Abstract
Chemical penetration enhancer (CPE) is a preferred approach to improve drug permeability through the skin, due to its unique advantages of simple use and high compatibility. However, CPEs efficiency and safety problems frequently arise, which greatly restrains the further application in transdermal drug delivery systems (TDDS). To get access to the root of problems, the efficiency and safety of CPEs are reviewed especially from molecular perspectives, which include (1) the possible factors of CPEs low efficiency; (2) the possible contribution of CPEs in the evolution of safety problems such as skin irritation and allergic reaction; (3) the interactive relationship between CPEs efficiency and safety, as well as the bottlenecks of achieving their balance. More importantly, based on these, recent advances are summarized in improving efficiency or safety of CPEs, which offers a guidance of rationally selecting CPEs in future research.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transdermal drug delivery has been an attractive alternative to conventional drug administration and made an important contribution to medical practice [1]. It can reduce first-pass hepatic metabolism, avoid gastrointestinal adverse reactions, and improve patients compliance [2]. Hence, transdermal drug delivery is regarded as a versatile and fascinating route for drug delivery [3].
However, human skin is a remarkably efficient barrier to protect our body by impeding the entry of xenobiotics into the body, which causes great difficulties for transdermal drug delivery systems (TDDS) [4]. In order to improve drug permeation through the skin, a variety of methods have been developed, including physical methods (e.g., ultrasound, laser, microneedle, iontophoresis) [5,6,7,8,9], chemical methods (e.g., CPEs, ion pairs, and prodrug design) [4, 8, 10, 11] and pharmaceutics methods (e.g., vesicular carriers) [3, 12, 13]. Among the above methods, CPEs are the most widely studied approaches in TDDS on account of the advantages they offered over other methods: (1) simplicity in application [14], (2) noninvasive way for the skin [15], (3) excellent compatibility in different formulations [3, 16], (4) relatively low cost [17]. However, the disadvantages of CPEs especially traditional ones were mainly unreachable enhancing efficacy to physical methods and potential safety problems in clinical or product applications [18]. According to the structure, the traditional CPEs have been classified into fatty acids and derivatives, terpenes, surfactants, glycols, alcohols, amide, pyrrolidones, and sulfoxides [19]. Scientists have been committed to improving the efficiency and safety of CPEs and have made great progress. Molecular level provides an essential and distinctive viewpoint in analyzing CPEs behavior in TDDS [20, 21]. Hence, this review summarized the limitations of traditional CPEs, especially providing a molecular perspective to analyze the problems of CPEs efficiency and safety as well as their relationships. More importantly, this review focuses on the advanced research progress of CPEs for improving their efficiency and safety. It may be helpful to deepen the understanding of CPEs for their further applications in TDDS.
Understanding efficiency problems of CPEs
About 300 molecules have been identified as CPEs to facilitate drug permeation through the skin, including alcohols, amides, amines, esters, fatty acids, glycols, surfactants, terpenes, terpenoids, and essential oils [22, 23]. In product application, the phenomenon usually occurs that some CPEs are difficult to exhibit their efficiency of enhancing drug permeation through the skin [20, 24,25,26]. In this part, the structure of the skin barrier function and CPEs action mechanisms will be reviewed at a molecular level, based on which the current understanding of CPEs efficiency problem will be summarized.
Structure of the skin barrier function
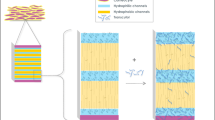
The epidermis of the skin, specifically its external layer, stratum corneum (SC), functions as a permeability barrier [17, 27]. The specific composition element and the ordered structure make SC the main impediment to diffusion and penetration of drug molecules through skin [17, 28]. As illustrated in Fig. 1, SC is composed of the dead but keratin-rich cells, corneocytes, which were issued from the terminal differentiation of keratinocytes from the viable epidermis [29, 30]. The organization of SC barrier structure is commonly referred to as the “brick and mortar” mode, in which the corneocytes resemble the bricks cemented by the intercellular lipid matrix [31, 32]. Besides, the corneocytes are connected by corneodesmosomes, thus maintaining cohesion between them [33]. The intercellular lipid matrix is composed of ceramide, fatty acid, and cholesterol molecules in a roughly 1:1:1 molar ratio [34, 35]. These molecules form a highly ordered bilayer arrangement, in which the ceramides are in splayed chain conformation, with the hydrocarbon tails pointing in opposite directions centered on the polar head groups [36]. Both cholesterol and fatty acid are distributed selectively: the cholesterol at the end of ceramide sphingoid and the fatty acid at the end of ceramide fatty acid [37]. Based on this bilayer arrangement, the organization of the lipid lamellar layer is parallel to the flat plane of the corneocytes [38]. This lamellar organization, as determined by the lateral packing of lipid head groups, plays a crucial role in the barrier properties of SC. Besides, there are hydrogen bond networks between ceramide head groups both in the plane of the bilayer and across the lamellar layers [31].
Molecular structure of the skin barrier function and molecular action mechanism of chemical penetration enhancers, modified from Andrej [18]
With the abovementioned structure of SC, the skin only allows drugs with suitable physicochemical properties (molecular weight of below 500 Dalton and satisfactory lipid solubility) to permeate through it [39]. Even though the hair follicles provide a pathway for drugs to the dermal microcirculation, the limited account in total skin area hindered the amount of drugs that can be transported through this pathway [40,41,42]. Hence, it was necessary to introduce strategies like CPEs to enhance drug permeation.
Action mechanisms of CPEs
As illustrated in Table 1, different action mechanisms of CPEs and the corresponding examples were listed.
Action on SC lipids.
Most of the CPEs are designed to reduce the barrier resistance by disrupting the highly ordered SC lipid bilayer structure, which can be classified into three different manners, as depicted in Fig. 1: (1) Extraction of lipids [43]. Some CPEs can extract lipids from the skin thereby forming diffusion pathways for drugs to permeate through [44]. Ethanol at concentrations between 40 and 80%, hexanol, and octanol may have the lipid extraction effects [4, 34, 43, 45]; (2) Fluidization of lipids [38, 44, 46]. Amphipathic CPEs possess a similar molecular structure to the lipid, generally having lipid fluidization effects [47]. Their hydrophobic chains enable molecules to penetrate the intercellular lipids of SC, and the polar heads interact with the hydrophilic region of the lipid bilayers via Van der Waals forces and H-bonds [17]. In this way, SC lipid fluidity increases, which may improve drug permeation; (3) Disorganization of lipid lamellae, SC lipids in a normal state are arranged in an orthorhombic lateral packing, which is the most tightly packed form as a barrier [48]. The change or disruption of this lipid lamellae organization caused by CPEs may result in higher drug transdermal permeability [49].
Action on SC protein.
CPEs interact with the SC intracellular protein-named keratin and denature it or modify its conformation. Hydrogen bonds are essential for keeping keratin in their natural structures. Competitive hydrogen bonding from CPEs may potentially change the natural hydrogen bonding in keratin, resulting in a reduction of SC barrier function and an improvement of drug permeation [44, 50].
Action on solvent nature of SC.
Altering the solvent nature of SC can modify the drug partitioning into the skin, which may contribute to the enhancement of drug permeation through the skin [51].
Action on desmosomes and tight conjunction.
Desmosomes maintain the main cohesion properties between epidermal keratinocytes, which will be transformed into corneodesmosomes and thus provide stronger intercellular adhesion [33]. Some CPEs may affect desmosomes, but the specific action mechanism of these CPEs is still unclear [51].
Action on formulations.
CPEs can enhance drug permeation through the skin via indirect action on formulations, such as modification of the thermodynamic activity of the vehicle [4, 51]. Recently, several reports revealed that CPEs can also increase drug permeation via improving drug release behavior from formulation matrix [4, 21, 52,53,54].
Apart from the above mechanisms, some CPEs were reported to improve drug permeation via trans-appendage route such as hair follicle [55, 56]. They could interact with sebum in the hair follicle, in turn improving drug solubilization in the “sebum-enhancer” matrix, thereby improving drug permeation through the skin [55].
Understanding CPEs efficiency problem
As illustrated in Fig. 2, CPEs locate in a multifactored TDDS microenvironment, which involves complicated interactions among drug, formulation, the skin, and CPEs [58]. In the initial state of TDDS, both drug molecules and CPE molecules are in the position of the formulation. First, CPEs either play a role in changing a property of the formulation matrix or simply release to the surface of the skin, and drugs simultaneously release to the surface of the skin [21, 52, 59, 60]. Then, CPEs at the surface of the skin influences SC [4, 61, 62]. Finally, drugs can permeate through the skin via the influenced SC [62, 63]. Based on the above process, the factors influencing CPEs efficiency can be reviewed from the following aspects.
Unsatisfactory release profile of CPEs
Initially, CPEs locate in the polymer of formulation in TDDS as shown in Fig. 2. Thus, the release behavior of CPEs from the polymer (such as release degree and speed) was likely to restrain the efficiency of CPEs, especially for those CPEs whose site of action is the skin [20]. It was found that some kinds of CPEs (such as fatty acids and their esters) were of difficulty in releasing from patches [20, 64]. In comparison to direct skin pretreatment with 5% fatty acids and their esters, the efficiency of fatty acids and their esters contained in the patch was reduced significantly in enhancing felodipine skin permeation [20]. This suggested that the decreased efficiency of fatty acids and their esters was just due to the problem of themselves releasing from formulations. Furthermore, Zeng et al. reported that the release rate of fatty acids and their esters in adhesives were very slow, which to some extent verified that the efficiency of CPEs was likely to be influenced by their release behavior from formulations [20].
Unsatisfactory release profile of drugs
A drug must release from formulations before percutaneous absorption [61]. The release behavior of drugs could also be a non-negligible factor restraining the efficiency of CPEs [21]. If the drugs were restrained and cannot release from the matrix of formulations, CPEs may fail to enhance drug permeation just by acting on the skin SC [65]. For example, both Azone (AZ) and N-methyl pyrrolidone (NMP) are CPEs acting on SC, which could increase SC lipid fluidity and improve drug partitioning into SC, respectively [66, 67]. The combination of AZ and NMP revealed a significant enhancing effect on bisoprolol tartrate (BSP-T) percutaneous absorption from its saturated solution, but their combination could hardly enhance BSP-T skin permeation in its transdermal patches. In other words, the efficiency of CPEs could be decreased when the formulation was switched from solution to transdermal patches, just because that BSP-T had a problem in releasing from its transdermal patches, while this problem usually did not happen in its solution [21]. The improvement of drug release from adhesive of patches could greatly contribute to the enhancement of drug transdermal permeation [21, 65, 68, 69].
Unmatched behavior between CPE and drug
The matched behavior between CPE and drug means “the consistency between CPE and drug in their skin permeation or their skin residence time,” which is in favor of CPEs efficiency of enhancing drug permeation [45]. To investigate the “matched” relationship between drugs and CPEs, the logarithm of the apparent oil–water partition coefficient (log P) of a drug is often researched as an important parameter. For a specific CPE, its efficiency is constantly changing which depends on the individual characteristics of drugs [70]. The efficiency of camphor (log P = 2.38) as a CPE was evaluated for the transdermal delivery of drugs possessing different log P (from − 0.95 to 3.8). It was shown that the efficiency of camphor was in a parabolic curve relationship with log P values of the drugs, which showed the best efficiency for hydrophilic or weak lipophilic drugs (an estimated log P value of 0) [71]. Similarly, the “matched” CPEs for a specific drug were also evaluated with log P. The skin permeation enhancing effect of isoniazid (INH) (log P = − 0.7) was found positively correlated with the different log P of CPEs (from − 0.42 to 4.58) [72]. And it was much the same with the research of zaltoprofen (ZAL) (log P = 3.55) transdermal delivery, in which the correlation coefficient between ZAL permeation enhancement and log P values of CPEs (from − 1 to 7) was up to 0.919 [73].
Unsatisfactory penetration behavior of CPEs in SC
As illustrated in “Action mechanisms of CPEs,” some CPEs achieve their enhancing efficacy by mainly acting on the SC of the skin. Hence, the retention amounts of chemical enhancers in SC may be important in influencing their efficacy. Unfortunately, the amounts of chemical enhancers in SC or epidermis were scarcely reported. Time-of-flight secondary ion mass spectrometry bioimaging analysis was performed to visualize and analyze the distribution of fatty acids in the skin. It was found that there was a large difference between the amounts of saturated fatty acids and unsaturated fatty acids in the epidermis [74]. Moreover, in some researches, the relevance between the permeation of chemical enhancers in SC and the extent of decreasing SC barrier was confirmed by transepidermal water loss [75]. Some researchers even reported it was an indispensable factor of their efficacy whether they can only be restricted to the top few layers of SC or penetrate into a deeper part of the SC [76]. If they show poor permeation across SC, their concentration reduces in the deeper part of the SC, and their enhancing efficiency decreases as well.
Understanding safety problems of CPEs
CPEs can significantly enhance drug permeation through the skin, but at the same time, CPEs may also cause safety concerns [19]. The main safety concerns are skin irritation and allergic reaction, which greatly limits their clinical application [76].In this part, the possible roles of CPEs in the evolution pathway of skin irritation and allergic reaction are illustrated from molecular perspectives (Fig. 3). The involved important cytokines in both these concerns are summarized in Table 2.
The skin irritation
The clinical symptoms of skin irritation range from slight scaling, erythema, edema, and erosions, to eczema [77]. As a nonimmune, nonspecific topical skin reaction, the skin irritation results from direct damage to the skin SC, which occurs faster than the self-repair of the skin itself [78].
The first step that CPEs may involve is the destruction of the SC barrier mainly in two modes: one is extracting SC lipids, also called delipidizations; the other is denaturing SC protein such as keratin, which exposes new water-binding sites and causes high hydration of the SC [79, 80].
The second step that CPEs may involve is the release of pro-inflammatory primary cytokines, which have two distinctive pathways. When CPEs continue to penetrate deep into the skin, they can contact the membrane of keratinocytes in viable epidermis and cause the release of interleukin-1α (IL-1α) and tumor necrosis factor-α (TNF-α) [79,80,81]. In contrast to this pathway, other CPEs may indirectly or directly cause keratinocytes to produce excessive reactive oxygen species (ROS), which causes dysregulation of the cellular signaling pathway. ROS activates transcription factors and promote the synthesis of IL-1α and TNF-α [82, 83].
The successive final step of skin irritation caused by CPEs is the inducing of secondary mediators. IL-1α accumulates in the cytoplasm of keratinocytes in epidermal layers and is only released from leaky cells following cell damage or membrane perturbation [84]. IL-1α is a vital inflammatory mediator in the skin and is believed to be the main switch triggering the inflammatory cascade. Briefly, IL-1α induces the expression of further cytokines IL-6 and IL-8, followed by changes in the skin morphology and finally the typical onset of irritation symptoms [79]. In addition, the keratinocytes also produce tumor necrosis factor-α (TNF-α), which is not related to the release of IL-1α. It has been reported that TNF can increase the production of inflammatory cytokines, including IL-1β, IL-6, etc. [85]. These cytokines activate Langerhans cells (LC), dermal dendritic cells (dDC), and endothelial cells. Their overall role is to recruit neutrophils, lymphocytes, macrophages, and mast cells to the damaged site, eventually inducing histological alterations followed by the clinical manifestation of eczema [86, 87].
The skin allergic reaction
The skin allergic reaction is a T-lymphocyte-mediated delayed hypersensitivity reaction that occurs after the skin exposure to a specific allergen in previously sensitized individuals [78]. The symptoms are erythema, edema, vesicles, oozing, and notably intense pruritus [88].
The sensitization phase is the first phase of the skin allergic reaction [89]. At this phase, the skin barrier is firstly disrupted by CPEs just in the same way as “The skin irritation”. Then, CPEs may penetrate the skin, come into contact with immune cells, and cause an immune response [80]. After they penetrate through SC, they either covalently bind to or form complexes with endogenous proteins, which is essential for the activation of the innate immune system, and for the effective initiation of T cells [90]. Then, keratinocytes are activated and produce various cytokines such as IL-1α, IL-1β, IL-8, and TNF-α, which promote the migration and activation of Langerhans cells (LCs) [89, 91]. LCs capture the hapten and migrate to the draining lymph nodes (dLNs), where they present hapten to T cells. Then, these T cells become effectors or memory T cells and are distributed through the blood circulation. This phase usually takes about several days.
Next is the elicitation phase when the same CPEs diffuses into the skin and stimulates keratinocytes to produce pro-inflammatory cytokines such as IL-1β and TNF-α [88]. These cytokines then activate vascular endothelial cells to express adhesion molecules, thereby directing the migration of T cells from the blood to tissues [89]. When T cells infiltrate into the skin, they can be activated by cutaneous LCs and then produce pro-inflammatory cytokines, such as IFN-γ and IL-17. These cytokines activate keratinocytes and cause further recruitment of T cells, which exacerbates the inflammation [92].
Comparison between the skin irritation and the skin allergic reaction
The mechanism processes of skin irritation and allergic action share strong similarities mainly in two respects. In the beginning, both processes require the destruction of the SC barrier, which is a prerequisite condition for their occurrence [80]. On the other hand, both processes cause inflammation via kinds of cytokines [87, 92].
In comparison, there are two key aspects of difference between the skin irritation and allergic action. The most pivotal difference is that the skin allergic reaction requires the participation of “allergen-specific T cells” as initiators of the skin inflammatory reaction, while the skin irritation does not [87]. Their second important difference is clinical symptoms. The skin irritation is sharply circumscribed to the area of contact while dissemination of the lesions can occur in an allergic reaction. The skin allergies are more prone to itching, while the skin irritations are more likely to present with burning and stinging [93]. Furthermore, following CPE removal, the skin irritation is characterized by the decrescendo phenomenon, while the revolution of the skin allergic reaction is slower than the skin irritation which is characterized by the crescendo phenomenon [94].
Key factors influencing the safety of CPEs
The mechanisms of CPEs disrupting the skin barrier were very crucial. As showed in CPEs examples in Table 1, among these action mechanisms, only the extraction of SC lipids and the degeneration of SC protein were designated as the first step of skin irritation and allergic reaction. In other words, CPEs of these two action mechanisms were more likely to cause safety risks. For instance, termed as a “Universal Solvent,” DMSO enhanced drug percutaneous absorption via denaturing the skin proteins, resulting in erythema, stinging, and burning sensation [57]. Similarly, as a volatile solvent, ethanol at high concentrations may extract SC lipids, which caused skin safety problems [4]. Therefore, from the molecular perspectives of skin safety, it was necessary to avoid the selection of CPEs with these two action mechanisms.
In addition, the cytotoxicity of CPEs was also a key factor influencing their safety. After disrupting the SC barrier, CPEs penetrated into the viable epidermis. They can interact with the keratinocytes, the living cells, and cause different cytotoxicity, thereby resulting in safety issues. Cytotoxicity assay was often investigated to roughly evaluate the skin irritation irritancy potential [71]. When comparing the cytotoxicity of laurocapram and camphor, their different cytotoxicity was regarded as a great possibility of their different irritancy potential. In a report on the cytotoxicity of 22 CPEs, 5 CPEs were found to be nontoxic at the moderate concentration of 1 mg/mL, including oleic acid, 4-octanone, octanal, cis-4-hexen-1-ol and 2,4,6-collidine [95]. Other CPEs like octanoic acid, 1-methyl-2-pyrrolidone, and pulegone all exhibited different cytotoxicity, which may lead to safety problems.
To sum up the above parts of “Understanding efficiency problems of CPEs” and “Understanding safety problems of CPEs,” disrupting the skin barrier is not only an effective mechanism for enhancing drug permeation but also an important primary step for the occurrence of skin irritation and allergic action. For some CPEs with poor permeating ability across SC, their activity is restricted to the top few layers of SC, thus resulting in an unsatisfactory enhancement efficiency of CPEs [76]. Inversely, other CPEs with excellent enhancement efficiency are good at disrupting SC, but they cannot confine their activity to SC and ultimately further diffuse into the viable epidermis. In a viable epidermis, CPEs can interact with the keratinocytes, the living cells of the epidermis, and pose safety risks [87].
Generally, the potency of CPEs in causing skin irritation or allergic reaction increases in proportion to their efficiency of causing the skin disruption [76]. However, the safety risk of CPEs may be different, which depends on the action mechanisms in disrupting the skin barrier. Karande et al. evaluated the relationship between enhancing efficiency (ER) and irritation potential (IP) of one hundred and two CPEs chosen from 10 categories [96]. According to the results, they found that these CPEs fell into two classes. The first class, where ER increases proportionately with IP, includes nonionic surfactants, zwitterionic surfactants, Azone-like compounds, and sodium salts of fatty acids. The second class, where ER exhibits poor correlation with IP, comprises fatty acids, fatty amines, fatty esters, anionic surfactants, cationic surfactants, and others. Interestingly, the mechanism of CPEs in the first class is typically the extraction of SC lipids, while it is fluidization of SC lipids in the second class. It was found that IP was related to lipid extraction and SC protein denaturation. At this point, the design of CPEs possessing high efficiency and low safety risk is possible.
Current developments to improve efficiency and safety of CPEs
CPEs structure modification
Some investigators have succeeded in modifying the CPEs structure for efficiency improvement without increasing safety risk. In structure, hydrogen bond, lipophilicity, carbon-chain length, double bonds, etc., all contribute to the efficiency [96,97,98,99,100]. Angela Abruzzo et al. synthesized two surfactants (C12-OPK and C18-OPK) by condensation of fatty amines and itaconic acid, with C12 and C18 alkyl chains separately. They studied the effect of the tail length on hydrocortisone skin permeation and found that compared with C12-OPK, C18-OPK obtained a higher enhancing efficiency on drug permeation as a result of its hydrophobic properties [99]. Yang Chen et al. synthesized the saturated long-chain isopulegol (ISO) esters by the esterification reaction between ISO and saturated fatty acids [101]. Compared with saturated fatty acids and ISO, saturated long-chain ISO esters significantly increased the skin permeability of amlodipine and flurbiprofen with low safety risk. The results of CLSM and ATR-FTIR substantiated that the saturated long-chain esters of ISO were suitable CPEs for TDDS, and only the esters could disorder the alkyl chains of skin lipids [101]. In the research of Sanjeev Rambharose et al., in comparison to unsaturated fatty acids, the dendritic esters exhibited better efficiency with increasing lipophilicity. In electron micrographs, they showed a greater disruption and fluidization on SC and stretching/expansion of the tight junctions/desmosomes [98]. In the report of Rahul S. Kalhapure et al., the connection of dendrons and oleic acid by covalent bonds increased the enhancing efficiency of oleic acid, similarly due to the increase of lipophilicity [102].
Combined application of CPEs
The combined application of CPEs was proved to be more effective than the individual CPEs (Fig. 4) [103]. Scientists have tested approximately 5000 unique binary combinations of CPEs; however, only a small number of them showed highly synergistic behavior [104]. Shah et al. pointed that the combined application of lipophilic and hydrophilic CPEs played a vital part in the percutaneous formulations and was recommended for their satisfactory synergistic action, just because that the environment of SC and viable epidermis ( as well as the dermis) is lipophilic and hydrophilic, respectively [105]. Viewed from another angle, J. Mueller et al. investigated the effect of combining two hydrophilic CPEs, urea and taurine on SC lipid models. They found that the penetration enhancing effect might result from the large water-binding capacity of urea and a consequent osmotic pressure in the presence of taurine, which causes large amounts of water to diffuse into the corneocytes [106].
Synergistic effect of oleic acid and ethanol on the in vitro skin permeation profiles of S-methyl-L-methionine (SMM). Reprinted with permission from Kim et al. [103]
Synthesis of novel CPEs
Due to the limitations of traditional CPEs, researchers have been striving to search for novel CPEs that are not only efficient but also safe to the skin in recent years. Novel CPEs, such as the skin-penetrating peptides, ionic liquids, dendrimers and biodegradable enhancers have been currently studied.
The skin-penetrating peptides (SPPs)
SPPs have received considerable attention in recent years [107,108,109,110]. In comparison to conventional CPEs, the unique advantage of SPPs is that they can promote the penetration of small and large drug molecules into the skin, even further into various cells [107, 110, 111]. Besides, SPPs are generally considered safe [110,111,112,113]. Some SPPs exhibited nontoxic at the concentration range from 0.1 to 1.0 mg/mL [111], while other SPPs like hexamer and octamer SPPs were nontoxic at a concentration as high as 10 mg/mL [110]. The short peptide TD-1 was the first used SPP, which was specifically discovered to penetrate the SC [111, 112]. TD-1 can promote penetration with or without drug connection. Conjugated with drugs, TD-1 can act on Na–K-ATPase, which affects the tight junction of cells (Fig. 5) [112]. Polyarginine can enhance transdermal drug permeation by covalent and non-covalent methods, which was reported to promote both skin penetration and cell penetration [108, 112]. It is fit for the transdermal delivery of protein and hydrophilic drugs, but not for low molecule drugs [114]. Tracy Hsu et al. synthesized the skin-penetrating and cell-entering (SPACE) peptide and then conjugated the peptide to streptavidin and siRNA, respectively [111]. They found that the permeation of streptavidin across porcine SC was greatly enhanced, and SPACE exhibited an increased penetration into all viable cells including fibroblasts, keratinocytes, and endothelial cells. The transdermal permeation of siRNA was explored using green fluorescent protein (GFP)-expressing endothelial cells as model cell lines in vitro [111]. They found that SPACE peptide-conjugated siRNA caused a significant knockdown of GFP. However, no obvious knockdown was observed in all cell lines using siRNA alone [111].
a Interaction between TD-1 and Na + , K + -ATPase beta subunit on epidermal cell. The interaction influences the penetration of protein drugs. b TD-1 disrupts the tight junction of epidermal cell observed under transmission electron microscopy. The intercellular space obviously increases after treating TD-1. Reprinted with permission from Ruan et al. [112]
The mechanisms by which SPPs mediated transdermal delivery of macromolecular drugs are not yet fully clarified. Nowadays, mainly two hypotheses of the possible functional mechanisms have been proposed. One is the pore formation of the skin via the skin shunt pathway [109]. Magainin, a peptide with 23 amino acids, obtained from the skin of an African clawed frog, is a typical example of these hypotheses [112]. When magainin interacts with a negatively charged membrane, it can form an α-helical structure, causing a brief disturbance of the membrane and forming a transmembrane pore [113]. Magainin itself exhibits weak permeation ability and can only penetrate through a single bilayer lipid. Therefore, it is usually used in combination with other CPEs like surfactants to increase the permeation. The other hypothesis is the interaction with the component of skin via the transcellular pathway, such as the interaction hypothesis model of Kumar et al. They suggested that SPPs did not transform the skin lipid barrier, which was measured by the skin resistance, transepidermal water loss (TEWL), and Fourier transform infrared (FTIR) spectroscopic analysis. On the contrary, SPPs interacted with the skin proteins and induced alterations in the secondary structure of the skin proteins [109].
Ionic liquids
As novel CPEs for transdermal drug delivery, ionic liquids have gained great interest in pharmaceutical applications [115, 116]. Ionic liquids are defined as salts obtained by a neutralization reaction between large and asymmetric organic cations and organic or inorganic anions, which are liquid at room temperature [115, 117]. Simply change the anion/cation combination or introduce specific functional groups on them, and they can be synthesized for specific applications or present specific physicochemical properties [115]. Additionally, several ionic liquids like choline based salts provide peculiar properties such as solubilizing ability and antibacterial activity [115, 118, 119]. However, the toxicity of ionic liquids is a crucial factor when considering their applications. Some ionic liquids have been reported to possess low toxicity, while in different biological systems, they might display significant toxicological effects [120, 121]. It has been reported that the toxicity of ionic liquids is relevant to the length of the alkyl side chain in the cation, the nature of functional groups in the cation, the nature of the anion and cation as well as their interactions [120, 121]. The ionic liquids with longer chain lengths were proven to be more toxic [122], and cholinium-based ionic liquids were found to be much less toxic than regular ionic liquids [123]. Therefore, it is promising to design an ionic liquid with low toxicity based on the abovementioned relationships between the toxicity and structure.
Currently, the ionic liquids composed of highly biocompatible materials are used as CPEs [124], and previous findings have shown that there was no skin tissue injury in the course of the skin permeation caused by such ionic liquids [117]. Ionic liquid has been shown to enhance the transdermal permeation of quite a few drugs [125, 126]. For these reasons, ionic liquids are considered as safe and powerful promoters of drugs skin permeation and have gained successful uses in a wide range of applications recently. Qi et al. reported a significant improvement of dextrans transdermal transport employing choline and geranic acid-based ionic liquid [127]. Such ionic liquids belonged to bioinspired ionic liquids, which performed desirable biodegradation as well as decreased toxicity profiles. The mechanisms through which ionic liquids can be beneficial to TDDs are summarized as follows: extraction of lipid components in the SC, creation of diffusional pathways, and disruptions of cellular integrity as well as fluidization (Fig. 6) [122]. According to Sidat et al., ionic liquids with enhancing effects can be classified as hydrophobic and hydrophilic [122]. As for hydrophobic ionic liquids, they act by increasing the partition into the epithelial membrane via providing channels, thereby facilitating transcellular transport in lipid regions. While hydrophilic ionic liquids can open tight junctions in the SC, thus accelerating paracellular transport via enhancing fluidization primarily in protein and lipid regions [128].
a Double-tail lipid-mimic imidazolium-based IL; b glycerol-phospholipid subregions with large tail lengths. Theirs structures are similar and allow for their ability to intercalate within the phospholipid membrane structure. Reprinted with permission from a Wang et al. [116] and b https://www.nature.com/scitable/topicpage/cell-membranes-14052567/
Dendrimers
Dendrimers are monodisperse hyperbranched polymers with core–shell structures, which contain high-density adjustable surface functional groups [129, 130] (Fig. 7). Due to the unique characteristics such as multivalency, nanoscale, spherical structure, precise molecular weight, and surface functional groups [131], dendrimers have been successfully investigated as drug carriers for different administration routes. Drugs can be conjugated to the surface functional groups of dendrimers or encapsulated in the core.
Schematic presentation of a G4 dendrimer containing four generations, modified from Chauhan [129]
Also, dendrimers can be used alone as novel CPEs, which have been reported to successfully improve transdermal drug delivery [129]. However, some widely used dendrimers like PAMAM dendrimers were reported to be toxic. To overcome this drawback, peptide dendrimers were synthesized, possessing negligible toxicity and biodegradation to harmless endogenous amino acids [131, 132]. The action mechanisms of dendrimers are thought to be acting on formulations and altering the skin barrier function [131, 132]. Dendrimers can increase the oil/water partition coefficient of drugs and improve drug partitioning into SC, thereby enhancing drug permeation [132]. Dendrimers interact with the skin lipids as well as proteins, thus weakening the skin barrier function [131]. Besides, it has been reported that dendrimers could interact with free fatty acids and ceramides of the skin and interact with negatively charged phosphate groups of phospholipids subsequently causing lipids fluidization [132]. Yang et al. studied various dendrimers for transdermal delivery of therapeutic agents and found that dendrimers with carboxyl or acetyl surface groups showed higher skin permeability than that with amine groups, and smaller-sized dendrimers showed higher skin permeability than larger ones [130]. According to them, for transdermal delivery of non-covalently attached drugs, larger-sized cationic dendrimers are more suitable, while smaller-sized anionic dendrimers are more appropriate for covalently bound drugs. Jyothsna et al. synthesized arginine-terminated peptide dendrimers and found that the dendrimers significantly improved the transdermal permeation of ketoprofen [132]. Furthermore, the excised mouse skin after permeation studies with dendrimers showed no major toxic reactions, indicating nontoxicity of the tested dendrimers. The results indicated that dendrimers enhanced the ketoprofen transdermal permeation by improving the partition of ketoprofen into SC.
Biodegradable penetration enhancers
Biodegradable CPEs have also been researched to avoid the skin safety risks while maintaining enhancement, which can be metabolized into inactive components in the viable epidermis after they act on SC. Amino-acid derivatives are reported to be one of the most promising soft CPEs, peculiarly those with a hydrophobic “tail” connected to the “head” of the amino acid via a biodegradable linkage like ester bond. Its unstable bond could be hydrolyzed by epidermal esterase, releasing known nontoxic compounds with much less irritation [133]. Besides, other types of CPEs such as terpene alcohol alkyl esters have also been designed as biodegradable CPEs [134]. Liu et al. studied the efficiency and safety of biodegradable O-acylmenthol derivatives. They demonstrated that such biodegradable CPEs could significantly improve the transdermal permeation of flurbiprofen, and their influences on the skin barrier function as well as the skin irritation were reversible [14]. As for biodegradable O-ethylmenthol ester with different carbon-chain lengths, it was found that an O-ethylmenthol ester with C6-C10 chain length was more effective for lipophilic drugs, while a C14 O-ethylmenthol ester seemed to be more beneficial for hydrophilic drugs [135]. Vavrova et al. found that biodegradable CPE-like tranexamic acid decyl ester could increase the theophylline permeation amounts while mediating to restore the skin barrier easily through the action of released tranexamic acid (TXA) (Fig. 8) [136]. In addition, it is worth noting that if an ester CPE is degraded into a fatty acid or corresponding alcohol, it will be another type of CPE.
Hypothesized action of TX12 on the skin, modified from Vavrova et al. [136]
Extraction from natural plants
Natural plant extracts such as natural oils are considered to be promising nontoxic, nonirritating permeation enhancers compared with the traditionally used synthetics chemicals [76]. Permeation effects for both hydrophilic and hydrophobic drugs can be increased by essential oils and their constituents with low cytotoxicity. Geeta Aggarwal et al. investigated the effect of CPEs on transdermal permeation of olanzapine [137]. They chose different natural oils as CPEs and found that corn oil with unsaturated fatty acids was the best CPE and caused no skin irritation. Faqir Muhammad et al. reported that the penetration of caffeine and salicylic acid would be enhanced when some plant extracts were subsequently applied [138]. Only a few studies have compared the activities of essential oil components with typical CPEs in transdermal drug delivery [76]. Vytis et al. found that natural oils especially soybean and olive oils could efficiently increase the penetration of dihydroquercetin [139]. Pomegranate seed oil was found to enhance the transdermal absorption of resveratrol [140] and evening primrose oil exhibited a similar effect to flurbiprofen [24]. The permeability mechanism of these natural agents may be related to the fatty acids contained in them [24, 139, 140].
Other methods
Searching for a method that can reduce the concentration of CPEs meanwhile maintaining efficient penetration enhancement has become a current trend. Several studies have reported other methods for improving the efficiency and safety of CPEs. Aharon Azagury et al. compared free CPEs in solution with CPEs encapsulated in nanoparticles using chorioamnion membrane as model, and they found that the enhancing effect of the latter was significantly increased [141]. The efficacy enhancement was explained that the nanoparticle with average diameter of 135 ± 60 nm could penetrate into the chorioamnion membrane. Thus, the nanoparticle in membrane continued to release chemical enhancers and prolong their effects, thereby increasing their efficacy. Besides, CPE encapsulated in nanoparticles resulted in a reduction of required mass of CPE, which maintains a balance between efficiency and safety [141]. Notably, chorioamnion membrane was more permeable than the skin. But the SC of skin could also be penetrated by the nanoparticle with very small particle size [142]. In this way, this approach of CPEs encapsulation in nanoparticles was likely to apply in the enhancement of efficacy through the skin.
Based on previous studies, CPEs can be combined with iontophoresis to achieve higher efficiency. In addition, combining CPEs with iontophoresis could modulate the required mass of CPEs, thereby decreasing the irritation caused by CPEs and increasing the safety of CPEs [143, 144]. Unlike CPEs, iontophoresis does not disrupt the skin barrier structure that may cause skin irritation under low current density [145, 146]. Besides, studies showed that the potential skin irritation was proportional to the current intensity of iontophoresis [145]. However, there might be a potential risk of cell damage after membrane discharge by electroporation [146]. According to the study of Keng-Chih Liu et al., compared to the control group without any enhancing strategy, there was only a slight increase in sodium fluorescein permeation using limonene/ethanol, and triple sodium fluorescein permeation amount was achieved when cathodal iontophoresis was applied [143]. However, when combined CPEs with cathodal iontophoresis, there was a fourfold increase in sodium fluorescein permeation amount, which demonstrated that CPEs efficiency was significantly improved with the application of iontophoresis [143]. The histological findings of S. Arunkumar et al. suggested that the combination of CPEs and iontophoresis could minimize skin irritation [144].
In summary, finding a suitable way to achieve the goal of safety and efficiency is a huge challenge that still requires unremitting efforts.
Outlook/future directions
The efficiency and safety of traditional CPEs are two important problems that greatly restrain their further wide application in transdermal drug delivery. Usually, the efficiency of traditional CPEs is low or unpredictable for different drugs and there are security risks in causing skin discomfort. More troubling, there exists interaction and mutual restriction between CPEs efficiency and safety. It is not easy to improve one aspect without influencing the other aspect. Only understanding the superficial phenomenon of CPEs efficiency and safety problem is far from solving the above problems. The lack of understanding of CPEs efficiency and safety from the molecular mechanism directly resulted in the blindness and failure in CPEs research and product application. Molecular-level provides an essential and distinctive viewpoint and therefore helps to understand the essence of CPEs efficiency and safety and to select CPEs rationally.
The efficiency mechanism of CPEs to enhance drug permeation relies on their influence on the skin barrier structures as well as formulation properties. SC lipid, SC protein, SC solvent nature, and desmosomes and tight conjunction are primary action sites of CPEs that may affect skin barrier function. Different CPEs possess their own action sites in the skin, while different drug candidates have different trouble sites in the skin restricting their permeation through the skin. An exact match relationship between “reached site” and “trouble site” is important for the satisfactory efficiency of CPEs. Apart from the action site, the matching behavior of CPEs and drugs in the skin permeation and the skin residence also influences CPEs efficiency. Additionally, the release behavior of CPEs and drugs from matrix polymers was also an indispensable factor, which is easy to be ignored in CPEs selection.
The safety risks of CPEs mainly include skin irritation and skin allergic reaction. Both of them initiate with the step of the destruction of the SC barrier, which is also the efficiency mechanism of CPEs enhancing drug permeation. Similarly, they eventually cause inflammation via kinds of cytokines. But there are still some differences between them, such as the initiators of inflammatory skin reaction and their clinical symptoms. The different mechanisms by which CPEs disrupting the SC barrier are essential to balance efficiency and safety. For example, SC lipids extraction, fluidization, and lipid lamellae disorganization are three pathways of CPEs altering SC lipid barrier, thereby bringing efficiency. But the SC lipids extraction is more likely to cause skin irritation and allergic reaction. Hence, the efficiency mechanism of CPEs ought to be distinguished and paid attention to. Notably, for some CPEs, their efficiency is proportional to their skin risk. But for other CPEs, there is no such relationship between their efficiency and the skin risk. From this point, it is very possible to search for a CPE with high efficiency and high safety. To maintain the balance between CPEs efficiency and safety, the following aspects should be paid attention to:
-
1)
The action mechanisms of CPEs disrupting the skin barrier should be distinguished. It is necessary to avoid the selection of CPEs with action mechanisms of extracting SC lipids or denaturing SC protein.
-
2)
Cytotoxicity of CPEs is often relevant to the skin irritation of CPEs.
-
3)
It is satisfactory to design CPEs that exert their effect exclusively in SC with little or without influence on the living cells of the epidermis. It is hoped that biodegradable CPEs would solve this problem.
-
4)
To some CPEs, their safety problems increased with the increasing of CPEs concentration. The combined application of CPEs is promising to balance between CPEs efficiency and safety.
In current developments to improve efficiency and safety, CPEs structure modification has been employed to maximize the efficiency, which may be owed to the adjustment of matched behavior between CPE and drug. As for the combined application of CPEs, it is a satisfactory method to both improve efficiency and reduce skin risk via promoting CPEs skin permeation behavior, but the choice of CPE combinations is arduous. Novel CPEs exhibited higher efficiency than traditional CPEs, some of which even can enhance the permeation of macromolecules. Most of these CPEs improved their skin permeation for a more satisfactory efficiency. For these highly efficient novel CPEs, the safety risk should be paid close attention to and the applicability ought to be researched for different kinds of drug candidates.
To sum up, possessing the unique advantage of simple use and high compatibility, CPE is a more economic and accessible approach than any other enhancing technologies in product development. In the long term, CPEs will be researched as a hotspot and still occupy an important position in the market of the transdermal product. As for enhancing the permeation of small molecule drugs, the balance between CPEs efficiency and safety has not yet reached their potential. The understanding of CPEs molecular mechanism is expected to provide rational guidance for the selection of CPEs.
Conclusion
Effectiveness and safety issues limit the applications of CPEs in TDDS. Understanding the essence of CPEs efficiency and safety helped to improve the problems of low, unpredictable efficiency and safety risk, thereby contributing to a rational selection of CPEs. Although CPEs efficiency and safety have a strong association with each other, it is still possible to acquire a CPE with high efficiency and low safety risk. The current developments in the field provided potent and safe CPEs; however, these progresses suggested that the balance between CPEs efficiency and safety has not yet reached its potential. CPEs are expected to have a bright research perspective and occupy an important position in the market of transdermal products.
References
Battisti M, Vecchione R, Casale C, Pennacchio FA, Lettera V, Jamaledin R, et al. Non-invasive production of multi-compartmental biodegradable polymer microneedles for controlled intradermal drug release of labile molecules. Front Bioeng Biotechnol. 2019;7:296. https://doi.org/10.3389/fbioe.2019.00296.
Dai X, Yin Q, Wan G, Wang R, Shi X, Qiao Y. Effects of concentrations on the transdermal permeation enhancing mechanisms of borneol: a coarse-grained molecular dynamics simulation on mixed-bilayer membranes. Int J Mol Sci. 2016;17(8):1349. https://doi.org/10.3390/ijms17081349.
Sahu P, Kashaw SK, Jain S, Sau S, Iyer AK. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: in vitro and ex vivo studies. J Control Release. 2017;253:122–36. https://doi.org/10.1016/j.jconrel.2017.03.023.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37. https://doi.org/10.1016/j.addr.2012.09.032.
Azagury A, Khoury L, Enden G, Kost J. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev. 2014;72:127–43. https://doi.org/10.1016/j.addr.2014.01.007.
Chih-Hung Lin IAAJ-YF. Lasers as an approach for promoting drug delivery via skin. Expert Opin Drug Deliv. 2014;11(4):599–614.
Chen Y, Chen BZ, Wang QL, Jin X, Guo XD. Fabrication of coated polymer microneedles for transdermal drug delivery. J Control Release. 2017;265:14–21. https://doi.org/10.1016/j.jconrel.2017.03.383.
Puri A, Murnane KS, Blough BE, Banga AK. Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride. Int J Pharm. 2017;528(1–2):452–62. https://doi.org/10.1016/j.ijpharm.2017.06.041.
Seong KY, Seo MS, Hwang DY, O’Cearbhaill ED, Sreenan S, Karp JM, et al. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J Control Release. 2017;265:48–56. https://doi.org/10.1016/j.jconrel.2017.03.041.
Xi H, Cun D, Xiang R, Guan Y, Zhang Y, Li Y, et al. Intra-articular drug delivery from an optimized topical patch containing teriflunomide and lornoxicam for rheumatoid arthritis treatment: does the topical patch really enhance a local treatment? J Control Release. 2013;169(1–2):73–81. https://doi.org/10.1016/j.jconrel.2013.03.028.
N’Da DD. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules. 2014;19(12):20780–807. https://doi.org/10.3390/molecules191220780.
Jain S, Patel N, Shah MK, Khatri P, Vora N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci. 2017;106(2):423–45. https://doi.org/10.1016/j.xphs.2016.10.001.
Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release. 2018;270:203–25. https://doi.org/10.1016/j.jconrel.2017.11.049.
Liu X, Quan P, Li S, Liu C, Zhao Y, Zhao Y, et al. Time dependence of the enhancement effect of chemical enhancers: molecular mechanisms of enhancing kinetics. J Control Release. 2017;248:33–44. https://doi.org/10.1016/j.jconrel.2017.01.017.
Watkinson AC, Kearney MC, Quinn HL, Courtenay AJ, Donnelly RF. Future of the transdermal drug delivery market–have we barely touched the surface? Expert Opin Drug Deliv. 2016;13(4):523–32. https://doi.org/10.1517/17425247.2016.1130034.
Vitorino C, Almeida J, Goncalves LM, Almeida AJ, Sousa JJ, Pais AA. Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release. 2013;167(3):301–14. https://doi.org/10.1016/j.jconrel.2013.02.011.
Hui Zhang YZ. Xiaoye Yang and Guangxi Zhai. Breaking the skin barrier achievements and future directions. Curr Pharma Des. 2015;2015(21):2713–24.
Andrej Kováčik MKKV. Permeation enhancers in transdermal drug delivery benefits and limitations. Expert Opin Drug Deliv. 2020. https://doi.org/10.1080/17425247.2020.1713087.
Lopes LB, J Garcia MT, Lb Bentley MV. Chemical penetration enhancers. Ther Deliv. 2015:1053–61. https://doi.org/10.4155/tde.15.61.
Zeng L, Song W, He W, Zhang J, Wang Y, Bian J, et al. Unconventional passive enhancement of transdermal drug delivery: toward a mechanistic understanding of penetration enhancers releasing from acrylic pressure-sensitive adhesive of patches. Pharm Res. 2020;37(9):169. https://doi.org/10.1007/s11095-020-02901-0.
Song W, Quan P, Li S, Liu C, Lv S, Zhao Y, et al. Probing the role of chemical enhancers in facilitating drug release from patches: mechanistic insights based on FT-IR spectroscopy, molecular modeling and thermal analysis. J Control Release. 2016;227:13–22. https://doi.org/10.1016/j.jconrel.2016.02.027.
Abid Hussain GMK, Abdul Wahab, Muhammad Akhlaq, Saif ur Rahman, Hamza Altaf, Naheed Akhtar and Muhammad Imran Qayyum. Potential enhancers for transdermal drug delivery: a review. Int J Basic Med Sci Pharm. 2014;4(1).
Serna-Jimenez CE, del Rio-Sancho S, Calatayud-Pascual MA, Balaguer-Fernandez C, Femenia-Font A, Lopez-Castellano A, et al. Development of antimigraine transdermal delivery systems of pizotifen malate. Int J Pharm. 2015;492(1–2):223–32. https://doi.org/10.1016/j.ijpharm.2015.07.033.
van Zyl L, du Preez J, Gerber M, Plessis J, Viljoen J. Essential fatty acids as transdermal penetration enhancers. J Pharm Sci. 2016;105(1):188–93. https://doi.org/10.1016/j.xphs.2015.11.032.
Liu W, Teng L, Yu K, Sun X, Fan C, Long C, et al. Design of hydrogels of 5-hydroxymethyl tolterodine and their studies on pharmacokinetics, pharmacodynamics and transdermal mechanism. Eur J Pharm Sci. 2017;96:530–41. https://doi.org/10.1016/j.ejps.2016.10.024.
Ameen D, Michniak-Kohn B. Development and in vitro evaluation of pressure sensitive adhesive patch for the transdermal delivery of galantamine: effect of penetration enhancers and crystallization inhibition. Eur J Pharm Biopharm. 2019;139:262–71. https://doi.org/10.1016/j.ejpb.2019.04.008.
Bakshi P, Vora D, Hemmady K, Banga AK. Iontophoretic skin delivery systems: success and failures. Int J Pharm. 2020;586: 119584. https://doi.org/10.1016/j.ijpharm.2020.119584.
Jiang T, Xu G, Chen G, Zheng Y, He B, Gu Z. Progress in transdermal drug delivery systems for cancer therapy. Nano Res. 2020;13(7):1810–24. https://doi.org/10.1007/s12274-020-2664-5.
Sjovall P, Skedung L, Gregoire S, Biganska O, Clement F, Luengo GS. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci Rep. 2018;8(1):16683. https://doi.org/10.1038/s41598-018-34286-x.
Yang D, Chen M, Sun Y, Jin Y, Lu C, Pan X, et al. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021;121:119–33. https://doi.org/10.1016/j.actbio.2020.12.004.
Notman R, Anwar J. Breaching the skin barrier–insights from molecular simulation of model membranes. Adv Drug Deliv Rev. 2013;65(2):237–50. https://doi.org/10.1016/j.addr.2012.02.011.
Barbero AM, Frasch HF. Effect of stratum corneum heterogeneity, anisotropy, asymmetry and follicular pathway on transdermal penetration. J Control Release. 2017;260:234–46. https://doi.org/10.1016/j.jconrel.2017.05.034.
Ishida-Yamamoto A, Igawa S, Kishibe M, Honma M. Clinical and molecular implications of structural changes to desmosomes and corneodesmosomes. J Dermatol. 2018;45(4):385–9. https://doi.org/10.1111/1346-8138.14202.
Nina Dragicevic-Curic HIM. Percutaneous penetration enhancers Chemical enhancers in penetration enhancement. Berlin Heidelberg New York Dordrecht London: Springer; 2015.
Van Smeden J, Bouwstra JA. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol. 2016;49:8–26. https://doi.org/10.1159/000441540.
Beddoes CM, Gooris GS, Foglia F, Ahmadi D, Barlow DJ, Lawrence MJ, et al. Arrangement of ceramides in the skin: sphingosine chains localize at a single position in stratum corneum lipid matrix models. Langmuir: ACS J Surf Colloids. 2020;36(34):10270–8. https://doi.org/10.1021/acs.langmuir.0c01992.
Iwai I, Han H, den Hollander L, Svensson S, Ofverstedt LG, Anwar J, et al. The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. J Invest Dermatol. 2012;132(9):2215–25. https://doi.org/10.1038/jid.2012.43.
Mudra Saurabh Kapoor SG, Banerjee R. Stratum corneum modulation by chemical enhancers and lipid nanostructures: implications for transdermal drug delivery. Ther Deliv. 2017;8(8):701–18.
Chen X. Current and future technological advances in transdermal gene delivery. Adv Drug Deliv Rev. 2017;127:85–105. https://doi.org/10.1016/j.addr.2017.12.014.
Chen Y, Feng X, Meng S. Site-specific drug delivery in the skin for the localized treatment of skin diseases. Expert Opin Drug Deliv. 2019;16(8):847–67. https://doi.org/10.1080/17425247.2019.1645119.
Patzelt A, Lademann J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin Drug Deliv. 2019;17(1):49–60. https://doi.org/10.1080/17425247.2020.1700226.
Zorn-Kruppa M, Vidal YSS, Houdek P, Wladykowski E, Grzybowski S, Gruber R, et al. Tight Junction barriers in human hair follicles - role of claudin-1. Sci Rep. 2018;8(1):12800. https://doi.org/10.1038/s41598-018-30341-9.
Pan Q, Yu Y, Chen D, Jiao G, Liu X. Enhanced penetration strategies for transdermal delivery. Front Chem Sci Eng. 2020;14(3):378–88. https://doi.org/10.1007/s11705-019-1913-1.
Mansour RSH, Sallam AA, Hamdan II, Khalil EA, Yousef I. Elucidation of penetration enhancement mechanism of Emu oil using FTIR microspectroscopy at EMIRA laboratory of SESAME synchrotron. Spectrochim Acta A Mol Biomol Spectrosc. 2017;185:1–10. https://doi.org/10.1016/j.saa.2017.05.026.
Lane ME. Skin penetration enhancers. Int J Pharm. 2013;447(1–2):12–21. https://doi.org/10.1016/j.ijpharm.2013.02.040.
Carvalho ALM, Silva JA, Lira AAM, Almeida EDP, Nunes RS, Sarmento VHV, et al. Third-generation transdermal delivery systems containing zidovudine: effect of the combination of different chemical enhancers and a microemulsion system. AAPS PharmSciTech. 2018;19(7):3219–27. https://doi.org/10.1208/s12249-018-1160-7.
Pham QD, Bjorklund S, Engblom J, Topgaard D, Sparr E. Chemical penetration enhancers in stratum corneum - Relation between molecular effects and barrier function. J Control Release. 2016;232:175–87. https://doi.org/10.1016/j.jconrel.2016.04.030.
Kapoor MS, GuhaSarkar S, Banerjee R. Stratum corneum modulation by chemical enhancers and lipid nanostructures: implications for transdermal drug delivery. Ther Deliv. 2017;8(8):701–18.
Moghadam SH, Saliaj E, Wettig SD, Dong C, Ivanova MV, Huzil JT, et al. Effect of chemical permeation enhancers on stratum corneum barrier lipid organizational structure and interferon alpha permeability. Mol Pharm. 2013;10(6):2248–60. https://doi.org/10.1021/mp300441c.
Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A. 2005;102(13):4688–93. https://doi.org/10.1073/pnas.0501176102.
Vasyuchenko EP, Orekhov PS, Armeev GA, Bozdaganyan ME. CPE-DB: an open database of chemical penetration enhancers. Pharmaceutics. 2021;13(1). https://doi.org/10.3390/pharmaceutics13010066.
Ruan J, Wan X, Quan P, Liu C, Fang L. Investigation of effect of isopropyl palmitate on drug release from transdermal patch and molecular dynamics study. AAPS PharmSciTech. 2019;20(5):174. https://doi.org/10.1208/s12249-019-1370-7.
Li N, Quan P, Wan X, Liu C, Liu X, Fang L. Mechanistic insights of the enhancement effect of sorbitan monooleate on olanzapine transdermal patch both in release and percutaneous absorption processes. Eur J Pharm Sci. 2017;107:138–47. https://doi.org/10.1016/j.ejps.2017.07.006.
Chu T, Wang C, Wang J, Wang H, Geng D, Wu C, et al. Chiral 4-O-acylterpineol as transdermal permeation enhancers: insights of the enhancement mechanisms of a transdermal enantioselective delivery system for flurbiprofen. Drug Deliv. 2020;27(1):723–35. https://doi.org/10.1080/10717544.2020.1760403.
Caserta F, Brown MB, McAuley WJ. The use of heat and chemical penetration enhancers to increase the follicular delivery of erythromycin to the skin. Eur J Pharm Sci. 2019;132:55–62. https://doi.org/10.1016/j.ejps.2019.02.030.
Abd E, Benson HAE, Roberts MS, Grice JE. Follicular penetration of caffeine from topically applied nanoemulsion formulations containing penetration enhancers: in vitro human skin studies. Skin Pharmacol Physiol. 2018;31(5):252–60. https://doi.org/10.1159/000489857.
Roy N, Agrawal M, Chaudhary S, Tirkey V, Dhwaj A, Mishra N. Review article on permeation enhancers: a major breakthrough in drug delivery technology. Int J Pharm Sci Res. 2017;8(3):1001–11. https://doi.org/10.13040/ijpsr.0975-8232.8.
Kopecna M, Kovacik A, Kucera O, Machacek M, Sochorova M, Audrlicka P, et al. Fluorescent penetration enhancers reveal complex interactions among the enhancer, drug, solvent, and skin. Mol Pharm. 2019;16(2):886–97. https://doi.org/10.1021/acs.molpharmaceut.8b01196.
Simon A, Amaro MI, Healy AM, Cabral LM, de Sousa VP. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int J Pharm. 2016;512(1):234–41. https://doi.org/10.1016/j.ijpharm.2016.08.052.
Yang D, Liu C, Quan P, Fang L. A systematic approach to determination of permeation enhancer action efficacy and sites: molecular mechanism investigated by quantitative. J Control Release. 2020;322:1–12. https://doi.org/10.1016/j.jconrel.2020.03.014.
Alexander A, Dwivedi S, Ajazuddin GTK, Saraf S, Saraf S, et al. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164(1):26–40. https://doi.org/10.1016/j.jconrel.2012.09.017.
Brychtova K, Opatrilova R, Raich I, Kalinowski DS, Dvorakova L, Placek L, et al. Investigating the activity of 2-substituted alkyl-6-(2,5-dioxopyrrolidin-1-yl)hexanoates as skin penetration enhancers. Bioorg Med Chem. 2010;18(24):8556–65. https://doi.org/10.1016/j.bmc.2010.10.025.
Ibrahim SA, Li SK. Efficiency of fatty acids as chemical penetration enhancers: mechanisms and structure enhancement relationship. Pharm Res. 2010;27(1):115.
Wang C, Liu R, Tang X, Han W. A drug-in-adhesive matrix based on thermoplastic elastomer: evaluation of percutaneous absorption, adhesion, and skin irritation. AAPS PharmSciTech. 2012;13(4):1179–89. https://doi.org/10.1208/s12249-012-9849-5.
Lv S, Quan P, Wang W, Fang L. Two steps modification for improvement of cyclobenzaprine transdermal delivery: releasing from patch and penetrating through skin. Drug Dev Ind Pharm. 2016;42(12):2070–7. https://doi.org/10.1080/03639045.2016.1195402.
Jampilek J, Brychtova K. Azone analogues: classification, design, and transdermal penetration principles. Med Res Rev. 2012;32(5):907–47. https://doi.org/10.1002/med.20227.
Ameen D, Michniak-Kohn B. Transdermal delivery of dimethyl fumarate for Alzheimer’s disease: effect of penetration enhancers. Int J Pharm. 2017;529(1–2):465–73. https://doi.org/10.1016/j.ijpharm.2017.07.031.
Shen M, Liu C, Wan X, Farah N, Fang L. Development of a daphnetin transdermal patch using chemical enhancer strategy: insights of the enhancement effect of Transcutol P and the assessment of pharmacodynamics. Drug Dev Ind Pharm. 2018;44(10):1642–9. https://doi.org/10.1080/03639045.2018.1483391.
Wang W, Liu C, Luo Z, Wan X, Fang L. Investigation of molecular mobility of pressure-sensitive-adhesive in oxybutynin patch in vitro and in vivo: effect of sorbitan monooleate on drug release and patch mechanical property. Eur J Pharm Sci. 2018;122:116–24. https://doi.org/10.1016/j.ejps.2018.06.016.
Pupe CG, Do Carmo FA, De Sousa VP, Lopes M, Abrahim-Vieira B, Ribeiro AJ, et al. Development of a doxazosin and finasteride transdermal system for combination therapy of benign prostatic hyperplasia. J Pharm Sci. 2013;102(11):4057–64. https://doi.org/10.1002/jps.23715.
Xie F, Chai JK, Hu Q, Yu YH, Ma L, Liu LY, et al. Transdermal permeation of drugs with differing lipophilicity: effect of penetration enhancer camphor. Int J Pharm. 2016;507(1–2):90–101. https://doi.org/10.1016/j.ijpharm.2016.05.004.
Caon T, Campos CE, Simoes CM, Silva MA. Novel perspectives in the tuberculosis treatment: administration of isoniazid through the skin. Int J Pharm. 2015;494(1):463–70. https://doi.org/10.1016/j.ijpharm.2015.08.067.
Cui H, Quan P, Zhou Z, Fang L. Development of a drug-in-adhesive patch combining ion pair and chemical enhancer strategy for transdermal delivery of zaltoprofen: pharmacokinetic, pharmacodynamic and in vitro/in vivo correlation evaluation. Drug Deliv. 2016;23(9):3461–70. https://doi.org/10.1080/10717544.2016.1196766.
Cizinauskas V, Elie N, Brunelle A, Briedis V. Fatty acids penetration into human skin ex vivo: A TOF-SIMS analysis approach. Biointerphases. 2017;12(1): 011003. https://doi.org/10.1116/1.4977941.
Zeng L, Yang G, Liu J, Quan D, Song W. Probing dynamic behavior of chemical enhancers passing in and out of the stratum corneum and modulation by biodegradable enhancer. AAPS PharmSciTech. 2021;22(4):139. https://doi.org/10.1208/s12249-021-02009-7.
Herman A, Herman AP. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: a review. J Pharm Pharmacol. 2015;67(4):473–85. https://doi.org/10.1111/jphp.12334.
Ale IS, Maibach HI. Irritant contact dermatitis. Rev Environ Health. 2014;29(3):195–206. https://doi.org/10.1515/reveh-2014-0060.
Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32(1):116–24. https://doi.org/10.1016/j.clindermatol.2013.05.033.
Welss T, Basketter DA, Schroder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol In Vitro. 2004;18(3):231–43. https://doi.org/10.1016/j.tiv.2003.09.009.
Proksch E, Brasch J. Abnormal epidermal barrier in the pathogenesis of contact dermatitis. Clin Dermatol. 2012;30(3):335–44. https://doi.org/10.1016/j.clindermatol.2011.08.019.
Guo JW, Lin TK, Wu CH, Wei KC, Lan CC, Peng AC, et al. Human sebum extract induces barrier disruption and cytokine expression in murine epidermis. J Dermatol Sci. 2015;78(1):34–43. https://doi.org/10.1016/j.jdermsci.2015.01.010.
Wagener FA, Carels CE, Lundvig DM. Targeting the redox balance in inflammatory skin conditions. Int J Mol Sci. 2013;14(5):9126–67. https://doi.org/10.3390/ijms14059126.
Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–34. https://doi.org/10.1016/j.lfs.2016.03.039.
Mayassa J, Bou-Dargham ZIK, Armand B. (2017) Cognetta and Qing-Xiang Amy Sang The role of interleukin-1 in inflammatory and malignant human skin diseases and the rationale for targeting interleukin-1 alpha. Med Res Rev. 2017;37(1):180–216. https://doi.org/10.1002/med.21406.
Ruize Qu XC, Jing Hu, Yufeng Fu, Jiangfan Peng, Yuhua Li, et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci Rep. 2019; 9(1348). https://doi.org/10.1038/s41598-018-38174-2.
Haur Yueh Lee MS, Nikhil Yawalkar and MasatoKakeda. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013. https://doi.org/10.1155/2013/916497.
Alexandra Leonard EG-Y. The unique molecular signatures of contact dermatitis and implications for treatment. Clin Rev Allergy Immunol. 2019;56:1–8. https://doi.org/10.1007/s12016-018-8685-0.
Kostner L, Anzengruber F, Guillod C, Recher M, Schmid-Grendelmeier P, Navarini AA. Allergic contact dermatitis. Immunol Allergy Clin North Am. 2017;37(1):141–52. https://doi.org/10.1016/j.iac.2016.08.014.
Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133(2):303–15. https://doi.org/10.1038/jid.2012.284.
Koppes SA, Engebretsen KA, Agner T, Angelova-Fischer I, Berents T, Brandner J, et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis. 2017;77(1):1–16. https://doi.org/10.1111/cod.12789.
Leonard A, Guttman-Yassky E. The unique molecular signatures of contact dermatitis and implications for treatment. Clin Rev Allergy Immunol. 2019;56(1):1–8. https://doi.org/10.1007/s12016-018-8685-0.
Rustemeyer TIMWvH, von Blomberg BME, Scheper RJ. Mechanisms of allergic contact dermatitis. Kanerva’s Occupational Dermatology. 2018:1–14. https://doi.org/10.1007/978-3-319-40221-5_14-2.
Maibach JH, Ca HI. Allergic contact dermatitis. Allergy and Asthma. 2019. https://doi.org/10.1007/978-3-319-58726-4_11-1.
Romita P, Foti C, Calogiuri G, Cantore S, Ballini A, Dipalma G, et al. Contact dermatitis due to transdermal therapeutic systems: a clinical update. Acta Biomed. 2018;90(1):5–10. https://doi.org/10.23750/abm.v90i1.6563.
Yerramsetty KM, Rachakonda VK, Neely BJ, Madihally SV, Gasem KAM. Effect of different enhancers on the transdermal permeation of insulin analog. Int J Pharm. 2010;398(1–2):83–92. https://doi.org/10.1016/j.ijpharm.2010.07.029.
Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22(2):192–7. https://doi.org/10.1038/nbt928.
Joonho Choi MKC, Saeho Chong, Suk-Jae Chung, Chang-Koo Shim, Dae-Duk Kim. Effect of fatty acids on the transdermal delivery of donepezil: in vitro and in vivo evaluation. Int J Pharm. 2012;422:83–90. https://doi.org/10.1016/j.ijpharm.2011.10.031.
Sanjeev Rambharose RSK, Akamanchic KG, Govender T. Novel dendritic derivatives of unsaturated fatty acids as promising transdermal permeation enhancers for tenofovir. J Mater Chem B. 2015;3(32):6662–75. https://doi.org/10.1039/C5TB00957J.
Abruzzo A, Armenise N, Bigucci F, Cerchiara T, Gosser MB, Samori C, et al. Surfactants from itaconic acid: toxicity to HaCaT keratinocytes in vitro, micellar solubilization, and skin permeation enhancement of hydrocortisone. Int J Pharm. 2017;524(1–2):9–15. https://doi.org/10.1016/j.ijpharm.2017.03.056.
Chaudhari KS, Akamanchi KG. Fatty acid esters of g0-(propyl ether imine) dendron as bicephalous heterolipids for permeation enhancement in transdermal drug delivery. ACS Biomater Sci Eng. 2018;4(12):4008–20. https://doi.org/10.1021/acsbiomaterials.8b00953.
Chen Y, Cun D, Quan P, Liu X, Guo W, Peng L, et al. Saturated long-chain esters of isopulegol as novel permeation enhancers for transdermal drug delivery. Pharm Res. 2014;31(8):1907–18. https://doi.org/10.1007/s11095-013-1292-0.
Kalhapure RS, Akamanchi KG. Oleodendrimers: a novel class of multicephalous heterolipids as chemical penetration enhancers for transdermal drug delivery. Int J Pharm. 2013;454(1):158–66. https://doi.org/10.1016/j.ijpharm.2013.07.028.
Kim KT, Kim JS, Kim MH, Park JH, Lee JY, Lee W, et al. Effect of enhancers on in vitro and in vivo skin permeation and deposition of S-Methyl-L-Methionine. Biomol Ther. 2017;25(4):434–40. https://doi.org/10.4062/biomolther.2016.254.
Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta (BBA) Biomembr. 2009;1788(11):2362–73. https://doi.org/10.1016/j.bbamem.2009.08.015.
Shah PP, Desai PR, Patlolla R, Klevans L, Singh M. Effect of combination of hydrophilic and lipophilic permeation enhancers on the skin permeation of kahalalide F. J Pharm Pharmacol. 2014;66(6):760–8. https://doi.org/10.1111/jphp.12206.
Mueller J, Oliveira JS, Barker R, Trapp M, Schroeter A, Brezesinski G, et al. The effect of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models. Biochim Biophys Acta. 2016;1858(9):2006–18. https://doi.org/10.1016/j.bbamem.2016.05.010.
Chen M, Gupta V, Anselmo AC, Muraski JA, Mitragotri S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J Control Release. 2014;173:67–74. https://doi.org/10.1016/j.jconrel.2013.10.007.
Ita KB. Chemical penetration enhancers for transdermal drug delivery- success and challenges. Curr Drug Deliv. 2015;12:645–51.
Kumar S, Zakrewsky M, Chen M, Menegatti S, Muraski JA, Mitragotri S. Peptides as skin penetration enhancers: mechanisms of action. J Control Release. 2015;199:168–78. https://doi.org/10.1016/j.jconrel.2014.12.006.
Menegatti S, Zakrewsky M, Kumar S, De Oliveira JS, Muraski JA, Mitragotri S. De Novo design of skin-penetrating peptides for enhanced transdermal delivery of peptide drugs. Adv Healthc Mater. 2016;5(5):602–9. https://doi.org/10.1002/adhm.201500634.
Tracy Hsu SM. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. 2011. https://doi.org/10.1073/pnas.1016152108/-/DCSupplemental.
Ruan R CM, Zou L,5, Wei P,6, Liu J,5, Ding W,5, Wen L. Recent advances in peptides for enhancing transdermal macromolecular drug delivery. Ther Deliv. 2016;7(2):89–100.
Lemos D, Carvalho V, Lopes LB. Potential of peptide-based enhancers for transdermal delivery. Curr Pharm Des. 2015;21(20):2814–22.
Candan G, Michiue H, Ishikawa S, Fujimura A, Hayashi K, Uneda A, et al. Combining poly-arginine with the hydrophobic counter-anion 4-(1-pyrenyl)-butyric acid for protein transduction in transdermal delivery. Biomaterials. 2012;33(27):6468–75. https://doi.org/10.1016/j.biomaterials.2012.04.056.
Monti D, Egiziano E, Burgalassi S, Chetoni P, Chiappe C, Sanzone A, et al. Ionic liquids as potential enhancers for transdermal drug delivery. Int J Pharm. 2017;516(1–2):45–51. https://doi.org/10.1016/j.ijpharm.2016.11.020.
Wang D, Richter C, Ruhling A, Drucker P, Siegmund D, Metzler-Nolte N, et al. A remarkably simple class of imidazolium-based lipids and their biological properties. Chemistry. 2015;21(43):15123–6. https://doi.org/10.1002/chem.201502333.
Kubota K, Shibata A, Yamaguchi T. The molecular assembly of the ionic liquid/aliphatic carboxylic acid/aliphatic amine as effective and safety transdermal permeation enhancers. Eur J Pharm Sci. 2016;86:75–83. https://doi.org/10.1016/j.ejps.2016.03.002.
Zakrewsky M, Lovejoy KS, Kern TL, Miller TE, Le V, Nagy A, et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc Natl Acad Sci U S A. 2014;111(37):13313–8. https://doi.org/10.1073/pnas.1403995111.
Chantereau G, Sharma M, Abednejad A, Neves BM, Sèbe G, Coma V, et al. Design of nonsteroidal anti-inflammatory drug-based ionic liquids with improved water solubility and drug delivery. ACS Sustain Chem Eng. 2019;7(16):14126–34. https://doi.org/10.1021/acssuschemeng.9b02797.
Moshikur RM, Chowdhury MR, Moniruzzaman M, Goto M. Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem. 2020;22(23):8116–39. https://doi.org/10.1039/D0GC02387F.
Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117(10):7132–89. https://doi.org/10.1021/acs.chemrev.6b00562.
Sidat Z, Marimuthu T, Kumar P, du Toit LC, Kondiah PPD, Choonara YE, et al. Ionic liquids as potential and synergistic permeation enhancers for transdermal drug delivery. Pharmaceutics. 2019;11(2). https://doi.org/10.3390/pharmaceutics11020096.
Adawiyah N, Moniruzzaman M, Hawatulaila S, Goto M. Ionic liquids as a potential tool for drug delivery systems. Med Chem Comm. 2016;7(10):1881–97. https://doi.org/10.1039/c6md00358c.
Medrx Co. L. External preparation composition comprising fatty acid-based ionic liquid as active ingredient. EP2014.
Tanner EEL, Ibsen KN, Mitragotri S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J Control Release. 2018;286:137–44. https://doi.org/10.1016/j.jconrel.2018.07.029.
Wang C, Zhu J, Zhang D, Yang Y, Zheng L, Qu Y, et al. Ionic liquid - microemulsions assisting in the transdermal delivery of Dencichine: Preparation, in-vitro and in-vivo evaluations, and investigation of the permeation mechanism. Int J Pharm. 2018;535(1–2):120–31. https://doi.org/10.1016/j.ijpharm.2017.10.024.
Qi QM, Mitragotri S. Mechanistic study of transdermal delivery of macromolecules assisted by ionic liquids. J Control Release. 2019;311–312:162–9. https://doi.org/10.1016/j.jconrel.2019.08.029.
Agatemor CI KN. Ionic liquids for addressing unmet needs in healthcare. Bioeng Transl Med. 2018;3:7–25. https://doi.org/10.1111/btm2.10083.
Chauhan AS. Dendrimer nanotechnology for enhanced formulation and controlled delivery of resveratrol. Ann N Y Acad Sci. 2015;1348(1):134–40. https://doi.org/10.1111/nyas.12816.
Yang J, Hu J, He B, Cheng Y. Transdermal delivery of therapeutic agents using dendrimers (US20140018435A1): a patent evaluation. Expert Opin Ther Pat. 2015;25(10):1209–14. https://doi.org/10.1517/13543776.2015.1044974.
Mutalik S, Shetty PK, Kumar A, Kalra R, Parekh HS. Enhancement in deposition and permeation of 5-fluorouracil through human epidermis assisted by peptide dendrimers. Drug Deliv. 2014;21(1):44–54. https://doi.org/10.3109/10717544.2013.845861.
Manikkath J, Hegde AR, Kalthur G, Parekh HS, Mutalik S. Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int J Pharm. 2017;521(1–2):110–9. https://doi.org/10.1016/j.ijpharm.2017.02.002.
Janusova B, Skolova B, Tukorova K, Wojnarova L, Simunek T, Mladenka P, et al. Amino acid derivatives as transdermal permeation enhancers. J Control Release. 2013;165(2):91–100. https://doi.org/10.1016/j.jconrel.2012.11.003.
Kopecna M, Machacek M, Novackova A, Paraskevopoulos G, Roh J, Vavrova K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci Rep. 2019;9. https://doi.org/10.1038/s41598-019-51226-5.
Zhao L, Fang L, Xu Y, Liu S, He Z, Zhao Y. Transdermal delivery of penetrants with differing lipophilicities using O-acylmenthol derivatives as penetration enhancers. Eur J Pharm Biopharm. 2008;69(1):199–213. https://doi.org/10.1016/j.ejpb.2007.10.015.
Vavrova K, Hrabalek A, Dolezal P, Holas T, Klimentova J. Biodegradable derivatives of tranexamic acid as transdermal permeation enhancers. J Control Release. 2005;104(1):41–9. https://doi.org/10.1016/j.jconrel.2005.01.002.
Geeta Aggarwal SD, HariKumar SL. Natural oils as skin permeation enhancers for transdermal delivery of olanzapine: in vitro and in vivo evaluation. Curr Drug Deliv. 2012;9:172–81.
Muhammad F, Wiley J, Riviere JE. Influence of some plant extracts on the transdermal absorption and penetration of marker penetrants. Cutan Ocul Toxicol. 2017;36(1):60–6. https://doi.org/10.3109/15569527.2016.1147456.
Cizinauskas V, Elie N, Brunelle A, Briedis V. Skin penetration enhancement by natural oils for dihydroquercetin delivery. Molecules. 2017;22(9). https://doi.org/10.3390/molecules22091536.
Liu W, Zhao Q, Lv L, Yan S, Song Q, Chen T, et al. Pomegranate seed oil enhances the percutaneous absorption of trans-resveratrol. J Oleo Sci. 2018;67(4):479–87. https://doi.org/10.5650/jos.ess17144.
Azagury A, Amar-Lewis E, Appel R, Hallak M, Kost J. Amplified CPEs enhancement of chorioamnion membrane mass transport by encapsulation in nano-sized PLGA particles. Eur J Pharm Biopharm. 2017;117:292–9. https://doi.org/10.1016/j.ejpb.2017.04.031.
Alba-Molina D, Giner-Casares JJ, Cano M. Bioconjugated plasmonic nanoparticles for enhanced skin penetration. Top Curr Chem. 2019;378(1):8. https://doi.org/10.1007/s41061-019-0273-0.
Liu K-C, Green CR, Alany RG, Rupenthal ID. Synergistic effect of chemical penetration enhancer and iontophoresis on transappendageal transport of oligodeoxynucleotides. Int J Pharm. 2013;441(1–2):687–92. https://doi.org/10.1016/j.ijpharm.2012.10.027.
Arunkumar S, Ashok P, Desai BG, Shivakumar HN. Effect of chemical penetration enhancer on transdermal iontophoretic delivery of diclofenac sodium under constant voltage. J Drug Del Sci Technol. 2015;30:171–9. https://doi.org/10.1016/j.jddst.2015.10.007.
Wang Y, Zeng L, Song W, Liu J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: an overview of recent progress. Drug Deliv Transl Res. 2021. https://doi.org/10.1007/s13346-021-00898-6.
Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. https://doi.org/10.1016/j.addr.2018.12.006.
Acknowledgements
We would like to acknowledge the National Natural Science Foundation of China (No. 81703452) and the Double first-class innovative team (CPU2018GY28).
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81703452) and the Double first-class innovative team (CPU2018GY28).
Author information
Authors and Affiliations
Contributions
Lijuan Zeng: conceptualization, investigation, visualization, writing—original draft. Feifei Huang: investigation, writing—original draft. Qin Zhang: investigation, writing—original draft. Jianping Liu: writing—review and editing. Danyi Quan: writing—review and editing. Wenting Song: conceptualization, investigation, supervision, visualization, writing—original draft, writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors consent for publishing this work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, L., Huang, F., Zhang, Q. et al. Molecular perspective of efficiency and safety problems of chemical enhancers: bottlenecks and recent advances. Drug Deliv. and Transl. Res. 12, 1376–1394 (2022). https://doi.org/10.1007/s13346-021-01044-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01044-y