Abstract
Tuberculosis (TB) classified as one of the most fatal contagious diseases is of prime concern globally. Mycobacterium tuberculosis is the causative agent that ingresses within the host cells. The approved conventional regimen, though the only viable option available, is unfavorably impacting the quality of life of the affected individual. Despite newer antibiotics gaining light, there is an unending demand for more therapeutic alternatives. Therefore, substantial continuous endeavors are been undertaken to come up with novel strategies to curb the disease, the stepping stone being nanotechnology. This approach is instrumental in overcoming the anomalies associated with conventional therapy owing to their intriguing attributes and leads to optimization of the therapeutic effect to a certain extent. This review focusses on the different types of nanocarrier systems that are being currently explored by the researchers for the delivery of anti-tubercular drugs, the outcomes achieved by them, and their prospects.

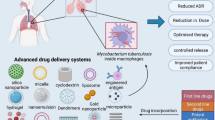
Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
TB, being one of the most prevalent infectious and contagious disease caused by Mycobacterium tuberculosis (Mtb), has a huge global burden accounting to about 10 million new cases in 2017. According to the WHO Report 2019, around 1.6 million deaths take place due to TB. Additionally, also one-third of the world population is latently affected by it. The worst affected geography is the Asian subcontinent with India reporting the highest tally [1,2,3,4,5,6]. Owing to the limited number of approved anti-TB drugs, the emergence of multi and extensively drug-resistant strains of TB has been a problematic issue. In the case of drug-susceptible cases, the initial phase consists of 6 months of combinatorial treatment commonly called “drug cocktail” which consists of isoniazid (INH), rifampicin (RIF), ethambutol (EMB), and pyrazinamide (PZA). This is further followed by a continuation phase of 2 months comprising of INH and RIF. However, there are drug-resistant strains that follow a different treatment regimen. The drug resistance occurs principally due to mutations occurring in drug target genes. The other causes might be attributable to the impermeability of the Mtb cell wall or the activity of efflux pumps. Multidrug resistance occurs when patients become resistant at least to INH or RIF. On the other hand, extensive drug resistance occurs on developing resistance to INH and RIF along with fluoroquinolones. In the case of INH resistance, the treatment regimen incorporates 6 months of RIF, EMB, PZA, and levofloxacin [7,8,9].
An extensive literature survey was carried out on databases such as Scopus, PubMed, ScienceDirect, and Google Scholar. Primarily research conducted on anti-tubercular nano-formulations within the duration of 2015–2019 was considered for incorporation within the manuscript. Few breakthrough review articles, however, limited in number, have also been incorporated to give the readers a clear concept.
Nanotechnological approach for combating TB
The lungs appear as an attractive target for the therapeutic delivery of anti-TB drugs. The inhalational route of drug administration serves as the primary option for delivering dry powder inhalers or aerosols, since they provide a preferential accumulation of drugs at the target site in addition to being non-invasive and overcoming gastrointestinal drug degradation. They can thus also restrict drug exposure to healthy cells. However, they were also associated with certain loopholes such as bolus drug release culminating into severe lung toxicity, short residence time, and poor aqueous solubility [10]. The other contemporary TB treatment includes oral and parenteral dosage forms. However, they lead to sub-therapeutic levels attributable to poor drug distribution to the lungs, ultimately causing drug-resistant strains [11]. The commercially available conventional anti-TB therapy is associated with innumerable glitches such as metabolic instability, low solubility, low permeability, a large number of allied side effects especially hepatotoxicity, and high drug load [3, 12]. Moreover, the combination therapy also leads to complex dosing schedules frequently causing inadequate therapeutic effect [7]. Therefore, these drawbacks with conventional therapy led to the paradigm shift towards nanotechnology-engineered delivery systems. The nanocarrier systems have established their supremacy over conventional therapy since they have the ability to incorporate both lipophilic and hydrophilic drugs. They offer advantages such as the drastic reduction in size hence providing high surface volume ratio, the protection of drug moiety from degradation, the targeted and site-specific delivery, follow a controlled release mechanism, and could be easily functionalized to modify drug therapeutic profile. Additionally, one of the prime outlooks might be the biocompatible nature of these systems [13, 14]. Furthermore, the lipid-based systems hold special significance, since the pathogen, i.e., Mtb, relies on the lipidic membrane to ingress with the host body and egress from the same [15]. Furthermore, nanoparticulate systems possess intrinsic antimycobacterial activity along with serving as a vehicle for various anti-tubercular drugs, hence being instrumental in reducing the dosing frequency along with associated side effects. Additionally, nano-formulations incorporating inorganic metals have anti-bacterial activity. Silver and gold nanoparticles (NPs) demonstrated promising in vitro antimycobacterial activity [16]. Moreover, targeted site-specific delivery could be obtained via inhalational NPs [6].
Nanotechnology primarily focusses on “technology transfer,” whereby novel techniques are implemented on a conventional treatment regimen to boost the therapeutic process. However, establishing the toxicity and safety profile of these nano-formulations is a daunting task and needs to be investigated further [17].
Therefore, the nano-based anti-tubercular therapy system overcomes the obsolete techniques of conventional regimen and revamps the drug delivery systems, hereafter promoting the healthcare. Impetus must be laid on implementing novel prospects for restricting the boundaries for the propagation of TB.
No doubt, plethora of anti-bacterial agents is existing, which bears activity against Mtb; however, these synthetic antimicrobials are associated with unavoidable glitches, most prominent among them being drug resistance. Consequently, researchers have been exploiting this domain to come up with novel techniques and anti-TB leads. Hence, plant-derived alternatives suit fashion and serve as probable leads, which warrants tremendous exploration [18]. Researches have come to light of late, which explicitly establishes the prospects of herbal origin drugs when incorporated in nano-formulations.
The different categories of nano-based systems that have been researched for the treatment of TB have been undermentioned.
Nanoparticles
NPs have gained impetus owing to their small dimension of around 100 nm. However, they comprise primarily of three layers, i.e., the outer layer, which is functionalized by either polymers or metal ions which give specific characteristics to these NPs, thus dividing them into different categories. The middle layer forms the shell of the NPs, while the inner layer is the core [19]. Thus, this section on NPs deals with their different types such as polymeric NPs or metallic NPs.
Polymeric NPs have garnered speculations attributable to their remarkable credentials such as passive deposition in the target site, biocompatibility, physicochemical stability, and modified release. Furthermore, the hydrophilic outer core and hydrophobic inner core allow for varied drugs to be entrapped within it [20]. All these abilities explicitly justify the use of NPs in TB therapy. Therefore, NPs have been the favorite among the researchers, and abundant of them are available in the public domain. Praphakar et al. tailored RIF silver NPs on chitosan (CS)-grafted cetyl alcohol-maleic anhydride-PZA polymer for ameliorated drug efficacy. It was observed that 90% of the drug was released within a span of 12 days and the anti-bacterial activity was ameliorated by the formulation. The VERO cell line was used for the cytotoxicity analysis, while THP-1 cells were employed for cell apoptosis assay. The results established the supremacy of NPs [21]. Tenland et al. studied the effect of a novel antimycobacterial peptide, NZX, on the colonization of Mtb. A slow release of 7.6% was observed from the NPs in the duration of 48 h. Primary macrophages and THP-1 cells used for uptake studies unraveled the fact that primary macrophage cell lines had greater cellular uptake. There was decline in CFU values, i.e., 84% reduction in the NZX group; 88% in the NP group; and 90% in the RIF-treated group, which was taken as standard, thus establishing the effectiveness of the prepared formulation [22]. In the research carried out by Bachhav et al., RIF-loaded NPs were fabricated by conjugation with GantrezAN-119 and using ethyl cellulose as polymer. Lymph-mediated lung targeting was elucidated from the fluorescence microscopy. A 2-fold increment in the AUC/minimum inhibitory concentration (MIC) ratio was observed for the prepared NP, whereas there is a 182.4 ± 22.6% increase in relative bioavailability (BA). Additionally, hepatotoxicity was augmented by the formulation [23]. Bhusal and colleagues developed glycan-coated magnetic NPs for the detection of acid-fast bacilli in sputum by making use of NP-based colorimetric biosensing assay. This biosensing technique might bear fruitful results through adequate implementation and facilitating the health care process. The outcome clarified that the designed system was in conformation with the established benchmark Xpert Mtb/RIF in terms of specificity and selectivity [24]. In the research carried out by Saifullah et al., EMB was loaded in graphene oxide–iron oxide magnetite NPs. A sustained in vitro release was observed along with potent anti-tubercular activity [25]. Rawal et al. developed RIF CS NP to be delivered as an inhalational system. The safety analysis was carried out using J774macrophage cells, whereby the % cell viability was calculated to be 75–80% at a dose of 0.125 mg/mL. The formulation also did not show any trace of toxicity [26]. In a different study envisaged by Thomas et al., they developed alginate cellulose nanocrystal hybrid NPs of RIF. A slow initial drug release of only 10–15% was observed in 2 h, which gradually increased up to 100% in 12 h on increasing the pH to 7.4. The L929 cell lines were used to perform the MTT assay, which demonstrated the % cell viability to be nearing 100% [27]. In a different study, Viswanathan and his colleagues developed mannosylated gelatin NP of licorice. The in vitro studies revealed enhanced cellular uptake in RAW 264.7 from the formulation. Furthermore, the pharmacokinetic (PK) study of the formulation showed the existence of the optimal drug level in the body even after 24 h of drug administration. The in vivo anti-tubercular activity was confirmed since there was a significant decline in bacterial load in the lungs and spleen when Mtb H37Rv-infected mice were treated with mannosylated NP on the contrary to the untreated mice [28]. Malik and colleagues formulated poly (lactic-co glycolic) acid (PLGA) NPs by loading bivalent H1 antigen, which is a fusion of Mtb Ag85B and ESAT6 proteins. The developed NPs were internalized by THP-1 human macrophages and later used for immunizing C57BL/6J mice. There was a significant increase in the total serum IgG level when compared with H1 alone. A 2.4-fold increase in cytokine level and 1.6-fold increment in IL4 level were also observed demonstrating the efficacy of the NPs [29].
Apart from the aforementioned studies, there were uncountable researches undertaken pertaining to NP. However, few of them with outstanding outcomes have been elaborated in Table 1.
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are lipidic carrier systems encompassing solid lipids and surfactants. They have garnered recognition lately by virtue of their attributes, namely, easy to scale up, inhibit drug degradation, ameliorating the pharmacological profile of the drug, desirable physical stability, and controlled or modified drug release [43]. In order to make the formulation accepted by the masses, numerous researches were undertaken. Costa and associates fashioned a conventional SLN formulation of INH and also by functionalization with mannose for contrasting evaluation. The outcome revealed that both the SLNs prepared curtailed the possibility of any toxicity on the human lung epithelial cell line (NCI-H441) and differentiated THP-1. Nevertheless, the functionalized SLN has enhanced uptake caliber in macrophagic cells and demonstrated receptor-dependent internalization [44]. Gaspar et al. developed RFB SLN using two different lipid constituents, namely, glyceryl dibehenate and glyceryl tristearate. In vitro uptake studies from THP1 cells in the macrophagic system showed 46 ± 3% for glyceryl dibehenate and 26 ± 9% for glyceryl tristearate, directing preferable proportion towards glyceryl dibehenate. The cell viability study conducted on A549 and Calu-3 cells gave evidence of low cytotoxicity nearing 20% [45]. Nemati et al., taking into consideration the astounding characteristics of SLN, developed EMB SLN as an inhalational delivery system. A549 cells were employed for the evaluation of toxicity, which depicted no significant cytotoxicity. A sustained release of 37% was reported from SLN, while the free drug has a release of 43% [46]. Singh and associates developed INH SLN for ocular delivery. The SLN showed 1.6-fold enhanced corneal permeability in addition to 4.6-fold increase in ocular BA when compared with free drug. An overall 2.6-fold improvement in mean resident time was evident from the formulation. The MIC value was diminished by 5 folds. Ten-fold increase in uptake was attained in HCJE cells, while 10.8 fold in HCLE cells [47]. Shipli et al. fabricated RIF SLN, which was conjugated with lactoferrin. Preliminary characterization was carried out. In vitro release studies exhibited enhanced output from the unconjugated system while the in vivo biodistribution study revealed a 3.5-fold increment in BA and amplification in drug uptake from the conjugated system [48]. In the study conducted by Banerjee et al., dual drug-loaded (RIF and INH) lipid nanoparticulate system was tailored, which demonstrated sustained in vitro release pattern. The developed carrier system effectively localized within the different compartments of THP-1. The in vivo studies revealed that the relative BA from the SLN was 7.5 fold higher than the drug suspension, proving the effectiveness of the developed formulation [49].
There are certain other pertinent researches that were carried out and governed by optimistic results as shown in Table 2.
Nanostructured lipid carriers
Nanostructured lipid carrier (NLC) is a newer generation of lipidic carrier systems that were devised to overcome the lacunas of SLN, which restricts its extensive adoption. They are the amalgamation of both solid and liquid lipids, hence possessing diminution in crystallinity and a loosely packed matrix system. This leads to the overall increase in drug entrapment capability and superior stability necessitating its further investigation [55]. Innumerable studies have been steered globally, which established its futuristic commercial applicability, nevertheless demanding exhaustive research. Carneiro et al. developed RIF NLC functionalized with a tuftsin-modified peptide. The drug release profile showed an initial burst release followed by a controlled release. The fluorescent labeled NLC depicted improved cellular uptake, enhanced cell internalization, and a 2-fold increase in activity against Mtb in contract to the free drug [56]. NLC of 3 different copper complexes was fabricated by Sato and colleagues. The in vitro activity of the formulation against Mtb H37Rv showed a value of 55.4, 27.1, and 41.1 folds along with a reduction in toxicity profile [57]. Table 3 represents certain other researches which need to be brought forward.
Nanoemulsion
Nanoemulsion (NE), being a robust carrier, incorporates a wide range of therapeutic agents along with offering stupendous advantages such as increased drug loading and stability, improved bioavailability, and controlled release. When administered via the oral route, NEs not only enhance the solubility of the hydrophobic drugs but also prolong the GIT residence time and improve lymphatic uptake, thereby avoiding the first-pass metabolism [62].
Shah et al. took note of the qualities of NE and fabricated 1st-generation RIF NE and its subsequent functionalization with CS and CS-folate. The inhalational efficiency was greater than 75% for the decorated NE coupled with high lung content, an increase in cell internalization, and improvement in cytotoxic profile [63]. Halicki et al. designed RIF NE and conducted Resazurin Microtiter Assay to determine its antimycobacterial potential. The MIC value for the developed NE was 7.8 μg/mL, while the free drug was 1024 μg/mL. The incorporation of the drug within the lipidic framework of NE led to a decline in drug degradation, whereby improving the pharmacological drug profile [64]. Shobo and associates developed NE of pretomanid, which was to be administered intranasally. The peak concentration of the drug in the brain was estimated to be 12,062.3 ng/g, which was significantly greater than the optimal concentration required, henceforth proving the effectiveness of the prepared formulation [65].
Liposomes
Liposome (LP) delivery systems, a lipid-based vesicular system, display tremendous intrinsic potentialities viz. biocompatibility, biodegradability, could easily be tailored and engineered, and immunogenic in addition to being formulated using phospholipids. LPs are known to be engulfed by the macrophagic cells to meet their fate, hence being a probable candidate for anti-TB drugs since Mtb invests the macrophages. These attributes account for their versatile nature and urge for further exploration [66, 67]. Liu et al. designed thermoresponsive LP in the hydrogel system for delivering INH at the target site for bone TB. Local drug delivery systems offer stupendous advantage by maximal drug delivery at the target site and negligible systemic exposure. The in vivo studies manifested rapid drug availability in synovial fluid post-injection. Furthermore, the cytotoxicity study conducted using the MTT assay affirmed no toxicity, thus substantiating the use of the prepared formulation in bone TB [68]. Hamed and his co-workers fabricated spray-dried nano-LPs incorporated in microparticles of MXF for improved lung deposition of the drug. The surface was modified using 4-aminophenyl-alphaD–manno-pyranoside, which facilitated improved drug uptake by alveolar macrophages. The biphasic drug release mechanism was observed from the formulation coupled with improved MIC values [69]. In the study carried out by Viswanathan et al., inhalational LPs were prepared from Glycyrrhiza glabra, which contains licorice in it. The in vivo lung deposition studies in mice revealed that 46% of the drug directed reaches the lungs, while 16% still persists there post 24-h duration. The study also established the decline in bacterial count and growth in the lungs and spleen of TB infected Balb/c mice. No toxicity was evident even on administering 20 times the normal dose, thus paving the way for its use as an effective anti-TB drug [70]. Table 4 puts forth a few additional commendable researches and their remarkable outcomes.
Micelles
Micelles have principally been explored for hydrophobic drugs attributable to their amphiphilic nature [75]. Tripodo et al. tailored micelles of RIF based on inulin modified with vitamin E. The MIC values obtained post formulation administration showed superior activity against bacterial strains as compared with free drug. Cytocompatibility on human alveolar macrophages demonstrated a higher value of more than 60% [76]. Prabhakar and his colleagues constructed polymeric micelles loaded with RIF and INH. After a duration of 12 days, the % drug release of INH and RIF was found to be 89.21% and 79.37% respectively at pH 5.5. LRP assay was performed to determine the anti-TB activity which depicted the inhibition of Mtb H37Rv by the formulation. They also assessed the cell viability against L929 and U937 cells by MTT assay. Fluorescence microscopy corroborated cell deformation. Furthermore, the in vivo localization study in zebrafish and hemolysis assay conformed to the standards and revealed its potential to enhance the therapeutic and pharmacological aspects of the drug [77]. The RIF-loaded pulmonary nano-based micellar system was developed by Grotz et al. The in vitro aerodynamic study revealed the suitability in alveolar delivery. The in vitro bactericidal activity was increased up to 2.5 folds in Mtb-infected THP-1 macrophages when compared with RIF solution [78].
Other promising researches have been mentioned in Table 5.
Certain other prominent researches undertaken
In addition to the nano-formulations described above, the area has been laden with surplus other nanocarriers, which are yet to be investigated to their fullest or are underexploited. However, few potential researches carried out globally have been undermentioned in Table 6.
Based on the results obtained from the researches carried out, it could rightly be said that the nano-based tubercular systems could revitalize the drug delivery process and improve the therapeutic profiling of the drugs.
Future perspective
Since TB is a condition affecting people globally with a soaring morbidity and mortality index, there is an upsurge in the researches to deduce novel approaches for combating the disease. Focus ought to be laid on the development of newer antimicrobial peptides for therapeutic intervention and to overcome the lacunas of the conventional commercially available therapies [90, 91]. Photodynamic therapies could also be employed in drug-resistant cases, since they yield reactive oxygen species [92]. Gastric resistant systems and drug depot formulations would give a radical solution to some of the relevant issues pertaining to the therapy [93]. Newer functionalization and surface modification techniques to suit the purpose should be repeatedly tried to pave the way for the development of newer products. It could also be rightly stated that herbal origin derivatives could be instrumental in delivering a holistic formulation with minimal side effects, though necessitating in-depth research. Additionally, it could be formulated in conjugation with synthetic drugs proposing a synergistic relation, thus positioning them as a strong competitor in future researches. A major breakthrough in anti-TB dosage regimen will be nanocarrier-based depot formulation. This will be instrumental in improving patient compliance by reducing the dosing frequency and minimizing the side effects [93]. Furthermore, TB-associated diseases also cause a concern, e.g., in TB-associated HIV. Hence, integrating nanotechnology to develop combination preparation to treat both the conditions would bear fruitful results. Moreover, till date, not much has been explored in the arena of vaccine development. Therefore, nanotechnology-based TB vaccine, if developed in the future, will prove to be a breakthrough in the field of drug discovery [6]. Additionally, ligand targeting is one arena that needs to be exploited completely such as mycolic acid like targeting ligands [94]. Repurposing of established drugs can be employed for establishing a newer treatment regimen. For example, fluoroquinolones and clofazimine all were repurposed drugs. There are multiple drugs in clinical trials for MDR-TB. However, to overcome this epidemic, improvement in the management of susceptible TB needs to be taken care of [95].
Conclusion
The review anticipates the use of nanotechnology as a boon for the delivery of anti-tubercular drugs. A plethora of drugs, which is associated with issues hindering their pharmacological profiles, could be incorporated within these nanocarrier systems, henceforth leveraging an increase in therapeutic effectiveness. Although the state of the art for these systems warrants to be exploited to its fullest to make these systems come to reality and hit the markets for commercial applicability.
Abbreviations
- RIF:
-
Rifampicin
- PZA:
-
Pyrazinamide
- ETH:
-
Ethionamide
- D-CS:
-
D-cycloserine
- TB:
-
Tuberculosis
- INH:
-
Isoniazid
- Mtb :
-
Mycobacterium tuberculosis
- MIC:
-
Minimum inhibitory concentration
- NP:
-
Nanoparticle
- NLC:
-
Nanostructured lipid carrier
- LP:
-
Liposome
- MXF:
-
Moxifloxacin
- PK:
-
Pharmacokinetic
- RFB:
-
Rifabutin
- SLN:
-
Solid lipid nanoparticles
- EMB:
-
Ethambutol
- BA:
-
Bioavailability
- PLGA:
-
Poly (lactic-co glycolic) acid
- CS:
-
Chitosan
References
Bisht D, Sharma D, Sharma D, Singh R, Gupta VK. Recent insights into Mycobacterium tuberculosis through proteomics and implications for the clinic. Expert Rev Proteomics. 2019;16(5):443–56. https://doi.org/10.1080/14789450.2019.1608185.
Mehta P, Bothiraja C, Kadam S, Pawar A. Potential of dry powder inhalers for tuberculosis therapy: facts, fidelity and future. Artif Cells Nanomed Biotechnol. 2018;46(3):791–806. https://doi.org/10.1080/21691401.2018.1513938.
Shivangi, Meena LS. A novel approach in treatment of tuberculosis by targeting drugs to infected macrophages using biodegradable nanoparticles. Appl Biochem Biotechnol. 2018. https://doi.org/10.1007/s12010-018-2695-5.
WHO. Global Tuberculosis Report. (2019). https://www.who.int/tb/publications/global_report/en/. Last accessed on 3rd February, 2020; 12:46 pm.
WHO. UN General Assembly High-Level meeting on ending TB. (2018). https://www.who.int/tb/features_archive/UNGA_HLM_ending_TB/en/. Last accessed on 3rd February, 2020; 15:26 pm.
Donnellan S, Giardiello M. Nanomedicines towards targeting intracellular Mtb for the treatment of tuberculosis. J Interdiscip Nanomed. 2019;4(3):76–85.
Walvekar P, Gannimani R, Govender T. Combination drug therapy via nanocarriers against infectious diseases. Eur J Pharm Sci. 2018;127:121–41. https://doi.org/10.1016/j.ejps.2018.10.017.
Ghajavand H, Kamakoli MK, Khanipour S, Dizaji SP, Masoumi M, Jamnani FR, et al. Scrutinizing the drug resistance mechanism of multi- and extensively-drug resistant Mycobacterium tuberculosis: mutations versus efflux pumps. Antimicrob Resist Infect Control. 2019;8:70.
Gilpin C, Korobitsyn A, Migliori GB, Raviglione MC, Weyer K. The World Health Organization standards for tuberculosis care and management. Eur Respir J. 2018;51:1800098. https://doi.org/10.1183/13993003.00098-2018.
Anderson CF, Grimmete ME, Domalewski CJ, Cui H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. WIREs Nanomed Nanobiotechnol. 2019;e1586.
Bibhas CM, Gitanjali M, Subas CD, Narahari NP. Exploring the use of lipid based nano-formulations for the management of tuberculosis. J Nanosci Curr Res. 2017;2:112.
Aun R, Bruck SF, Cabot PJ, Faheem M, Roberts JA, Robert FJ. Solid nanoparticles for oral antimicrobial drug delivery: a review. Drug Discov Today. 2019;24:858–66. https://doi.org/10.1016/j.drudis.2019.01.004.
Patil K, Bagade S, Bonde S, Sharma S, Saraogi G. Recent therapeutic approaches for the management of tuberculosis: challenges and opportunities. Biomed Pharmacother. 2018;99:735–45.
Costa-Gouveia J, Aınsa JA, Brodin P, Lucıa A. How can nanoparticles contribute to antituberculosis therapy? Drug Discov Today. 2017;22(3):600–7.
Dumas F, Haanappel E. Lipids in infectious diseases–the case of AIDS and tuberculosis. Biochim Biophys Acta. 1859;2017:1636–47.
Costa-Gouveia J, Ainsa JA, Brodin P, Lucia A. How can nanoparticles contribute to antituberculosis therapy? Drug Discov Today. 2017;22(3):600–7.
Saravanan M, Ramachandran B, Hamed B, Giardiello M. Barriers for the development, translation, and implementation of nanomedicine: an African perspective. J Interdiscip Nanomed. 2018;3(3):106–10.
Hoagland DT, Li J, Lee RB, Lee RE. New agents for the treatment of drug resistant Mycobacterium tuberculosis. Adv Drug Deliv Rev. 2016;102:55–72. https://doi.org/10.1016/j.addr.2016.04.026.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–31.
Praphakar RA, Munusamy MA, Rajan M. Development of extended-voyaging anti-oxidant linked amphiphilic polymeric nanomicelles for anti-tuberculosis drug delivery. Int J Pharm. 2017;524:168–77.
Praphakar RA, Jeyaraj M, Ahmed M, Kumar SS, Rajan M. Silver nanoparticle functionalized CSg-(CA-MA-PZA) carrier for sustainable anti-tuberculosis drug delivery. Int J Biol Macromol. 2018;118:1627–38. https://doi.org/10.1016/j.ijbiomac.2018.07.008.
Tenland E, Pochert A, Krishnan N, Rao KU, Kalsum S, Braun K, et al. Effective delivery of the anti-mycobacterial peptide NZX in mesoporous silica nanoparticles. PLoS One. 2019;14(2):e0212858. https://doi.org/10.1371/journal.pone.0212858.
Bachhav SS, Dighe VD, Devarajan PV. Exploring Peyer’s patch uptake as a strategy for targeted lung delivery of polymeric rifampicin nanoparticles. Mol Pharm. 2018;15:4434–45.
Bhusal N, Shrestha S, Pote N, Alocilja EC. Nanoparticle-based biosensing of tuberculosis, an affordable and practical alternative to current methods. Biosensors. 2019;9:1. https://doi.org/10.3390/bios9010001.
Saifullah B, Maitra A, Chrzastek A, Naeemullah B, Fakurazi S, Bhakta S, et al. Nano-formulation of ethambutol with multifunctional graphene oxide and magnetic nanoparticles retains its anti-tubercular activity with prospects of improving chemotherapeutic efficacy. Molecules. 2017;22:1697.
Rawal T, Parmar R, Tyagi RK, Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents: an improved therapeutic approach for alveolar tuberculosis. Colloids Surf B: Biointerfaces. 2017;154:321–30. https://doi.org/10.1016/j.colsurfb.2017.03.044.
Thomas D, Latha MS, Thomas KK. Synthesis and in vitro evaluation of alginate-cellulose nanocrystal hybrid nanoparticles for the controlled oral delivery of rifampicin. J Drug Deliv Sci Technol. 2018;46:392–9.
Viswanathan V, Mehta H, Pharande R, Bannalikar A, Gupta U, Mukne A, et al. Mannosylated gelatin nanoparticles of licorice for use in tuberculosis: formulation, in vitro evaluation, in vitro cell uptake, in vivo pharmacokinetics and in vivo anti-tubercular efficacy. J Drug Deliv Sci Technol. 2018;45:255–63.
Malik A, Gupta M, Mani R, Bhatnagar R. Single-dose ag85B-esaT6–loaded poly(lacticco-glycolic acid) nanoparticles confer protective immunity against tuberculosis. Int J Nanomedicine. 2019;14:3129–43.
Kutscher HL, Morse GD, Prasad PN, Reynolds JL. In vitro pharmacokinetic cell culture system that simulates physiologic drug and nanoparticle exposure to macrophages. Pharm Res. 2019;36:44. https://doi.org/10.1007/s11095-019-2576-9.
Scolari IR, Páez PL, Sánchez-Borzone ME, Granero GE. Promising chitosan-coated alginate-tween 80 nanoparticles as rifampicin coadministered ascorbic acid delivery carrier against Mycobacterium tuberculosis. AAPS PharmSciTech. 2019;20:67. https://doi.org/10.1208/s12249-018-1278-7.
Horvati K, Gyulai G, Csampai A, Rohonczy J, Kiss E, Bosze S. Surface layer modification of poly(D,L-lactic-co-glycolic acid) nanoparticles with targeting peptide: a convenient synthetic route for pluronic F127−tuftsin conjugate. Bioconjug Chem. 2018;29:1495–9. https://doi.org/10.1021/acs.bioconjchem.8b00156.
Jirı T, Sergey KF, Martin H, Tomas M, Zdenka S, Dusan C, et al. System with embedded drug release and nanoparticle degradation sensor showing efficient rifampicin delivery into macrophages. Nanomedicine. 2016;13:307–15. https://doi.org/10.1016/j.nano.2016.08.031.
Rani S, Gothwal A, Pandey PK, Chauhan DS, Pachouri PK, Gupta UD, et al. HPMA-PLGA based nanoparticles for effective in vitro delivery of rifampicin. Pharm Res. 2019;36:19. https://doi.org/10.1007/s11095-018-2543-x.
Ge Z, Xu G, Chen Z, Zhang D, Wang Q, Hei L, et al. Development and in vitro release of isoniazid and rifampicin-loaded bovine serum albumin nanoparticles. Med Sci Monit. 2018;24:473–8. https://doi.org/10.12659/MSM.905581.
Prashanth GK, Prashanth PA, Trivedi P, Chaturvedi V, Nagabhushana BM, Ananda S, et al. Antitubercular activity of ZnO nanoparticles prepared by solution combustion synthesis using lemon juice as bio-fuel. Mater Sci Eng C. 2017;75:1026–33. https://doi.org/10.1016/j.msec.2017.02.093.
Marcianes P, Negro S, García-garcía L, Montejo C, Barcia E, Fernández-carballido A. Surface-modified gatifloxacin nanoparticles with potential for treating central nervous system tuberculosis. Int J Nanomedicine. 2017;12:1959–68.
Kumarasingam K, Vincent M, Mane SR, Shunmugam R, Sivakumar S, Devi KR. Enhancing antimycobacterial activity of isoniazid and rifampicin incorporated norbornene nanoparticles. Int J Mycobacteriol. 2018;7:84–8.
Kesavan MP, Ayyanaar S, Vijayakumar V, Raja JD, Annaraj J, Sakthipandi K, et al. Magnetic iron oxide nanoparticles (MIONs) cross-linked natural polymer-based hybrid gel beads: controlled nano anti-TB drug delivery application. J Biomed Mater Res A. 2018;106A:1039–50.
Petkar KC, Taylor KMG, Chavhan S, Kunda N, Sawant KK. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: optimization by factorial design. AAPS PharmSciTech. 2018;19:1758–72. https://doi.org/10.1208/s12249-018-0972-9.
Hakkimane SS, Shenoy VP, Gaonkar S, Bairy I, Guru BR. Antimycobacterial susceptibility evaluation of rifampicin and isoniazid benz-hydrazone in biodegradable polymeric nanoparticles against Mycobacterium tuberculosis h37rv strain. Int J Nanomedicine. 2018;13:4303–18.
Churilov L, Korzhikov-Vlakh V, Sinitsyna E, Polyakov D, Darashkevich O, Poida M, et al. Enhanced delivery of 4-thioureidoiminomethyl pyridinium perchlorate in tuberculosis models with IgG functionalized poly(lactic acid)-based particles. Pharmaceutics. 2019;11:2. https://doi.org/10.3390/pharmaceutics11010002.
Gaspar DP, Gaspar MM, Eleuterio CV, Grenha A, Blanco M, Gonçalves LMD, et al. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol Pharm. 2017;14:2977–90. https://doi.org/10.1021/acs.molpharmaceut.7b00169.
Costa A, Sarmento B, Seabra V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur J Pharm Sci. 2018;114:103–13.
Gaspar DP, Vasco F, Goncalves LMD, Pablo T, Carmen RL, Antonio JA. Rifabutin-loaded solid lipid nanoparticles for inhaled antitubercular therapy: physicochemical and in vitro studies. Int J Pharm. 2015;497:199–209. https://doi.org/10.1016/j.ijpharm.2015.11.050.
Nemati E, Mokhtarzadeh A, Panahi-Azar V, Mohammadi A, Hamishehkar H, Mesgari-Abbasi M, et al. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. AAPS PharmSciTech. 2019;20:120. https://doi.org/10.1208/s12249-019-1334-y.
Singh M, Guzman-Aranguez A, Hussain A, Srinivas CS, Kaur IP. Solid lipid nanoparticles for ocular delivery of isoniazid: evaluation, proof of concept and in vivo safety & kinetics. Nanomedicine. 2019;14:465–91. https://doi.org/10.2217/nnm-2018-0278.
Shilpi S, Vimal VD, Soni V. Assessment of lactoferrin-conjugated solid lipid nanoparticles for efficient targeting to the lung. Prog Biomater. 2015;4:55–63.
Banerjee S, Roy S, Bhaumik KN, Pillai J. Mechanisms of the effectiveness of lipid nanoparticle formulations loaded with anti-tubercular drugs combinations toward overcoming drug bioavailability in tuberculosis. J Drug Target. 2019;28:55–69. https://doi.org/10.1080/1061186X.2019.1613409.
Ghaderkhani J, Yousefimashouf R, Arabestani M, Roshanaei G, Asl SS, Abbasalipourkabir R. Improved antibacterial function of rifampicin-loaded solid lipid nanoparticles on Brucella abortus. Artif Cells Nanomed Biotechnol. 2019;47(1):1181–93. https://doi.org/10.1080/21691401.2019.1593858.
Chokshi NV, Khatri HN, Patel MM. Formulation, optimization and characterization of rifampicin loaded solid lipid nanoparticles for the treatment of tuberculosis. Drug Dev Ind Pharm. 2018;44:1975–89. https://doi.org/10.1080/03639045.2018.1506472.
Maretti E, Rustichelli C, Romagnoli M, Balducci AG, Buttini F, Sacchetti F, et al. Solid lipid nanoparticle assemblies (SLNas) for an anti-TB inhalation treatment—a design of experiments approach to investigate the influence of pre-freezing conditions on the powder respirability. Int J Pharm. 2016;511:669–79.
Nirbhavane P, Vemuri N, Kumar N, Khuller GK. Lipid nanocarrier-mediated drug delivery system to enhance the oral bioavailability of rifabutin. AAPS PharmSciTech. 2017;18(3). https://doi.org/10.1208/s12249-016-0559-2.
Vieira ACC, Chaves LL, Pinheiro S, Pinto S, Pinheiro M, Lima SC, et al. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int J Pharm. 2017;536:478–85. https://doi.org/10.1016/j.ijpharm.2017.11.071.
Pinheiro M, Ribeiro R, Vieira A, Andrade F, Reis S. Design of a nanostructured lipid carrier intended to improve the treatment of tuberculosis. Drug Des Dev Ther. 2016;10:2467–75.
Carneiro SP, Carvalho KV, de Oliveira A, Soares RD, Carneiro CM, de Andrade MHG, et al. Functionalized rifampicin-loaded nanostructured lipid carriers enhance macrophages uptake and antimycobacterial activity. Colloids Surf B: Biointerfaces. 2018;175:306–13. https://doi.org/10.1016/j.colsurfb.2018.12.003.
Sato MR, Junior JAO, Machado RT, de Souza PC, Campos DL, Pavan FR, et al. Nanostructured lipid carriers for incorporation of copper(ii) complexes to be used against Mycobacterium tuberculosis. Drug Des Dev Ther. 2017;11:909–21.
Matteis LD, Jary D, Lucía A, García-Embid S, Serrano-Sevilla I, Pérez D, et al. New active formulations against M. tuberculosis: bedaquiline encapsulation in lipid nanoparticles and chitosan nanocapsules. Chem Eng J. 2017. https://doi.org/10.1016/j.cej.2017.12.110.
Banerjee S, Roy S, Bhaumik KN, Kshetrapal P, Pillai J. Comparative study of oral lipid nanoparticle formulations (LNFs) for chemical stabilization of antitubercular drugs: physicochemical and cellular evaluation. Artif Cells Nanomed Biotechnol. 2018;46(1):540–58. https://doi.org/10.1080/21691401.2018.1431648.
Karmakar G, Nahak P, Guha P, Roy B, Nath RK, Panda AK. Role of PEG 2000 in the surface modification and physicochemical characteristics of pyrazinamide loaded nanostructured lipid carriers. J Chem Sci. 2018;130:42.
Vieira ACC, Magalhaes J, Rocha S, Cardoso MS, Santos SG, Borges M, et al. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomedicine. 2017;12:2721–36. https://doi.org/10.2217/nnm-2017-0248.
Tayeb HH, Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine. 2018;13:2507–25. https://doi.org/10.2217/nnm-2018-0088.
Shah K, Chan LW, Wong TW. Critical physicochemical and biological attributes of nanoemulsions for pulmonary delivery of rifampicin by nebulization technique in tuberculosis treatment. Drug Deliv. 2017;24(1):1631–47. https://doi.org/10.1080/10717544.2017.1384298.
Halicki PCB, Hadrich G, Boschero R, Ferreira LA, von Groll A, da Silva PEA, et al. Alternative pharmaceutical formulation for oral administration of rifampicin. Assay Drug Dev Technol. 2018;16:456–61. https://doi.org/10.1089/adt.2018.874.
Shobo A, Pamreddy A, Kruger HG, Makatini MM, Naicker T, Govender T, et al. Enhanced brain penetration of pretomanid by intranasal administration of an oil-in-water nanoemulsion. Nanomedicine (London). 2018;13(9):997–1008.
Tabaran AF, Catoi C. Macrophages targeted drug delivery as a key therapy in infectious disease. Biotechnol Mol Biol Nanomed. 2014;2:2330.
Tang Y, Zhang H, Lu X, Jiang L, Xi X, Liu J, et al. Development and evaluation of a dry powder formulation of liposome-encapsulated oseltamivir phosphate for inhalation. Drug Deliv. 2015;22(5):608–18.
Liu P, Guo B, Wang S, Ding J, Zhou W. A thermo-responsive and self-healing liposome-in-hydrogel system as an antitubercular drug carrier for localized bone tuberculosis therapy. Int J Pharm. 2019;558:101–9. https://doi.org/10.1016/j.ijpharm.2018.12.083.
Hamed A, Osman R, Al-Jamal KT, Holayel SM, Geneidi AS. Enhanced antitubercular activity, alveolar deposition and macrophages uptake of mannosylated stable nanoliposomes. J Drug Deliv Sci Technol. 2019;51:513–23.
Viswanathan V, Pharande R, Bannalikar A, Gupta P, Gupta U, Mukne A. Inhalable liposomes of Glycyrrhiza glabra extract for use in tuberculosis: formulation, in vitro characterization, in vivo lung deposition and in vivo pharmacodynamic studies. Drug Dev Ind Pharm. 2018;45:11–20. https://doi.org/10.1080/03639045.2018.1513025.
Kaul A, Chaturvedi S, Attri A, Kalra M, Mishra AK. Targeted theranostic liposomes: rifampicin and ofloxacin loaded pegylated liposomes for theranostic application in mycobacterial infections. RSC Adv. 2016;6:28919–26.
Nkanga CI, Krause RWM. Conjugation of isoniazid to a zinc phthalocyanine via hydrazine linkage for pH-dependent liposomal controlled release. Appl Nanosci. 2018;8:1313–23. https://doi.org/10.1007/s13204-018-0776-y.
Nkanga CI, Werner KR, Siwe NX, Bryan RW. Preparation and characterization of isoniazid loaded crude soybean lecithin liposomes. Int J Pharm. 2017. https://doi.org/10.1016/j.ijpharm.2017.04.074.
Mansury D, Ghazvini K, Jamehdar SA, Badiee A, Tafaghodi M, Nikpoor AR, et al. Enhancement of the effect of BCG vaccine against tuberculosis using DDA/TDB liposomes containing a fusion protein of HspX, PPE44, and EsxV. Artif Cells Nanomed Biotechnol. 2019;47(1):370–7. https://doi.org/10.1080/21691401.2018.1557674.
Praphakar RA, Murugan AM, Mariappan R. Development of extended-voyaging anti-oxidant linked amphiphilic polymeric nanomicelles for antituberculosis drug delivery. Int J Pharm. 2017;524:168–77. https://doi.org/10.1016/j.ijpharm.2017.03.089.
Tripodo G, Perteghella S, Grisoli P, Trapani A, Torre ML, Mandracchia D. Drug delivery of rifampicin by natural micelles based on inulin: physicochemical properties, antibacterial activity and human macrophages uptake. Eur J Pharm Biopharm. 2019;136:250–8. https://doi.org/10.1016/j.ejpb.2019.01.022.
Praphakar RA, Ebenezer RS, Vignesh S, Shakila H, Rajan M. A versatile pH-responsive chitosan-g-polycaprolactone/maleic anhydride-isoniazid polymeric micelle to improve the bioavailability of tuberculosis multi-drugs. ACS Appl Bio Mater. 2019;2:1931–43. https://doi.org/10.1021/acsabm.9b00003.
Grotz E, Tateosian NL, Salgueiro J, Bernabeu E, Gonzalez L, Manca ML, et al. Pulmonary delivery of rifampicin-loaded soluplus micelles against Mycobacterium tuberculosis. J Drug Deliv Sci Technol. 2019;53:101170.
Sheth U, Tiwari S, Bahadur A. Preparation and characterization of antitubercular drugs encapsulated in polymer micelles. J Drug Deliv Sci Technol. 2018;48:422–8. https://doi.org/10.1016/j.jddst.2018.10.021.
Hussain A, Singh SK, Verma PRP, Singh N, Ahmad FJ. Experimental design-based optimization of lipid nanocarrier as delivery system against Mycobacterium species: in vitro and in vivo evaluation. Pharm Dev Technol. 2016;22:910–27. https://doi.org/10.1080/10837450.2016.1212879.
Sumaila M, Ramburrun P, Kumar P, Choonara YE, Pillay V. Lipopolysaccharide polyelectrolyte complex for oral delivery of an anti-tubercular drug. AAPS PharmSciTech. 2019;20:107.
Carazo E, Sandri G, Cerezo P, Lanni C, Ferrari F, Bonferoni C, et al. Halloysite nanotubes as tools to improve the actual challenge of fixed doses combinations in tuberculosis treatment. J Biomed Mater Res A. 2019;00A:1–9.
Cui J, Wang L, Han Y, Liu W, Li Z, Hu Y, et al. ZnO nano-cages derived from ZIF-8 with enhanced anti mycobacterium-tuberculosis activities. J Alloys Compd. 2018;766:619–25.
Mehnath S, Sithika MAA, Arjama M, Rajan M, Praphakar RA, Jeyaraj M. Sericin-chitosan doped maleate gellan gum nanocomposites for effective cell damage in Mycobacterium tuberculosis. Int J Biol Macromol. 2019;122:174–84.
Mulla AS, Mabrouk M, Choonara YE, Kumar P, Chejara DR, du Toit LC, et al. Development of respirable rifampicin-loaded nano-lipomer composites by microemulsion spray drying for pulmonary delivery. J Drug Deliv Sci Technol. 2017;41:13–9. https://doi.org/10.1016/j.jddst.2017.06.017.
Bachhav SS, Dighe VD, Kotak D, Devarajan PV. Rifampicin lipid-polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int J Pharm. 2017;532:612–22. https://doi.org/10.1016/j.ijpharm.2017.09.040.
Ishikawa AA, Salazar JJV, Salinas M, Gaitani CM, Nurkiewicz TR, Negrete GR, et al. Self-assembled nanospheres for encapsulation and aerosolization of rifampicin. RSC Adv. 2016;6:12959–63. https://doi.org/10.1039/c5ra25044g.
Kulkarni P, Rawtani D, Barot T. Formulation and optimization of long acting dual niosomes using Box-Behnken experimental design method for combinative delivery of Ethionamide and D-cycloserine in tuberculosis treatment. Colloids Surf A Physicochem Eng Asp. 2019;565:131–42. https://doi.org/10.1016/j.colsurfa.2019.01.004.
van Zyl L, Viljoen JM, Haynes RK, Aucamp M, Ngwane AH, du Plessis J. Topical delivery of artemisone, clofazimine and decoquinate encapsulated in vesicles and their in vitro efficacy against Mycobacterium tuberculosis. AAPS PharmSciTech. 2019;20:33. https://doi.org/10.1208/s12249-018-1251-5.
AlMatar M, Makky EA, Yakıcı G, Var I, Kayar B, Köksal F. Antimicrobial peptides as an alternative to anti-tuberculosis drugs. Pharmacol Res. 2018;128:288–305.
Arranz-Trullén J, Lu L, Pulido D, Bhakta S, Boix E. Host antimicrobial peptides: the promise of new treatment strategies against tuberculosis. Front Immunol. 2017;8:1499. https://doi.org/10.3389/fimmu.2017.01499.
Chang J, Oak C, Sung N, Jheon S. The potential application of photodynamic therapy in drug-resistant tuberculosis. J Photochem Photobiol B Biol. 2015;150:60–5. https://doi.org/10.1016/j.jphotobiol.2015.04.001.
Verma M, Furin J, Langer R, Traverso G. Making the case: developing innovative adherence solutions for the treatment of tuberculosis. BMJ Glob Health. 2019;4:e001323. https://doi.org/10.1136/bmjgh-2018-001323.
Lemmer Y, Kalombo L, Pietersen R, Jones AT, Semete-Makokotlela B, Wyngaardt SV. Mycolic acids, a promising mycobacterial ligand for targeting of nanoencapsulated drugs in tuberculosis. J Control Release. 2015;211:94–104.
Tiberia S, Munoz-Torricoc M, Duarted R, Dalcolmoe M, D’Ambrosiof L, Zumla A, et al. New drugs and perspectives for new anti-tuberculosis regimens. Rev Port Pneumol. 2018;24:86–98. https://doi.org/10.1016/j.rppnen.2017.10.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabi, B., Rehman, S., Aggarwal, S. et al. Nano-based anti-tubercular drug delivery: an emerging paradigm for improved therapeutic intervention. Drug Deliv. and Transl. Res. 10, 1111–1121 (2020). https://doi.org/10.1007/s13346-020-00786-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00786-5