to the biological membrane that enables these carriers to fuse with lipophilic corneal epithelium for increased topical drug delivery [5]. The honeycomb structure of cubosomes allows them to retain for a prolonged duration on corneal membrane contrast to spherical vesicles, providing increased time-period for drug-loaded particles to permeate through the cornea. Cubosomes possess a high internal surface-area and present higher encapsulation as they have a higher portion of lipid, consequently exhibits a large surface area. They can be harnessed to encapsulate diverse molecules of amphiphilic, hydro-phobic, and hydro-philic drugs. They possess high thermodynamic stability, bioadhesive nature, bio-compatibility, bio-degradability, protect compounds from physical and enzymatic degradation, and sustained drug release behavior [6].
Therefore, with all the above aspects, vancomycin hydrochloride (VA)-loaded cubosomes were developed, optimized, and characterized in this investigation. Cubosomes were formulated for prolonged pre-corneal residence time, enhanced targeting, increased permeability, reduced dosing frequency, controlled drug release, reduced systemic side-effects, improved bioavailability, and high stability. To assess the efficacy of developed cubosomes, ex vivo corneal permeation studies, in vivo studies, ocular pharmacokinetic studies, and corneal toxicity studies were performed in rabbit’s eyes and compared with marketed eye-drops.
Materials and experimental methods
Materials
Vancomycin was obtained as a gift sample from Cadila Pharmaceuticals Limited, Ahmadabad, India. Glyceryl monooleate was procured from Gattefose, France. Poloxamer 407 and dialysis membrane was purchased from Sigma Chemical Company (Sigma-Aldrich Corp., St. Louis, MO, USA). All other solvents used were of analytical grade. Double-distilled water was utilized during the study.
Methodology
Optimization of formulation variables and process variables
Cubosomes were developed by employing the fragmentation method. Distinct concentrations of GMO ranging from 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5%, and 5% were used. A constant amount of drug was weighed and disseminate in a molten blend of GMO and poloxamer 407 (1% w/v). Similarly, different concentrations of surfactant (poloxamer 407) were taken, 0.1 to 1%, and the other parameters were kept constant. Likewise, different stirring time was evaluated to develop the cubosomes to optimize the best one. Different time for homogenization was taken as 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 min. The rest of the parameters such as concentration of GMO, poloxamer 407, and the stirring speed were kept constant. Stirring time was optimized with respect to entrapment efficiency, polydispersity index, and particle size of cubosomes. Varying stirring speeds from 1000 to 10,000 RPM were optimized so that the formulation doesn’t flocculate or aggregate [7]. The viscosity measurements were carried out by using Brookfield viscometer (LV-DVE model, Brookfield, USA).
Preparation of vancomycin-loaded nanostructured liquid crystalline particles
Cubosomes were developed by virtue of the fragmentation of glyceryl monooleate and poloxamer 407 bulk cubic phase gels. Primarily, GMO and poloxamer 407 were entirely liquefied at 60 °C in a hot water bath, and afterward, vancomycin was incorporated to amalgamate under ceaseless mixing. To accomplish a homogeneous state, water was included moderately and the blend was vortexed. The cubic phase gel was developed following equilibration for 48 h at room temperature. Subsequently, the cubic gel was distorted by adding nearly 20 mL of water during mechanical stirring. Thereupon, high-pressure homogenizer was utilized to homogenize the developed dispersion at high pressures and cycles to form an opalescent dispersion of cubosomes. The final dispersion of nanostructured liquid crystalline particles was stored at room temperature [7].
Taguchi statistical experimental design for optimization of vancomycin-loaded cubosomes
Taguchi method of design of experiment was employed for the optimization of the formulation, which uses standard orthogonal arrays for forming a matrix of experiments in such a way so as to extract the maximum important information with a minimum number of experiments. The fundamental principle of this method serves as screening filters that examine the effects of many process variables and identify those factors which have major effects on the process using a few experiments. It included four factors (formulation variables), i.e., lipid concentration (%v/v), surfactant concentration (%v/v), stirring time (min), and stirring speed (rpm). All the factors were assigned three levels, i.e., low, medium, and high as depicted in Table 1. Based on the number of factors and their levels, L9 (34) orthogonal array was employed as shown in Table 2. Optimization was done on the basis of 2 techniques, i.e., “smaller the better” and “larger the better” and signal to noise ratio (S/N ratio). The signal-to-noise ratio takes the mean as well as the variation between results. The smaller the better is usually chosen signal-to-noise ratio for un-desirable characteristics like “defects” when the desired ideal-value is zero, for example, size of particles and polydispersity index, whereas larger the better is chosen when desired-ideal value is larger, for example, entrapment efficiency [8].
Characterization of vancomycin-loaded nanostructured liquid crystalline particles
Drug excipient compatibility studies
For the ascertainment of compatibility studies of drug and excipients, Fourier transform-infrared spectroscopy (FT-IR) (Perkin Elmer RX1 model) was utilized. Spectra of vancomycin, glyceryl monooleate, poloxamer 407, and a mixture of drug and excipients were examined and then analogized with individual reference spectra. For analysis, 1 mg of test and KBr were blended in the proportion of 1:5, and pellets were developed at high compaction pressure. The spectra were acquired in the infrared region of 450–4000 cm−1 at a resolution of 4 cm−1 [9].
Particle shape and surface morphology
Morphological evaluation of VA-loaded cubosomes was performed utilizing transmission electron microscopy (TEM) (FEI Tecnai TF20). It is a fundamental instrument for legitimately imaging nanoparticles to acquire quantitative analysis of particle size, surface morphology, shape, and size distribution. To prepare the sample for TEM, a droplet of developed cubosomes was deposited on a 300-mesh copper lattice smeared by amorphous carbon film and then air-dried. Surplus fluid was eliminated by an adsorbent paper, and cubosomes were scrutinized at 250,000 × and 80,000 × magnifications at 200 kV voltage acceleration [10].
Particle size distribution and polydispersity index
Particle size distribution and polydispersity index (PDI) of cubosomes were analyzed by dynamic light scattering technique utilizing Malvern nano S90 (Malvern Instruments, Worcestershire, UK). Cubosomal formulations were diluted with de-ionized water and poured into the cuvette and then placed in analyzing chamber. The temperature was kept at 25 °C and the angle was fixed at 90° during the analysis [11].
Zeta potential measurement
Zeta potential is contemplated as a crucial indicator for the estimation of surface charge and physical stability of nanoparticles. Estimation of zeta potential of cubosomes was ascertained by electrophoretic mobility of particles. For measurement, 1 mL of sample was taken and diluted in 10 mL of de-ionized water. In a U-type tube, cubosomes were poured in Zetasizer (3000HS Malvern Instruments, Worcestershire, UK) at a temperature of 25 °C and analyzed [12].
Drug entrapment efficiency
Drug entrapment efficiency (EE) of VA-loaded cubosomes was determined by calculating the percentage of the amount of drug entrapped by cubosomes. The developed formulations were evaluated by centrifugation technique; in this method, cubosomes were centrifuged at 12,000 rpm for 30 min employing refrigerated centrifuge (Remi, C-24BL, New Delhi, India). The resultant suspension was then segregated, and the supernatant was accumulated. The absorbance of the supernatant was analyzed spectrophotometrically at absorption maxima (λ max) 281 nm by using a UV–visible spectrophotometer (UV-1800, Shimadzu, Japan) [13]. The % EE was calculated by using the formula:
In vitro drug release studies
The release of vancomycin from cubosomes was demonstrated by employing Franz diffusion (FD) cell. Dialysis membrane of molecular weight cut off 12,000–14,000 Da (Spectra Pro, Spectrum Chemical Manufacturing Corp., USA) and pore size was 2.4 nm. The surface area of the dialysis membrane for the release of the drug was 3.14 cm2. The receiver compartment was filled with simulated tear fluid (STF) having pH 7.4 as a medium. Dialysis membrane was positioned in the FD-cell and the donor compartment; cubosomal preparation was placed [14]. FD cell was thermostatically maintained at 37 °C ± 1 °C under constant magnetic stirring. A total of 5 mL of sample were drawn from the receiver chamber at pre-determined interludes and concurrently replenished with an equivalent quantity of buffer to perpetuate sink conditions. The withdrawn samples were appraised for cumulative percentage drug release by using a UV–visible spectrophotometer at 281 nm, and the graph was plotted [15].
Ex vivo transcorneal permeation studies
Ex vivo transcorneal permeation studies were carried out on rabbit cornea utilizing FD-cell. Cornea, and 4–5 mm of scleral tissues were cautiously extracted and rinsed with cold saline to eliminate protein adhered to the tissue. The freshly isolated cornea was mounted in-between donor and receptor chamber in such a manner that the epithelial side was positioned towards the donor chamber and the endothelial side towards the receptor chamber. Simulated tear fluid of pH 7.4 was added in the receptor chamber, and the temperature was thermoregulated at 37 °C ± 1 °C with persistent magnetic agitation. An aliquot of optimized VA-cubosomal formulation and VA-solution was introduced on the corneal surface for correlative examination. From the receptor compartment, 1 mL of the sample was drawn out at a pre-established schedule and afterward replaced with an equivalent volume of STF [16]. The samples were investigated by a UV–visible spectrophotometer at 281 nm. The drug permeation % was determined by the following;
Corneal hydration studies
The effect of the VA-loaded cubosomes and VA-solution on corneal hydration was determined. In brief, at the end of the experiment, the scleral tissue was removed from the cornea; its epithelial surface was wiped with filter paper and weighed (initial weight). The cornea was then soaked in 1 mL of methanol, dried overnight at 90 °C, and reweighed (final weight) [17]. From the difference in weight, corneal hydration (%) was calculated as follows:
Bioadhesion studies
Bioadhesion studies were performed to ascertain the potential of the developed formulation to cling with the corneal surface for a prolonged duration. Rabbit eyes were exercised and the cornea was extracted and cleaned. The extricated cornea was kept on petri-dish containing STF with mucosal side of the cornea outwards. An aliquot of the formulation was applied to the cornea followed by a droplet of water-soluble dye. The ocular tissue was kept hydrated by perpetuating the supply of STF. Bioadhesion was visually perceived for its integrity at designated interludes up to 24 h [18].
Ocular tolerance test
Hen’s Egg Test/Huhner-Embroynen Test on Chorioallantoic membrane (HET-CAM) is a substitute method to Draize test done on rabbit eyes. In this investigation, ocular irritancy, vascular response, hemorrhage, and injuries to the conjunctiva are observed for an ophthalmic product. Fresh fertilized hen’s eggs weighing 50–60 g were acquired from a poultry farm. Briefly, fertilized eggs were incubated for 3 days at 37 ± 0.5 °C and 55 ± 5% relative humidity [19]. On day 3, albumin (3 mL) was drawn-out from the eggs utilizing a sterilized approach, and the hole was fastened with paraffin sterilized with 70% alcohol. On day 10 of incubation, at the equator, 2 × 2 cm of eggshell was removed to create a window [20]. Normal saline (0.9%, negative control), sodium hydroxide (0.1 N, positive control), and VA-cubosomes (test formulation) were administered directly to the surface of CAM. At pre-established interims, CAM was observed for vascular responses/injuries and scores were specified as per the scoring scheme. No visible hemorrhage = 0 (Non-irritant), observable membrane discoloration = 1 (mild irritant), structures covered slightly because of membrane discoloration/hemorrhage = 2 (moderately irritant), and structures covered completely owing to membrane discoloration/hemorrhage = 3 (severe irritant) [21].
Anti-microbial studies
Comparative anti-microbial activity of VA-cubosomes and VA-solution were determined utilizing agar well-diffusion technique along with cup-plate technique. These studies were performed on Staphylococcus aureus and Pseudomonas aeruginosa. Media was composed by blending agar and nutrient broth and sterilized by autoclave at 15 lb/sq-inch at 121 °C for 60 min. In two distinct petri-dish, nutrient agar media was poured and was inoculated with S. aureus and P. aeruginosa in sterile conditions under a laminar airflow unit. Subsequently, in the inoculated solidified nutrient-agar plates, two wells of 8 mm were created with the assistance of a sterile cork-borer. The wells were loaded with vancomycin solution as control and developed cubosomes as test and then incubated for 24 h at 37 °C. The diameter of the inhibition zone was calculated and compared with the control [22].
Isotonicity evaluation
Isotonicity of ophthalmic preparation has to be maintained to avert tissue damage and corneal irritation. Isotonicity assessment was performed by the hemolytic method. The developed formulation of VA-cubosomes was blended with red blood cells of mice and perceived with an inverted microscope at a magnification of 45 × . The concentration of hypotonic solution was (0.45% w/v NaCl), hypertonic solution (3% w/v), and isotonic solution (0.9% w/v NaCl). The aforementioned method was replicated for isotonic solution (negative control), hypotonic solution (positive control) along hypertonic solution to observe bursting, swelling, and cremation effect on red blood cells [23].
Sterility studies
Sterility is a crucial requirement for ophthalmic preparation as contamination may cause ocular infections. Sterility studies are performed to detect the existence of pathogens in the developed ocular formulations [24]. Analysis was executed on the optimized cubosomal formulation by direct inoculation technique as described in Indian Pharmacopoeia 2010. In this study, 2 mL of VA-cubosomes were transferred to two distinct flasks encompassing 10 mL of soybean-casein digest medium and fluid thioglycollate medium. The inoculated media was blended with medium and was incubated at 20–25 °C for soyabean-casein digest media and 30–35 °C for fluid-thioglycollate medium for 14 days. After the end of 14 days, the incubated flasks were scrutinized for any indication of the growth of microorganisms [25].
Drug release kinetic studies
For the determination of drug release kinetics of developed formulation, various kinetic models were implemented. Data acquired from in vitro drug release studies were fitted into different kinetic models such as Korsmeyer-Peppas model (log percentage drug release v/s log time), Higuchi’s model (percentage of drug release v/s square root of time), first-order equation (log percentage of drug remaining v/s time), and zero-order equation (cumulative amount of drug release v/s time). For respective models, a correlation co-efficient was evaluated [26].
In vivo studies
Rabbits were provided by Institutional Animal House, Amity University, Noida, India. The study was approved by University Animal Ethical Committee with registration number 1327/PO/ReBi/S/10/CPCSEA. All institutional and national guidelines for the care and use of laboratory animals were followed. In vivo studies were performed on rabbit’s eyes which were induced with bacterial keratitis. In this investigation, twelve New Zealand albino rabbits weighing 2–3 kg with no sign of ocular inflammation and gross abnormalities were utilized. Rabbits were categorized into 3 groups of 4 rabbits. Group I (control) was instilled with sterile saline solution, group II was topically administered with VA-solution, and group III was administered with optimized VA-cubosomes. In group III, 50 µL of optimized cubosomes were introduced twice a day and in group II vancomycin solution four times a day [27].
S. aureus was grown aerobically in 10 mL of brain heart infusion broth (HiMedia, India) at 37 °C for 18 h. Microorganisms were collected by centrifugation (Remi, C-24BL, New Delhi, India) at 5000 rpm for 10 min and washed with phosphate buffer saline (PBS) pH 7.4, and suspended in 2 mL PBS. Keratitis was induced by inoculating 100 µL S. aureus suspension (containing nearly 107–108 colony-forming units) in the rabbit’s eyes by intrastromal injection with a 29-gauge needle. Manifestations of keratitis were developed after 24 h of microbial inoculation into the eyes [28, 29]. Rabbit’s eyes were evaluated for clinical signs of bacterial keratitis such as redness, mucoid discharge, lacrimal secretion, corneal ulcer, and swelling of the eyelid. The grading scale was followed on the scale from 0 to 4 as 0 (absent), 1 (mild), 2 (moderate), 3 (severe), and 4 (extensive). The grading of clinical signs was performed at intervals of 0, 24, 72, and 120 h. The formulations were instilled into the eyes of rabbits following the complete development of infection. The eyes were perceived for the recuperation of infected eyes on each successive day by the aforementioned categorization till complete recovery from keratitis. Comparative analysis of developed VA-loaded cubosomes with vancomycin solution was done, and the treatment results were compared. Significance was determined by applying an unpaired t-test [30].
Ocular pharmacokinetic studies
The rabbits were randomly divided into the following two groups: a group treated with vancomycin solution and a group treated with vancomycin cubosomes. Ocular absorption of VA-cubosomes was assessed on aqueous humor fluids. Pupils of rabbits were dilated with topical instillation of 0.25% tropicamide. Rabbits were anesthetized through-out the investigation using an injection of sodium pentobarbital (30 mg/kg) injected into marginal ear-vein. The rabbits were administered with 50 µL of optimized cubosomal formulation in the lower conjunctival sac of the right eyes and vancomycin solution in the left eyes. Aqueous humor samples (50–80 μL) were withdrawn at different time intervals at 0.25, 0.5, 1, 2, 3, 4, 5, and 6 h by anterior chamber paracentesis utilizing 1 mL insulin syringe attached with a 26-gauge needle. The aqueous humor samples were collected in glass tubes and stored at − 20 °C [31]. The study was repeated in triplicate, and samples were examined utilizing UV-spectrophotometer. The pharmacokinetic parameters such as area under the concentration–time curve (AUC0→t) and peak aqueous humor concentration (Cmax) were calculated from appropriate graphs using the linear trapezoidal rule (Kaleidagraph, Synergy Software) [32].
Corneal toxicity studies (histopathology studies)
Histopathology studies were done to corroborate the results obtained from in vivo studies on developed cubosomes and also to scrutinize corneal structure and integrity. Rabbits were euthanized with an injection of sodium pentobarbital, and then eyeballs were removed. Rabbit corneas were cautiously exercised and incubated with optimized VA-cubosomes (test sample), sodium dodecyl sulfate 0.1% w/v (positive control), and phosphate buffer saline (negative control) for 2 h. Following incubation, rabbit corneas were rinsed with phosphate buffer saline (pH 6.4). Subsequently, corneas were instantaneously fixed with an 8% v/v formalin solution. Corneal tissues were dehydrated with an alcohol gradient and placed in melted paraffin and then hardened to form a block. Cross-sections of blocks < 1 mm were slit and stained with haematoxyline and eosine. The slides were cover-slipped and assessed under a microscope [33].
Stability studies
The aim of stability studies was to determine that the developed formulation has preserved its efficacy and productivity with the progression of time, humidity, and temperature. Comprehensive stability data was collected for shelf-life determination and slope and degradation constant of VA-loaded cubosomes. The protocols for the determination of stability studies were in accord with ICH guidelines. For evaluation for stability, the developed cubosomal preparation was subjected to distinct humidity and temperature conditions. Optimized VA-cubosomes were kept in screw-capped amber colored glass vials and stored at 5 ± 1 °C, 25 ± 2 °C, 40 ± 2 °C, 60 ± 2 °C, and 75 ± 5% relative humidity for a period of 6 months. The samples were drawn out at different interims viz. 0, 30, 60, 90, and 180 days and investigated for entrapment efficiency, particle size distribution, polydispersity index, pH, in vitro drug release, and phase separation [34].
Results and discussion
Optimization of formulation variables and process variables
For optimization of formulation variables and process variables, different characterization parameters were considered such as particle size, polydispersity-index, and entrapment efficiency. In the optimization of lipid concentration, an increment in particle size and decreased drug entrapment was perceived on increasing the ratio of drug to lipid. On increasing the lipid concentration from 0.5 to 5% v/v, the size of the cubosomes increased from 46.24 ± 0.58 nm to 273.55 ± 0.43 nm. The formulations with the concentration of 1%, 1.5%, and 2% glyceryl monooleate demonstrated the best appropriateness for ocular delivery. On increasing the concentration of lipid from 0.5–2.5% v/v, increased entrapment was observed as a large number of cubosomes were formulated which led to higher drug entrapment. Increasing the quantity of glyceryl monooleate may have resulted in faster solidification of cubosomes. But on further augmentation of lipid from 3 to 5% v/v, drug entrapment was decreased which might be due to an increase in viscosity that might have hindered the diffusion of drug in the lipid matrix [35].
The concentration of surfactant was optimized so as to acquire cubosomes of small size with the highest drug entrapment. 0.1%, 0.5%, and 1% w/v concentration of surfactant was considered as optimization concentration as it led to low PDI, low particle size of 46.24 ± 0.58, 82.31 ± 0.26, and 68.22 ± 0.85 nm with better entrapment efficiency of 87.28%, 86.92%, and 89.25%. Poloxamer 407 provided steric stabilization in opposition to the accumulation of cubosomes particles by securing its poly-propylene oxide that causes impediment in polar-region or at the exterior of glyceryl monooleate bi-layer. Cubosomes were synthesized at a distinct stirring time, and 2, 2.5, and 3 min were the optimized stirring time. The stirring speed of homogenization was varied from 1000 to 10,000 rpm. The stirring speed of 4000, 5000, and 6000 rpm were optimized as small particle size, and high drug entrapment was attained. Higher shear was acquired on escalating the stirring speed that decreased the particle size [36].
Drug-excipient compatibility studies
FT-IR was done to analyze any physico-chemical influence on the drug and its excipients and to establish that chosen excipients were compatible with the drug. FT-IR spectroscopy of vancomycin, glyceryl monooleate, poloxamer 407, and physical mixture of drug-excipients are depicted in Fig. 1. Pure vancomycin manifested its characteristics sharp peak at 3418.51 cm−1 that was ascribed to N–H stretching, 1505.76 cm−1 due to C = C stretching, 1383.18 cm−1 indicated OH phenol group, and C–O–C stretching at 1230.26 cm−1 and 1062.47 cm−1 aliphatic amine for C-N stretching were in accordance with standard vancomycin FT-IR data [37, 38].
The FT-IR spectrum of pure GMO displayed characteristics peaks in the range of 3423–765 cm−1. Spectra of GMO exhibited a broad sharp peak at 3423 cm−1 that could be attributed to stretching of O–H group, peak at 2926 cm−1 were ascribed to CH2 stretching, and the band at 1597 cm−1 indicated the presence of C–C bonds. The sharp peak at 1384 cm−1 designated nitrate absorption band, and peak at 618 cm−1 was mainly due to C-H bending [39].
Pure poloxamer 407 exhibited a characteristic broad peak at 3425 cm−1 due to O–H group stretching, 2889 cm−1 peak indicated C-H stretching vibration, sharp peak at 1596 cm−1 corresponded to C–C stretching, and sharp peak at 1384 cm−1 indicated nitrate absorption band. The peak at 1113 cm−1 corresponded to C-O stretching vibration, and a slight peak at 766 cm−1 was related to C-N stretch of an aromatic, and the peak at 618 cm−1 was due to C-H bending [40, 41]. A combination of vancomycin and excipients exhibited absorbance in the range of 3419 to 617 cm−1. From the FTIR spectra, it was observed that there was compatibility between drug, GMO, and poloxamer 407. The combination exhibited similar functional groups with extremely slight shift demonstration outstanding compatibility, and no evidence of interaction between the physical mixture of drug and excipients [42].
Particle shape and surface morphology
VA-loaded cubosomes were envisaged for particle shape and surface morphology through transmission electron microscopy. TEM photomicrographs illustrated nearly cubic organization with well-dispersed individual particles as shown in Fig. 2. Cubosomes were uniformly distributed without any aggregation that could be accredited to the optimum combination of drug, excipient, stirring-time, and stirring-speed. A large concentration of surfactant adsorbed on the exterior of cubosomes acted as overlaying covering to stabilize cubosomes surface, whereas low surfactant quantity caused enlargement in particle-size due to expansion in inter-facial pressure that caused accumulation of particles [43, 44].
Particle size distribution and polydispersity index
Dynamic light scattering (DLS) measurements were employed to assess size distribution and polydispersity index of developed cubosomes. Particles size values were between 51.11 ± 0.96 nm to 158.73 ± 0.46 nm. Usually, molecular size should be less than 200 nm for easy permeation across the corneal membrane. The developed formulation had a molecular size smaller than 200 nm that ensures adequate bioavailability, good uniformity, and significant potential for effortless permeation. The outcome divulged that particle-size of cubosomes was inversely proportional to an increase in the quantity of surfactant [45]. High poloxamer quantity could have reduced surface tension of lipid-phase and aqueous phase that might have reduced particle size. A high poloxamer amount might have reduced steric hindrances. On minimizing the amount of surfactant, particle size was expanded because of decreased inter-facial stability that was due to inadequate concentration of poloxamer that caused agglomeration of cubosomes [46]. PDI was found to be in the range of 0.275 ± 0.53 to 0.525 ± 0.48. PDI is used to describe the degree of non-uniformity for the nanoparticle size distributions. PDI close to 0.5 indicates good monodispersity or fairly monodisperse particles. Particle size distribution and polydispersity index of formulation have been tabulated in Table 3.
Zeta potential measurement
Zeta potential measurement was analyzed to ascertain the surface charge of cubosomes that is of great significance for anticipating prolonged stability. Zeta potential revealed that vancomycin cubosomes carried a negative charge with mean values of − 21.53 ± 0.29 to − 30.36 ± 0.41 as represented in Table 3. A total of − 40 to + 40 mv is considered as ideal stability range of zeta potential for formulations. High zeta potential manifests high electric repulsion between dispersed particles which averts agglomeration and provides good stability and dispersion quality [47]. A negative charge could be due to the existence of fatty acid in glyceryl monooleate. Non-ionic surfactant might have caused a negative charge because of hydroxyl ions anchored on the exterior of cubosomes and association of hydroxyl group with an aqueous medium. Poloxamer 407 provided good steric stability as it stabilized the formulations by steric hindrances that avoided aggregation [48].
Drug entrapment efficiency
Drug entrapment efficiency was evaluated to determine the concentration of drug incorporated in VA-cubosomes. Entrapment efficiency was found between 72.181% ± 0.64 to 91.624% ± 0.42 as shown in Table 3. The strong affinity between drug and lipid in cubosomes might have acquired the drug into its inverted-type self-assembled nanostructure. Cubic crystalline structure promotes high drug entrapment as they have a higher portion of lipid that presents a large surface area. Entrapment efficiency was determined by the concentration of glyceryl monooleate and poloxamer [49]. The outcome indicated that with the increase in the concentration of lipid and surfactant, the entrapment was also increased. This could be attributed to the increased viscosity of the formulation, as the concentration of lipid was increased, cubosomal dispersion was solidified faster that could have prevented the diffusion of the drug to the external phase medium. On further escalating the concentration of lipid, reduction in entrapment was perceived which could be due to immoderate viscosity of cubosomal dispersion that obstructs dispersion of drug from the binary lipid matrices [50].
In vitro drug release studies
The release of vancomycin from developed cubosomes was carried-out utilizing FD-cell for 24 h. The release of drug-loaded formulations ranged from 79.43% ± 0.68 to 92.32% ± 0.29 for 24 h. The in vitro release profile of VA-cubosomes manifested that the formulations demonstrated initial burst release up to 14.42% ± 0.54 and sustained release up to 92.32% ± 0.29. One probable rationale for burst release could be attributed to the spreading of free-drug adsorbed onto cubosome particle surface which could easily diffuse [51], whereas drug encompassed into particle core would release over extended time due to firm entrapment of drug by lipid layer. This pattern of slow-release could be ascribed to sedated expulsion of drug from inner-water channels in the cubic phases. Both the properties of initial-burst release and sustained drug release were beneficial in the ocular delivery of drug. Initial-burst was favorable for ameliorating permeation of the drug and provided rapid onset drug action; however, sustained-release provided action of drug over a protracted time-span. The sustained-release pattern of drug release up to 24 h would facilitate reduced dosing frequency [52]. In vitro drug release of vancomycin from cubosomes is depicted in Fig. 3.
Ex vivo transcorneal permeation studies
Transcorneal permeation studies of VA-cubosomes and VA-solution were evaluated through FD cell on rabbit cornea. On the basis of small particle size, PDI, surface morphology, entrapment efficiency, and in vitro drug release, formulation F8 was selected as the optimized formulation. The permeation studies demonstrated that a linear-relationship between accumulative permeated vancomycin and time could be perceived. The apparent permeability co-efficient (Papp) of VA-loaded cubosomes and vancomycin solution was 4.48 ± 0.62 × 106 and 1.83 ± 0.84 × 106 µg/s/cm2, respectively. Steady state flux (Jss) was found to be 1.45 ± 0.26 × 103 and 0.67 ± 0.59 103 μg/cm2/h. Compared with vancomycin solution, VA-cubosomes demonstrated a nearly 2.4-fold increase in permeability co-efficient and 2.1-fold increase in a steady state flux. Significant higher corneal permeation of optimized formulation could be due to strong mucoadhesive property and nano-dimension of nanostructured liquid crystalline particles. Enhanced permeation of optimized cubosomes via paracellular route could be due to the presence of surfactant that could have led to disturbance in the tight junction of epithelial cells of the cornea. Ameliorated drug permeation could also be due to structural similarity between bi-continuous lipid bi-layer of cubosomes and corneal epithelial cells that might permit membrane fusion, facilitating direct drug absorption into corneal cells [53]. Transcorneal permeation profiles of VA-loaded cubosomes and VA-solution are graphically represented in Fig. 4.
Corneal hydration studies
The corneal hydration level of the normal mammalian cornea is between 75 and 83%, whereas 83–92% hydration level denotes damage to the cornea. The corneal hydration value of the optimized formulation and vancomycin solution was between 75 and 82%, which indicated that the formulation did not cause any harm to the corneal tissue. The corneal hydration level of VA-cubosomes was 81.68% ± 0.73, and VA-solution was 79.53% ± 0.29.
Bioadhesion studies
The purpose of the bioadhesion studies was to perceive the nature of developed cubosome adherence to cornea tissues to resolve the drainage issue in contrast to vancomycin solution. To improve the visibility of the formulation, indicator dye was used. It was observed that the intensity of color diminished progressively over 24 h time period. This indicated the adherence of cubosomal formulation to corneal surface over the entire duration of the study. The optimized formulation F8 remained intact in the corneal surface for 24 h and that could be attributed to the optimum concentration of lipid and surfactant that prolonged the bioadhesion time.
Ocular tolerance test
HET-CAM assay was employed to assess the predictive model for in vitro irritation by perceiving vascular damage, hemorrhage, hyperemia, coagulation, and computing irritation-score. Arteries, veins, and capillaries are encompassed in CAM that gives identical reaction as inflammation-induced in corneal tissue of rabbits during draize-eye irritancy test. With normal saline, a 0 mean score was observed [54]. Optimized VA-cubosomes were subjected as a test and showed 0 mean score up to 12 h, and 0 score was found up to 24 h. Results denoted that formulation was non-irritating in nature and could be well-tolerated and was safe for application to eyes. It could be accredited to the bio-compatible and non-immunogenic nature of cubosomes. Positive control reported hemorrhage and coagulation followed by lysis that confirmed severe irritant nature with a mean score of 3 [55]. Figure 5 represents photographs illustrating the results of the HET-CAM test.
Anti-microbial studies
The anti-microbial efficacy of optimized cubosomal formulation and vancomycin solution was executed against Staphylococcus aureus and Pseudomonas aeruginosa. VA-cubosomes displayed a higher growth inhibitory effect as compared to control. The anti-microbial properties exhibited that the diameter of zone of inhibition against S. aureus for VA-cubosomal formulation was 36 mm and against P. aeruginosa was 30 mm which was significantly higher than VA-solution. A higher zone of inhibition acquired by synthesized cubosomes indicated that the formulation had optimum viscosity that resulted in sustained drug release that maintained minimum inhibitory concentration for an extended time period [56, 57]. Pictorial presentation of anti-microbial studies is depicted in Fig. 6.
Isotonicity evaluation
Isotonicity test was performed to examine whether the tone of the cell would be influenced by the entry of water from the instilled solution as an instance of hypo-tonic solution or egress of water as an instance of the hyper-tonic solution. Hypertonic and hypotonic formulations tend to cause irritation, pain, and tissue damage to the eyes. Ideally, the ophthalmic formulations should be isotonic in nature so that they do not cause discomfort, irritation, or swelling. Microscopic observations illustrated that blood cells treated with hypertonic solution seemed shrunk, whereas hypotonic solution treated blood cells were swollen. Although RBCs treated with formulated cubosomes and isotonic solution were unaltered and did not show lysis or crenation of blood cells, the results implied that VA-cubosomes were isotonic with lachrymal fluid and blood and were found to be non-irritating with no ocular damage [58]. Microscopic images for isotonicity evaluation have been depicted in Fig. 7.
Sterility Studies
For sterility studies, fluid thioglycollate medium was employed to detect aerobic bacteria while soya-bean casein digest was used to detect anaerobic fungi and bacteria. Sterility testing corroborated that there was an absence of turbidity and no bacteriostatic and fungistatic activity was perceived after incubation for 14 days. Therefore, the optimized VA-cubosomes were found sterile and free from microorganisms [59].
Drug release kinetics studies
Formulation F8 was optimized on the basis of drug release studies to determine drug release kinetic. Data acquired from in vitro release profile was fitted into diverse kinetic models. The results manifested that the optimized cubosomal formulation was predominantly elucidated by first order (R2 = 0.9831, K = 0.048), followed by Higuchi’s equation (R2 = 0.9762, K = 19.045) and zero order (R2 = 0.8724, K = 3.683). The corresponding plot for Korsmeyer-Peppas equation indicated good linearity (R2 = 0.9817). The release exponent “n” was found to be 0.6032, which indicated non-Fickian or anomalous transport. In non-Fickian release diffusion, the rates of vancomycin release from cubosomes were governed by more than one process, i.e., diffusion controlled and swelling controlled release.
In vivo studies
The optimized VA-cubosomal formulation and VA-solution were subjected to in vivo studies, and treatments effects were compared. Criteria taken for the response to drug therapy were redness, lacrimal secretion, mucoid discharge, eye-lid swelling, and corneal ulcer. The eyes of rabbits were examined and recorded for ocular damage or abnormal signs to conjunctiva, iris, or cornea and systemic side-effects [60]. Scores of cubosomal formulation and VA-solution were compared, and results indicated that optimized formulation was efficacious in mitigating manifestation of keratitis as depicted in Table 4. After 120 h, the score of the redness in group II was 2.927 ± 0.59, whereas in group III was considerably lower 0.09 ± 0.0. Lacrimal secretion and mucoid discharge in group II was 2.032 ± 0.63 and 1.649 ± 0.19, whereas in group III, it was 0.07 ± 0.0 and 0.08 ± 0.0, respectively. The intensity of keratitis was reduced after 72 h by the optimized cubosomes, and after 120 h, the symptoms were significantly relieved. Images during treatment of infected rabbit eyes at different time intervals are depicted in Fig. 8. The rate of frequency of administration was also lowered as compared to the solution. Remarkable better results were acquired with vancomycin cubosomes in reducing the severity of keratitis [61]. Grading of parameters of keratitis was done, and the results of an un-paired t-test between 2 groups demonstrated that cubosomal formulation showed significantly superior response as shown in Table 5.
Ocular pharmacokinetic studies
Optimized cubosomal formulation and VA-solution were subjected to in vivo ocular pharmacokinetic studies to evaluate the level of drug in aqueous humor. Conventional ocular formulations have several problems associated with them such as pre-corneal drug loss and poor bioavailability. For a successful therapy, it is important to maintain the drug in aqueous humor to enhance the bio-availability of the drug. The results depicted that drug concentration in aqueous humor showed a maximum at 1 h after administration. A higher concentration of antibiotics is always preferable at an early time of infection. The area under curve (AUC) value of cubosomal formulation was 307.63 ± 0.43 µg min/mL, and VA-solution was 137.72 ± 0.21 µg min/mL, which was 2.1-fold higher, whereas the peak concentration (Cmax) of VA-cubosomes was 3.16 ± 0.27 µg/mL, whereas VA-solution was 1.24 ± 0.59 µg/mL which was nearly 2.5-fold higher (p < 0.01). The time to peak concentration (Tmax) of cubosomal formulation was 58.55 ± 0.38 min, which was about 1.9-fold higher. Mean residence time of VA-cubosomes was 107.87 ± 0.92 min, and VA-solution was 49.26 ± 0.46 min [62]. The transcorneal penetration of vancomycin might be increased due to interaction between cubosomes and cornea in the presence of glyceryl monooleate. Moreover, prolonged mean residence time and Tmax could be attributed to close adherence of cubosomes to ciliary muscle and the conjunctival sac that would provide subsequent absorption and surface-lipid exchange effect [63]. That might form a drug depot in the corneal area and would provide more drug to the anterior chamber of the eyes. Increased ocular-bioavailability could be ascribed to 3 factors: enhanced drug-loading capacity, permeation capability of the drug through the corneal membrane, and ocular contact-time of cubosomes. These factors corroborated intimate contiguity in-between drug and epithelial mucosa, averting tear-washout and therefore correlated with sustained-release of drug and protracted drug-retention [64]. Concentration–time profiles of vancomycin in the aqueous humor are graphically represented in Fig. 9.
Corneal toxicity studies (histopathology studies)
Histopathological studies confirmed the results obtained from in vivo studies. The cross-section of the ocular tissues of the rabbit was treated with optimized VA-cubosomes, sodium dodecyl sulfate, and phosphate buffer saline as illustrated in Fig. 10. Histopathological studies were performed to corroborate the existence of normal corneal tissues by retaining their morphology without causing impairment to tissues. In positive control, the epithelial layer was grievously damaged with absolute separation from the bowman’s layer at diverse regions of the corneal epithelium. Distorted cells were evidently observable with significant corneal swelling, whereas corneal tissues incubated with normal saline and VA-cubosomes demonstrated no cytotoxicity and intact epithelial layer with no changes in corneal cross-sections. The corneal layers displayed well-maintained morphology without any stromal swelling, consequently indicating the safety of formulation [65].
Stability studies
Stability studies were performed over a period of 6 months, and formulations were stored at varying temperatures 5 ± 1 °C, 25 ± 2 °C, 40 ± 2 °C, 60 ± 2 °C, and 75 ± 5% RH to analyze the stability. The optimized cubosomes were observed for entrapment efficiency, particle size distribution, polydispersity index, pH, in vitro drug release, precipitation, and phase separation. The formulation exhibited satisfactory results and remained stable for 6 months. There were no changes in the physical appearance, and no precipitation and phase separation was observed. Slight changes were perceived in the formulation at 60 °C. Table 6 illustrates the slope and degradation constant.
Shelf life determination (Arrhenius plot)
Shelf-life determination was calculated by Arrhenius plot. Value of log K was obtained − 4.2485 by extrapolation of Arrhenius plot. Optimized VA-cubosomes exhibited shelf-life in range of 3.5 years. Consequently, an arbitrary shelf-life of 3 years could be assigned to cubosomal formulation.
Conclusion
In the present study, novel nanostructured liquid crystalline particles were developed using glyceryl monooleate and poloxamer 407 by fabrication method. The prospect of cubosomes as a carrier for topical administration was established by the results acquired, demonstrating drug penetration. FTIR results revealed that there was no interaction between the drug and the excipient of the formulations. TEM confirmed cubic organization with well-dispersed individual particles. HET-CAM assay denoted that the formulation was non-irritating in nature and could be well-tolerated and was safe for application to eyes. Ex vivo studies revealed significantly high corneal permeation of optimized formulation, whereas in vivo studies demonstrated that the intensity of keratitis was reduced after 72 h, and after 120 h, the symptoms were significantly relieved. Ocular pharmacokinetic studies evaluated drug level in aqueous humor and results revealed that AUC, Tmax, Cmax, and MRT of cubosomal formulation were considerably higher than vancomycin solution. Histological evaluation revealed that vancomycin-loaded cubosomes demonstrated no cytotoxicity and corneal layers displayed intact epithelial layer without any deleterious influence on corneal structure or stromal swelling, consequently indicating the safety of formulation. It could be concluded that the developed VA-loaded cubosomes have great potential as a viable substitute as compared to conventional eye-drops.
Availability of data and materials
All data generated and analysed during this study are included in this article.
References
Anutra M, Somsanguan A, Phuriwat L, Chutiporn L, Robert M. Chitosan as an ocular drug delivery vehicle for vancomycin. J Appl Polym Sci. 2019;122:3160-3167. https://doi.org/10.1002/app.34323.
Zavarshani M, Ahmadi M, Saei HD, Tehrani AA, Naghadeh BD. Comparison therapeutic effects of ciprofloxacin, silver nanoparticles and their combination in the treatment of Pseudomonas keratitis in rabbit: an experimental study. Iran J Pharm Res. 2019;18(1);320–327. https://doi.org/10.22037/IJPR.2019.2328.
Pooja P, Roma M, Sreeja MK. Development and optimisation of quercetin cubosomes incorporated in glyceryl monooleate aided by design expert software. Ijppr.Human. 2018;11(4):80-106. https://www.ijppr.humanjournals.com/wpcontent/uploads/2018/04/8.Pooja-Poulose-Roma-Mathew-Sreeja-M.K.pdf.
Swati C, Neha G, Upendra N. Fabrication and evaluation of ultra deformable vesicles for atopic dermatitis as topical delivery. Int J Polym Mater Polym Bioma. 2018;68(5):266-277. https://doi.org/10.1080/00914037.2018.1443932.
Nilesh RR, Suprit DS, Nishikant AR, Jayashree BT, Pramod BK, Vivek SD. Nanostructured cubosomes in a thermoresponsive depot system: an alternative approach for the controlled delivery of docetaxel. AAPS PharmSciTech. 2016;17(2):436-445. https://doi.org/10.1208/s12249-015-0369-y.
Shreya K, Neha J, Jaya P, Upendra N. Investigating the retention potential of chitosan nanoparticulate gel: design, development, in-vitro & ex-vivo characterization. Recent Pat Antiinfect Drug Discov. 2020;15(1):41-67. https://doi.org/10.2174/1574891x14666191014141558.
Yuanfeng W, Jianjun Z, Yazhen Z, Yaxiang G, Meng F, Chengran L, Liang X, Changquan CS, Yuan G, Shuai, Q. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019;9:6287–6298. https://doi.org/10.1039/C8RA10302J.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19): 5748–56. https://pubs.acs.org/doi/abs/10.1021/la010161w.
Gulati N, Nagaich U, Saraf SA. Intranasal delivery of chitosan nanoparticles for migraine therapy. Sci Pharm. 2013;81(3):843–854. https://doi.org/10.3797/scipharm.1208-18.
Faiza F, Ayesha N, Shakil A, Syed THS, Muhammad IB. A green approach for the determination of selected anti-diabetic drugs in pharmaceutical formulation by transmission FTIR spectroscopy. J Braz Chem Soc. 2014;25(11):2033-2038. https://doi.org/10.5935/0103-5053.20140188.
Nasra M, Ghoraba MK, Abdelazem A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharmaceutica Sinica B. 2015;5(1):79–88. https://doi.org/10.1016/j.apsb.2014.12.001.
Bei D, Zhang T, Murowchick JB, Youan BB. Formulation of dacarbazine-loaded cubosomes. Part III. Physicochemical characterization. AAPS PharmSciTech. 2010;1(3): 1243–1249. https://doi.org/10.1208/s12249-010-9496-7.
Boyd BJ. Characterization of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003;260(2):239–247. https://doi.org/10.1016/s0378-5173(03)00262-x.
Rizwan SB, Hanley T, Boyd BJ, Rades T, Hook S. Liquid crystalline systems of phytantriol and glyceryl monooleate containing a hydrophilic protein: characterisation, swelling and release kinetics. J Pharm Sci. 2009;98(11):4191–4204. https://doi.org/10.1002/jps.21724.
Verma D, Kaul S, Jain N, Nagaich U. Fabrication and characterization of ocular phase transition systems for blepharitis: a novel approach. Drug Delivery Letters. 2020;10(1):24-37. https://doi.org/10.2174/2210303109666190614115304.
Meetali M, Pravin K. Preparation and in vitro/ex vivo evaluation of moxifloxacin-loaded plga nanosuspensions for ophthalmic application. Sci Pharm. 2013;81(2):591–606. https://doi.org/10.3797/scipharm.1204-16.
Neeraj M, Gurpreet K. Investigations on polymeric nanoparticles for ocular delivery. Advances in Polymer Technology. 2019;3:1-14. https://doi.org/10.1155/2019/1316249.
Waghulde V, Saudagar R. Formulation development and evaluation of pH triggered in situ ophthalmic gel of besifloxacin hydrochloride. JDDT. 2018;8(5):313-321. https://doi.org/10.22270/jddt.v8i5.1874.
Meraj A, Jovita K, Poonam P, Malti A, Alok Y, Shubhini S. Evaluation of a polymer-lipid-polymer system utilising hybrid nanoparticles of dapsone as a novel anti acne agent. Curr Drug Ther. 2016;11(2):86-100. https://doi.org/10.2174/1574885511666160818145920.
Maria CPPRM, Suzana GL, Cristal CC, Alane BV, Ronald SS, Octávio AFP, Álvaro ACL. Gilda GL, Eduardo RJ, Elisabete PS. In-vitro and in-vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Brazilian Journal of Pharmacognosy. 2016;26(2):251-258. https://doi.org/10.1016/j.bjp.2015.11.006.
Kumar D, Jain N, Gulati N, Nagaich U. Nanoparticles laden in situ gelling system for ocular drug targeting. J Adv Pharm Technol Res. 2013;4(1):9-17. https://doi.org/10.4103/2231-4040.107495.
Puranik KM, Tagalpallewar AA. Voriconazole in situ gel for ocular drug delivery. SOJ Pharm Pharm Sci. 2015;2(2):1-10. https://doi.org/10.15226/2374-6866/2/2/00128.
Narjes S, Saeide S, Hashem KB, Esmael S. In vitro antibacterial effects of silver nanoparticles synthesized using Verbena officinalis leaf extract on Yersinia ruckeri, Vibrio cholera and Listeria monocytogenes. Iran J Microbiol. 2018;10(6):400–408. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6414745/.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J Drug Targ.2011;19(6): 409-417. https://doi.org/10.3109/1061186X.2010.504268.
Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy. Natamycin solid lipid nanoparticles – sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomedicine. 2019;14:2515-2531. https://doi.org/10.2147/IJN.S190502.
Snehal SC, Jameel ASM. A novel corticosteroid cubosomes – for ocular drug delivery. Indo Am J Pharm Res. 2020;10(6):775-784. https://doi.org/10.5281/zenodo.3923714.
Neupane YR, Srivastava M, Ahmad N. Lipid based nanocarriers system for the potential oral delivery of decitabine: formulation design, characterization, ex vivo, and in vivo assessment. Int J Pharm. 2014;477(1):601–612. https://doi.org/10.1016/j.ijpharm.2014.11.001.
Soni K, Mujtaba A, Kohli K. Lipid drug conjugate nanoparticle as a potential nanocarrier for the oral delivery of pemetrexed diacid: formulation design, characterization, ex vivo, and in vivo assessment. Int J Biol Macromol. 2017;103:139–51. https://doi.org/10.1016/j.ijbiomac.2017.05.015.
Prabhu P, Dubey A, Parth V, Ghate V. Investigation of hydrogel membranes containing combination of gentamicin and dexamethasone for ocular delivery. Int J Pharma Investig. 2015;5(4):214-225. https://doi.org/10.4103/2230-973X.167684.
Guohua W, Qixia N, Chen Z, Baoxian Z, Qiong Z, Gan L, Shuang W. Self-assembled thermoresponsive nanogels prepared by reverse micelle/positive micelle method for ophthalmic delivery of muscone, a poorly water-soluble drug. J Pharm Sci. 2016;105(9): 2752-2759. https://doi.org/10.1016/j.xphs.2016.02.014.
Aggarwal D, Pal D, Mitra AK, Kaur IP. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int J Pharm. 2007;338(1-2):21-26. https://doi.org/10.1016/j.ijpharm.2007.01.019.
Shun H, Jin-qiu S, Yong G, Hai-ming G, Xin-xin Z, Chun-liu Z, Li G. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacologica Sinica. 2010;31(8):990–998. https://doi.org/10.1038/aps.2010.98.
Jin X, Zhang ZH, Li SL, Sun E, Tan XB, Song J, Jia XB. A nanostructured liquid crystalline formulation of 20(S)-protopanaxadiol with improved oral absorption. Fitoterapia. 2013;84: 64–71. https://doi.org/10.1016/j.fitote.2012.09.013.
Bei D, Marszalek J, Youan BB. Formulation of dacarbazine-loaded Cubosomes—part II: influence of process parameters. AAPS PharmSciTech. 2009;10(3):1040-1047. https://doi.org/10.1208/s12249-009-9296-0.
Siekmann B, Bunjes H, Koch MH, Westesen K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. Int J Pharm. 2002;244(1-2):33–43. https://doi.org/10.1016/s0378-5173(02)00298-3.
Kesavan K, Nath G, Pandit J. Preparation and in vitro antibacterial evaluation of gatifloxacin mucoadhesive gellan system. Daru. 2010;18(4):237-246. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3304351/.
Kaul S, Gulati N, Verma D, Mukherjee S, Nagaich U. Role of nanotechnology in cosmeceuticals: a review of recent advances. Journal of Pharmaceutics. 2018. https://doi.org/10.1155/2018/3420204.
Asep BDN, Rosi O, Risti R. How to read and interpret FTIR spectroscope of organic material. J Sci Technol 2019;4(1):97-118. https://doi.org/10.17509/ijost.v4i1.15806.
Sayeta G, Sinegre M, Reguiga MB. Development of a Fourier transform infrared spectroscopy coupled to UV-Visible analysis technique for aminosides and glycopeptides quantitation in antibiotic locks. Annales Pharmaceutiques Françaises. 2014;72(1):41-50. https://doi.org/10.1016/j.pharma.2013.10.002.
Venkatesh B, Indira S, Srinivas P. Formulation and evaluation of miconazole nitrate as a cubosomal topical gel. JGTPS. 2014;5(4):2037-2047. http://www.jgtps.com/admin/uploads/QP7hd3.pdf.
Worle G, Siekmann B, Koch MHJ, Bunjes H. Transformation of vesicular into cubic nanoparticles by autoclaving of aqueous monoolein/poloxamer dispersions. Eur J Pharm. Sci. 2006;27(1):44-53. https://doi.org/10.1016/j.ejps.2005.08.004.
Dawoud MZ, Nasr M. Comparison of drug release from liquid crystalline monoolein dispersions and solid lipid nanoparticles using a flow cytometric technique. Acta Pharmaceutica Sinica B 2016;6(2):163-169. https://doi.org/10.1016/j.apsb.2016.01.004.
Esposito E, Cortesi R, Drechsler M, Paccamiccio L. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22(12):2163–2173. https://doi.org/10.1007/s11095-005-8176-x.
Seyedeh PA, Iris RR, Ben JB, Watson L. Impact of preparation method and variables on the internal structure,morphology, and presence of liposomes in phytantriol-Pluronic®F127cubosomes. Colloids and Surfaces B: Biointerfaces. 2016;145:845–853. https://doi.org/10.1016/j.colsurfb.2016.05.091.
Kojarunchitt T, Hook S, Rizwan S, Rades T, Baldursdottir S. Development and characterisation of modified poloxamer 407 thermoresponsive depotsystems containing cubosomes. Int J Pharm. 2011;408(1-2):20–26. https://doi.org/10.1016/j.ijpharm.2011.01.037.
Salwa S, Azza AM, Amany OK. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017;24(1):846–856. https://doi.org/10.1080/10717544.2017.1326539.
Xin P, Ke H, Xinsheng P, Zhiwen Y, Lingzhen Q, Chune Z, Xintian H, Xuan S, Linghui D, Ming L, Chuanbin W. Nanostructured cubosomes as advanced drug delivery system. Curr Pharm Des. 2013;19(35):6290–6297. https://doi.org/10.2174/1381612811319350006.
Jian-Chun L, Na Z, Jin-Xiu Z, Wen-Jing Z, Hong-Min Z, Qing-Qing W, Xiao-Xiang W, Xiu W, Jin Z, Ji-Fu H. Self-assembled cubic liquid crystalline nanoparticles for transdermal delivery of paeonol. Med Sci Monit. 2015;21:3298-3310. https://doi.org/10.12659/msm.894484.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (part 2). Trop. J. Pharm. Res. 2013;12(2):265–273. https://doi.org/10.4314/tjpr.v12i2.20.
Sadhu VR, Beram NS, Kantamneni P. A review on cubosome: the novel drug delivery system. GSC Biological Pharm Sci. 2018;5(1):76–81. https://doi.org/10.30574/gscbps.2018.5.1.0089.
Larsson K. Cubic lipid-water phases: structures and biomembrane aspects. J Phys Chem. 1989;93(21):7304–14. https://doi.org/10.1021/j100358a010.
Zheng DD, Dai WT, Zhang DR, Duan CX, Jia LJ, Liu Y. In vivo studies on the oridonin-loaded nanostructured lipid carriers. Drug Deliv. 2012;19(6):286–291. https://doi.org/10.3109/10717544.2012.704096.
Achouri D, Sergent M, Tonetto A, Piccerelle P, Andrieu V, Hornebecq V. Self-assembled liquid crystalline nanoparticles as an ophthalmic drug delivery system. Part II: optimization of formulation variables using experimental design. Drug Dev Ind Pharm. 2015;41(3): 493–501. https://doi.org/10.3109/03639045.2014.884113.
Abhishek KS, Preeti KS, Vinod KV. PLGA nanoparticles for ocular delivery of loteprednol etabonate: a corneal penetration study. Artifical cells. Nanomedicine and Biotechnology, 2017;45(6):1156-1164. https://doi.org/10.1080/21691401.2016.1203794.
Aminu I, Maznah I, Mustapha UI, Rozi M, Ismaila MS, Zuki ABZ. Toxicity evaluation, HET-CAM irritation, and anti-irritant potential of rice bran wax policosanol nanoemulsion. J Nano Res. 2017;49:44-55. https://doi.org/10.4028/www.scientific.net/JNanoR.49.44.
Spielmann, H. HET-CAM test. Methods in molecular biology. 1995;43:199-204. https://doi.org/10.1385/0-89603-282-5:199.
Nazoori ES, Kariminik A. In vitro evaluation of antibacterial properties of zinc oxide nanoparticles on pathogenic prokaryotes. J Appl Biotechnol Rep. 2018;5(4):162-165. https://doi.org/10.29252/JABR.05.04.05.
Young-Ki J, Byung HK, Geunhwa J. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Disease. 2009;93(10):1037-1043. https://doi.org/10.1094/PDIS-93-10-1037.
Ankit A, Prakash G, Renukaradhya C, Basavaraj MD. Preparation of gellan gum and chitosan based in-situ gel of timolol maleate for ophthalmic drug delivery and evaluation of physicochemical properties and drug release profile. Acta Scientific Pharm Sci. 2019; 3(2):68–78. https://www.actascientific.com/ASPS/pdf/ASPS-03-0205.pdf.
Shanmugam S, Valarmathi S, Satheesh K. Sterility testing procedure of ophthalmic ocusert aciclovir used for treating herpes simplex virus. Asian J Pharm Clin Res. 2017;10(10):344–346. https://doi.org/10.22159/ajpcr.2017.v10i10.19216.
Evren HG, Giuseppina S, Sait E, Bonferoni, MC, Güneri T, Caramella C. Cyclosporine A-loaded solid lipid nanoparticles:ocular tolerance and in vivo drug release in rabbit eyes. Curr Eye Res. 2009;34(11):996-1003. https://doi.org/10.3109/02713680903261405.
Jialu W, Fang Z, Rui L, Jingjing C, Qinghua Z, Ruijuan L, Ze W, Xin J, Changxiao Liu. Novel cationic lipid nanoparticles as an ophthalmic delivery system for multicomponent drugs: development, characterization, in vitro permeation, in vivo pharmacokinetic, and molecular dynamics studies. Inter J Nanomed. 2017;12:8115- 8127. https://doi.org/10.2147/IJN.S139436.
Anand BS, Atluri H, Mitra AK. Validation of an ocular microdialysis technique in rabbits with permanently implanted vitreous probes: systemic and intravitreal pharmacokinetics of fluorescein. Int J Pharm. 2004;281(1-2):79-88. https://doi.org/10.1016/j.ijpharm.2004.05.028.
Macha S, Mitra AK. Ocular pharmacokinetics in rabbits using a novel dual probe microdialysis technique. Exp Eye Res. 2001;72(3):289-299. https://doi.org/10.1006/exer.2000.0953.
Rui L, Shuangshuang W, Shiming F, Jialu W, Jingjing C, Xingguo H, Xin H, Changxiao L. Liquid crystalline nanoparticles as an ophthalmic delivery system for tetrandrine: development, characterization, and in vitro and in vivo evaluation. Nanoscale Research Letters. 2016;11:254-266. https://doi.org/10.1186/s11671-016-1471-0.
Lyla PA, Seevakan K. Advancement of cubosomes nanomedicine. International Journal of Pure and Applied Mathematics. 2018;119(12):7847–7854. https://acadpubl.eu/hub/2018-119-12/articles/3/683.pdf.
Author information
Authors and Affiliations
Contributions
Shreya Kaul: Methodology, Investigation, Writing — original draft. Upendra Nagaich and Navneet Verma: Conceptualization, Supervision, Writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Institutional Animal Ethical Committee with registration number 1327/PO/ReBi/S/10/CPCSEA. All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent for publication
All authors agree to the submission of this research article to the journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaul, S., Nagaich, U. & Verma, N. Preclinical assessment of nanostructured liquid crystalline particles for the management of bacterial keratitis: in vivo and pharmacokinetics study. Drug Deliv. and Transl. Res. 12, 1719–1737 (2022). https://doi.org/10.1007/s13346-021-01072-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01072-8