Abstract
Wounds have always been considered as one of the most common physical damages. Therefore, various researches have been conducted to find an appropriate method to improve wound healing process. Among various materials, since hydrogels have appropriate properties for wound healing, they are widely used for this purpose. In this study, to develop a potential wound dressing, different concentrations of naringenin (0%, 1%, 10% and 20%) were incorporated in alginate hydrogel followed by evaluating its characters such as morphology, swelling properties, weight loss, antibacterial activity, releasing profile of the naringenin, hemo-, and cytocompatibility. Finally, to evaluate the effect of developed hydrogels on wound healing, the full-thickness dermal wound model in rat was used. Our results provided that the prepared hydrogels have appropriate porosity (86.7 ± 5.3%) with the interconnected pores. Moreover, weight loss assessment confirmed that fabricated hydrogels have suitable biodegradability (about 89% after 14 days). MTT assay also revealed the positive effect of hydrogels on cell viabilities, and they have no toxicity effect on cells. In vivo study indicated that the prepared hydrogels had better wound closure than the gauze-treated wound (the control), and alginate/20% naringenin group had the best wound closure among other groups. All in all, this study concluded that alginate/naringenin hydrogel has positive effect on wound healing process, and it can be used to treat skin injuries in the clinic.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During human history, skin damage has been one of the most common physical injury [1, 2]. The number of people suffered from skin damages such as trauma, burns, skin infections increased, and therefore, needing an appropriate treatment for skin healing is growing [3, 4]. Self-regeneration of skin defects always needs a long time, thus, it is better to use various methods to speed up skin defect repair [5]. Among various methods, using wound dresser because of its appropriate properties is used more than other methods [6]. An appropriate wound dressing should provide an appropriate environment for wound healing, and it has the same structure and biological characteristics of the skin’s extracellular matrix (ECM) [7].
Among different wound dressers which have been widely used in wound healing, hydrogels because of their special properties have great promise as wound dressings. They mimic the ECM features and provide a template for cell migration, adhesion, and growth during tissue regeneration which is crucial for skin regeneration [8, 9]. Moreover, hydrogel makes the wound site moist and cool which leads to a decline in the pain and therefore, it has high patient acceptability [10, 11].
Alginate (Alg) is an anionic polysaccharide that can form to hydrogel at room temperature and in the lack of toxic solvents easily [12, 13]. Because of appropriate properties of alginate such as biocompatibility, biodegradability, relatively low cost, low toxicity, and gelling properties, it has been widely used and investigated in different biomedical studies [14, 15]. Since alginate hydrogel has suitable properties like permeability, water content, elasticity, and make the wound site moisty, it has been widely used for healing different types of wound [16,17,18,19,20,21]. Alginate hydrogel is usually fabricated by external gelation method and it is cross-linked by using calcium ions. The gel networks formed by cation interacts and binds with guluronate blocks of alginate chains [22]. Calcium alginate hydrogel has two important features that make it suitable for using in wound healing: the ability of the gel to use as a matrix for the aggregation of platelets and erythrocytes and hemostatic properties of the calcium ion ([11, 23, 24]). Furthermore, the porosity of alginate hydrogel makes a suitable environment that entrapment/immobilization of therapeutic agents (drugs, growth factors, etc.) were released in the wound site [25, 26].
Flavonoids are present in many plant-based foods regularly consumed by humans. These natural materials have shown an extensive range of pharmacological and biological activities that have been attributed to their abilities to inhibit or modulate certain enzymes and to their antioxidant properties ([27,28,29,30]). Naringenin (4,5,7-trihydroxyflavanone) (Nar) is one of the flavonoids that can be found in many citrus fruits. It has antioxidant, antibacterial, anti-inflammatory, antiproliferative, and antimutagenic properties that make it a good material for using in biomedical application ([30,31,32,33]). Based on these properties, naringenin has high effects on chronic diseases such as diabetes, hypertension, inflammation, and allergy [34].
The purpose of this study is to develop an alginate hydrogel with different concentrations of naringenin and to evaluate its ability to use as a skin wound dressing. The properties of the alginate hydrogel without and with different percentages of naringenin were evaluated by using different in vitro tests. Finally, the wound healing efficacy of the fabricated hydrogels were compared with conventional sterile gauze in the full-thickness excisional wound of the rat model.
Materials and methods
Materials
Sodium alginate (pharmaceutical grade, molecular weight 120,000, viscosity ≥ 2), naringenin, penicillin, streptomycin, calcium chloride (CaCl2) were purchased from Sigma-Aldrich (St. Louis, USA). 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide (MTT), Dulbecco’s modified Eagle’s medium nutrient mixture F-12 (DMEM/F12), and fetal bovine serum (FBS) were purchased from Gibco, BRL (Eggenstein, Germany).
Hydrogel fabrication
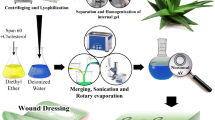
To fabricate alginate hydrogel, sodium alginate at the final concentration of 4% w/v was dissolved in deionized water. Next, the various concentrations of naringenin (0, 1, 10, and 20% weight of polymer alginate) was added to the alginate solution. Subsequently, in order to initiate the gelation process, calcium chloride (CaCl2) (50 mM) at neutral pH value was used as a source of calcium ions. The fabricated sodium alginate/naringenin (Alg/Nar) solution was mixed and then vortexed with CaCl2 suspension for 1 min to activate the gelation process.
Lyophilization
The prepared solutions were first frozen for 16 h at − 80 °C. To fabricate the porous hydrogels, the frozen solutions were lyophilized by using a freeze drier (Telstar, Terrassa, Spain) for 48 h at − 54 °C.
Hydrogel characterization
Morphological properties
The hydrogel sample was freeze-dried, and the dry sample was cut in the diameter of 7 mm. The prepared samples were coated with gold by a sputter coater (SC7620, Quorum Technologies, England) for 250 s. After that, to evaluate the ultrastructure of alginate hydrogel, a scanning electron microscope (SEM AIS2100, Seron Technology, South Korea) at an accelerating voltage of 20 kV was used.
Swelling studies
It is widely accepted that swelling is an important property of materials used in drug delivery applications [35]. The prepared hydrogel was lyophilized until a constant weight of the gel and after that, the 1-mL dried hydrogel containing 20% naringenin was immersed in buffered saline (PBS) solution in 5 mL beaker (pH = 7.4) at 37 °C and kept for 3 days. The swelling proportion of samples were measured every 60 min for the first 6 h and then every 2 h for the remaining hours. At provided time points, the sample was extracted from the solution and weighted quickly. The measurements were done, and Eq. 1 was used to calculate mass swelling percentages [36]:
In Eq. 1, m0 and m1 are the dried mass weight and the mass swollen weight of the gel, respectively.
Release of naringenin
The UV-visible spectroscopy was used to assess the release of naringenin from alginate hydrogel. For this purpose, Alg/20% Nar hydrogel (1 mL) was loaded in simulated body fluid (SBF) (5 mL) under 37 °C. At various time intervals, the supernatants were extracted and centrifuged, and to determine the amount of released naringenin, the absorption intensity of supernatants was recorded at 292 nm [37].
Weight loss analysis
To evaluate the degradation rate of alginate hydrogel, the mass loss method was used [36]. In this method, each dried hydrogel disk was weighted and equal-size samples were immersed in a falcon tube containing PBS at 37 °C. At the specific time points (7 and 14 days), triplicate specimens for each group were taken out from water and dried, and the weight loss measurement was recorded. Equation 2 was used to determine the degree of degradation [16]:
In the above equation, W0 is the primary weight of hydrogels and W1 is the dry weight after removal from the water.
Blood compatibility
For evaluating blood compatibility of fabricated hydrogels, 2 mL of human fresh anticoagulated blood mixed with 2.5 mL of normal saline was used. After that, 0.2 mL of the diluted blood was added to the prepared hydrogels. After keeping the mixture at 37 °C for 60 min, they were centrifuged at 1500 rpm for 10 min to extract supernatant. The extracted supernatant was transferred to a 96-well plate, and a microplate reader (Anthos 2020, Biochrom, Berlin, Germany) at 545 nm was used to measure their absorbance. In this test, 0.2 mL diluted blood in 10 mL deionized water was used as positive control and 0.2 mL diluted blood in 10 mL normal saline was used as negative control. Finally, Eq. 3 was used to calculate hemolysis degree [36]:
where Dt and Dnc are the absorbance of the sample and the absorbance of the negative control, respectively. In addition, Dpc is the absorbance of the positive control.
Time-kill assay
The time-kill assay was used to evaluate antibacterial properties of alginate hydrogel with and without different amounts of naringenin against two bacterial species (Staphylococcus aureus and Pseudomonas aeruginosa). Briefly, a bacterial suspension was adjusted to 1 × 107 CFU/mL. The prepared hydrogels were added to the suspension at concentrations of 1/2-fold of the minimum inhibition concentrations (MIC). The 0.5 mL suspension of each group was incubated at 37 °C with gentle agitation in a shaking water bath. After 24 h of incubation, 10 μL of the suspension was serially diluted and inoculated on agar plates. After 1, 2, 4, and 24 h of aerobic incubation at 37 °C, the number of viable bacteria colonies was counted [38]. The bacterial growth assays were done in triplicates.
Cell viability studies
To evaluate cell viability of the prepared hydrogels, the MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide, GIBCO-BRL, Eggenstein, Germany) assay was used. Briefly, L929 murine fibroblast cell line was cultured at the density of 1 × 104 cells on hydrogel in DMEM/F12 supplemented with 10% (v/v) FBS, 100 unit/mL of penicillin, and 100 μg/mL of streptomycin in a humidified incubator at 37 °C with 5% CO2. The positive control was the cells cultured on the tissue culture plate (TCP). At the specific time points (1 and 3 days after cell seeding), the culture medium was replaced with 150 μL of MTT (0.5 mg/mL) in each well of 96-well plate, and the cells were incubated at 37 °C for 3–4 h in a dark place. After that, the solution was removed, and 0.1 mL DMSO was added to each well [39, 40]. The formed purple formazan crystals were dissolved in DMSO, and a microplate reader Anthos 2020 (Biochrom, Berlin, Germany) was used to measure the absorption at wavelength of 570 nm. The mean for the triplicate wells for each sample was reported.
In vivo wound healing study
To evaluate the effect of alginate hydrogel with and without different amounts of naringenin in wound healing, a full-thickness excisional wound model in the rat was used. For this purpose, thirty healthy adult male Wistar rats (200–220 g and 2 months old) were purchased from the Pasteur Institute (Tehran, Iran). Animal experiments were approved by the ethics committee of the Tehran University of Medical Sciences (ethical code IR.TUMS.VCR.REC.1396.2876) and were carried out in accordance with the university’s guidelines. For anesthesia, a mixture of ketamine (Alfasan, Woerden, The Netherlands; 100 mg/1 kg body weight) and xylazine (Alfasan, Woerden, The Netherlands; 10 mg/1 kg body weight) was injected into the peritoneal cavity of each Wistar rat. Afterward, a scalpel blade was used to excise a 1.50 × 1.50-cm2 full-thickness wound on the back skin of rats near the neck posterior surface. The animals were randomly divided into five groups (6 rats in each group), and the wounds were treated with alginate hydrogel without naringenin, Alg/1% Nar, Alg/10% Nar, Alg/20% Nar, and sterile gauze as the negative control. In addition, rats without any wounds on the back skin were considered as positive control group. The hydrogels were then embedded on the injury site using an elastic adhesive bandage.
A digital camera (Canon Inc., Tokyo, Japan) was used to record reduction in the wound size in 7 and 14 days after treatment, and the wound area was measured by an image analyzing program (Digimizer, Ostend, Belgium). Finally, the wound closure was calculated via Eq. 4 [41]:
Histopathology study
Six animals from each group were euthanized 14 days post-treatment, and the skin tissues were harvested and immediately fixed in the 10% neutral buffered formalin (PH. 7.26) for 48 h. Then, the fixed tissue samples were processed, embedded in paraffin, and sectioned to 5 μm thickness. Finally, the sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome (MT). The histological slides were evaluated by the independent reviewer, using light microscopy (Olympus BX51; Olympus, Tokyo, Japan). Epithelialization, angiogenesis, inflammatory cell infiltration, fibroplasia, and granulation tissue formation have assessed in different groups, comparatively.
Histomorphometry analysis
Epithelialization in day 14 was assessed semi quantitatively on 5-point scale: 0 (without new epithelialization), 1 (25%), 2 (50%), 3 (75%), and 4 (100%). The results of these parameters were reported by comparative analysis of one independent observer who was blind to the treatment groups.
Statistical analysis
The Origin Pro software (Version 9, Origin Lab, Northampton, MA) using a statistic on rows and two-sample t test on rows was used to statistically analyze the results, and the data were expressed as a mean ± standard deviation. In all of the evaluations, p < 0.05 was considered statistically significant.
Results
Morphological studies
The SEM image was used to evaluate morphology of alginate hydrogel. The SEM image (Fig. 1) shows the inner structure of the developed hydrogel that has a highly porous structure with interconnected pores that were created during the phase separation in the lyophilization process. The amount of porosity in alginate hydrogel was approximately 86.7 ± 5.3%. The Image J (National Institutes of Health, Bethesda, USA) and Origin Pro 2015 software (Origin Lab, Northampton, USA) were used to calculate average diameters of pores. Moreover, prepared hydrogel had a pore size in the range of 75 to135 μm, and based on the previous studies, it is favorable for cell attachment and migration [42].
Swelling of alginate hydrogels
To evaluate the potential of prepared hydrogel to be used in applications requiring permanent contact with water or biological fluids, such as blood and wound exudate, the swelling test was used at 37 °C. Figure 2a depicts the swelling behavior of fabricated hydrogel. The results of the mass swelling ratio illustrated that the highest swelling percentage was 342 ± 18% at 240 min after the incubation.
Release
Figure 2b indicates the cumulative release profile of naringenin. A total of 16.08 ± 1.28% and 23.1 ± 4.46% of the naringenin was released in the first 6 and 12 h, respectively, followed by a sustained release of 74.09 ± 8.71% over 14 days.
Weight loss measurements
Figure 3a shows the weight loss percentages of the alginate hydrogel without and with different amounts of naringenin in PBS. Degradation analysis showed that this hydrogel had an acceptable degradation profile (around 70% during 14 days). Moreover, the results showed that almost 89% of the primary weight of the fabricated hydrogels have been lost after 2 weeks. By adding naringenin, the weight loss percentage was decreased because of the hydrophobic nature of naringenin [43].
a Weight loss percentage of the fabricated hydrogels at the different time points (7 and 14 days). b Blood compatibility histogram of the experimental samples. c MTT assay histogram after 24 and 72 h post-cell seeding. Values represent the mean ± SD, n = 3, *p < .05, **p < .01, and ***p < .001. SD, standard deviation
Blood compatibility
Hemolysis test indicated the amount of hemoglobin that was released into plasma due to damage of erythrocytes, and it is directly related to the blood compatibility of materials [44]. Figure 3b shows that the hemolysis proportions of all samples were significantly less than the positive control. This test also proved that by adding naringenin into the hydrogel, the absorbance (hemolysis) did not change significantly.
Time-kill assay
To evaluate the effect of the antimicrobial activity of alginate hydrogel with and without naringenin, the time-kill assay was used. The number of colonies after 1, 2, 4, and 24 h of starting assay was measured against Pseudomonas aeruginosa and Staphylococcus aureus, the most common Gram-positive bacteria found in wounds (Table 1). Reduction number of colonies by adding naringenin indicated that naringenin has antibacterial activity. It is also indicated that by increasing the amount of naringenin, the number of colonies decreased significantly.
Cell culture studies
To evaluate cells viability of alginate hydrogel without and with different amounts of naringenin, MTT assay was carried out at 24 and 72 h (Fig. 3c). After 24 h, the MTT results indicated that the cell viability of Alg/20% Nar group was higher than other groups. In addition, the results provided that the cellular activity of this group was more than the positive control group (wells of the plate without hydrogel). Moreover, 72 h post-cell seeding, results also indicated that cell viability of Alg/20% Nar group was significantly higher than other groups.
Wound healing study
The in vivo study was used to evaluate the healing effects of the prepared hydrogel, and the results are presented in Fig. 4a. It shows the macroscopic appearance of the wounds 7 and 14 days post-surgery for negative control (wound covered with the sterile gauze) and alginate hydrogel without and with different amounts of naringenin. The alginate hydrogel without naringenin group had mild infection and inflammation, so it did not let the wound to be treated. By adding naringenin to the hydrogel, wound healing was improved, and the best group was Alg/20% Nar that had no sign of infection and inflammation and wound treated very well.
In order to quantify the wound healing process, wound closure was determined (Fig.4b). The alginate hydrogel and commercial wound dressing control had the wound closure of 47.8 ± 3.09%, 63.5 ± 2.04 43.8 ± 1.82%, and 48.7 ± 0.24% after 7 and 14 days after surgery. By adding naringenin, the best group was Alg/20% Nar which had the wound closure of 68.21 ± 1.39 and 95.3 ± 1.09 at 7 and 14 days after wounding, respectively.
Histopathological results
Histological analysis of the skin wounds was done by H&E and Masson’s trichrome staining as shown in Figs. 5 and 6, respectively. Micrographs of positive control (histologic sections of normal skin tissue) are indicated in Fig. 5A–C. In the negative control group, the wounds do not treat and the histopathological evaluation of this group illustrated polymorphonuclear inflammatory cells (PMNs) infiltration and granulation tissue formation 14 days after surgery, however, the epidermal layer has not been formed and the wound was covered by a crusty scab (Fig. 5D–F).
H&E stained microscopic sections of healed incisions in different treatment groups, Thick arrows, crusty scab; thin arrows, epithelial layer; arrowheads, skin appendages; asteroid, infiltration of inflammatory cells. Neg ctrl, negative control; Pos ctrl, positive control; Alg, alginate; Nar, naringenin. (a) × 40. (b) × 100. (c) × 400
MT stained microscopic sections of healed incisions in the positive control group, Thick arrows, crusty scab; thin arrows, infiltration of inflammatory cells; arrowheads, epithelial layer. Neg ctrl, negative control; Pos ctrl, positive control; Alg, alginate; Nar, naringenin. (a) × 40. (b) × 100. (c) × 400
Histopathological evaluation of alginate hydrogel–treated animals at day 14 showed healing of the defect area. A narrow layer of the epithelial cell has formed, and inflammatory cells were significantly reduced in comparison with the negative control group (Fig. 5G–I).
Micrographs of the Alg/1% Nar-treated group at 14 days post-treatment revealed a close similarity to the alginate group; a narrow layer of the epithelial cell has formed and the inflammation was considerably reduced in wound area (Fig. 5J–L).
Histopathology of wounds was treated with alginate hydrogel which incorporated with 10% naringenin-depicted epidermal proliferation and growth in the epidermal layer at 14 days post-treatment. The inflammatory response and granulation tissue were slightly declined during 14 days of treatment with this treatment. Moreover, the skin appendages were started to rejuvenate in this group (Fig. 5M–O).
Histopathological evaluation of Alg/20% Nar depicted a significant decrease in inflammation reduction after 14 when compared with the negative control group. This group showed more resemblance to normal skin, with a thin epidermis and presence of normal rete ridges, rejuvenation of skin appendages (hair follicles, sebaceous glands), and normal thickness of skin layers (Fig. 5P–R). It seems that the alginate hydrogel incorporated with 20% of naringenin indicated the best results in comparison with the negative control and other experimental groups.
The results of MT staining (Fig. 6) demonstrated that Alg/20% Nar displayed a better collagen synthesis and deposition among experimental groups during the wound treatment period. Moreover, the results indicated that the rate of collagen fiber synthesis and deposition in wound site was the lowest in the case of negative control and hydrogel without naringenin groups, respectively. It could be inferred that the rate of collagen synthesis was enhanced by increasing the percentage of naringenin.
Histomorphometric analysis
The histomorphometric analysis was performed 14 days after skin injury, and the results have been presented in Table 2. Among all experiment groups, in the negative control group, as it was mostly filled with immature granulation tissue, the re-epithelialization was minimum (P < 0.05). The re-epithelialization of all treatment groups was significantly developed in comparison with the negative control group (P < 0.05).
Overall, the healing condition of the alginate hydrogels incorporated with 20% naringenin was more similar to that of the positive control group at day 14, which has the best cosmetic appearance with normal thickness of epidermal layer and rejuvenation of the hair follicles and skin appendages.
Discussion
The main purpose of the current study was to evaluate the effect of naringenin in wound healing. To clarify this issue, alginate hydrogel without and with different amounts of naringenin was prepared and characterized. The prepared hydrogels were used to cover wound site in rat model, and different tests were used to evaluate its effect on the wound healing.
Recently, different studies have focused on designing and fabricating an effective wound dresser that can be helpful in wound healing process. One of the most effective wound dressing is hydrogel-based biomaterials that show good results in wound healing [11, 45]. One of the best is alginate hydrogel that is widely used as wound dressing because of its advantage features [10, 46, 47].
Traditional wound dressings like gauze keep the wound site dry and it lets evaporation of wound exudates and prevents entry of the pathogen into the wound [11]. In contrary, modern dressings (e.g., hydrogel dressings) make the wound site moisty that can improve wound healing process [48]. Alginate hydrogel is usually prepared by ionic cross-linking of alginate solution with calcium ions, followed by processing to fabricate freeze-dried porous sheets (i.e., foam), and fibrous non-woven dressings [12]. The dry form of alginate absorbs wound fluid to re-gel, and the gel form of alginate can supply water to the wound site. It not only keeps the wound site moisty but also decreases bacterial infection at the wound site. Previous study indicated a mechanism that alginate can enhance wound healing. In this mechanism, alginate stimulates monocytes to generate high levels of tumor necrosis factors like an interleukin-6 (IL-6). These cytokines at the wound site causes in a pro-inflammatory stimulus advantageous to wound healing [49].
Phenolic compounds, such as naringenin, inhibit growth factor cascades, such as NF-kB, and therefore it has suppressive effect on cancerous cell proliferation [50, 51]. Al-Roujayee showed that thermal burn wounded rats treated with naringenin because of its antioxidative potential that can enhance antioxidant system in skin samples and reduce lipid peroxidation [52]. Moreover, another study indicated that naringenin not only lower the deleterious generation of reactive oxygen species and inhibit TNF-a but also inhibit superoxide anion and cytokine fabrication in vitro and in vivo studies [53]. Furthermore, Shan et al. demonstrated that treat wound healing and wound inflammation with naringenin inhibited the CD4+ T lymphocyte and CD68+ cells (monocyte/macrophage) retention, as well as proinflammatory cytokine levels, such as IL-1β, IL-6, and TNF-α [54]. They concluded that naringenin inhibited inflammatory cell recruitment and fibroblast activation. In addition, they indicated that following naringenin treatment, mRNA and protein expression levels of TNF-α, IL-1β, IL-6, and TGF-β1 were downregulated [54].
The porous structure of hydrogel helps cell attachment and propagation. Also, the interconnected space in the hydrogel makes an appropriate environment for gaseous exchange and nutrient diffusion [55]. In a study, Yannas et al. showed that a better average pore size for wound healing purposes must be in the range of 20 to 120 μm [56]. Other study depicted that cells could migrate and home better in this pore size range [57]. The analytical results of our study provided that the pore size of fabricated hydrogel is in the range of 75 to 135 μm, which was favorable for cell migration and proliferation [42].
Structural stability and swelling behavior of hydrogel are important factors that consider for using different hydrogels in tissue engineering. Previous study provided that primary swelling is favorable because it can increase pore size and improve cell attachment and growth in a three-dimensional structure [58]. Moreover, the gel which demonstrate a high grade of swelling during the calcium cross-linking and better shrinking has lower stability in a long time [59]). In our study, the highest swelling percentage happened after 240 min and after that it decreased. The reason might be because of the interaction of carboxylic acid functional groups of alginate with the hydrophilic medium [60].
Since alginate is not naturally enzymatically degraded in mammals, a long time is needed that alginate hydrogel is completely removed from implantation sites [61]. Different methods are considered to control degradation kinetics of alginate hydrogels such as using various alginate molecular weight, chemical structure, and by covalent crosslinking. In the current study, the weight loss percentage was decreased by adding naringenin. In addition, by increasing the amount of naringenin in fabricated hydrogel, the weight loss percentage decreased. It is due to the hydrophobic nature of naringenin [43].
Hemolysis displays the amount of hemoglobin released into the plasma due to the damage in erythrocytes. Previous study claimed that hemolysis rate has direct connection to the blood compatibility of different materials [62]. In this study, the hemolysis rate of the alginate hydrogel with various percentages of naringenin was the same, and they were less than the positive control. Therefore, it is indicated that by adding naringenin to the fabricated hydrogel, the hemolysis rate did not change.
Previous studies showed the antibacterial effect of active flavonoids ([63,64,65]). Wang et al. indicated antibacterial behaviors of naringenin against E. coli and S. aureus cells. They claimed that bacteria may have the ability to develop possible adaptive mechanisms to create the endurance to the antibacterial activity of naringenin at low levels [66]. Our study confirmed this point that by increasing the amount of naringenin in the alginate hydrogel, the antibacterial effect of hydrogel increase too.
The MTT assay results provided that the naringenin can increase the cell viabilities. Moreover, the highest cell viabilities belonged to the alginate hydrogel containing 20% naringenin. It has been shown that the low dose of naringenin increase the insulin-like growth factor-1 (IGF-1) expression, and it inhibits the apoptosis and improve the fibroblast proliferation [54]. While the high dosages of naringenin have cytotoxic effect [67]. Moreover, because of hydrophobic nature of naringenin, by increasing its dosage, cell attachment decrease [68].
The in vivo study was used to evaluate the effect of fabricated hydrogel without and with different amounts of naringenin as a wound dressing. The wound closure in the groups contained naringenin is better than alginate hydrogel and control groups, and the best group was alginate/20% naringenin hydrogel group. It is because of antioxidative properties of naringenin, and based on this, it is widely used numerous physiological processes, thereby protecting cells from oxidative damage [34].
Conclusion
In the current study, we fabricated and evaluated alginate hydrogel with different amounts of naringenin (0%, 1%, 10%, and 20%) for the treatment of full-thickness excisional wound in rat model. Our results showed that cell viabilities in the alginate hydrogel containing 20% naringenin is significantly higher than other groups. This results also confirmed with in vivo study, and it indicated that the wounds treated with alginate hydrogel containing 20% naringenin was almost closed after 2 weeks. Thus, the results of this experiment provide evidence to show favorable effect of Alg/Nar hydrogel for wound treatment.
Availability of data and material
Not applicable.
References
Xu R, et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials. 2015;40:1–11.
Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47.
Drosou A, Kirsner RS, Kato T, Mittal N, Al-Niami A, Miller B, et al. Use of a bioengineered skin equivalent for the management of difficult skin defects after pediatric multivisceral transplantation. J Am Acad Dermatol. 2005;52:854–8.
Gloster HM Jr. The use of full-thickness skin grafts to repair nonperforating nasal defects. J Am Acad Dermatol. 2000;42:1041–50.
Zhu C, et al. Novel enzymatic cross-linked hydrogels that mimic extracellular matrix for skin wound healing. J Mater Sci. 2018;53:5909–28.
Dhivya S, Padma VV, Santhini E. Wound dressings–a review. BioMedicine. 2015:5.
Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J Appl Polym Sci. 2019;136:47738.
Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering Part B: Reviews. 2008;14:149–65.
Park J, Lakes RS. Biomaterials: an introduction. Berlin: Springer Science & Business Media; 2007.
Balakrishnan B, Mohanty M, Umashankar P, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26:6335–42.
Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–923.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26.
Puppi D, Chiellini F, Piras A, Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403–40.
Alizadeh R, et al. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr Polym. 2019b;224:115161.
Ehterami A, et al. Chitosan/alginate hydrogels containing alpha-tocopherol for wound healing in rat model. J Drug Deliv Sci Technol. 2019;51:204–13.
Bagher Z, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. Journal of Drug Delivery Science and Technology. 2019; 101379.
Chiu C-T, Lee J-S, Chu C-S, Chang Y-P, Wang Y-J. Development of two alginate-based wound dressings. J Mater Sci Mater Med. 2008;19:2503–13.
Shalumon K, Anulekha K, Nair SV, Nair S, Chennazhi K, Jayakumar R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol. 2011;49:247–54.
Suzuki Y, et al. In vivo evaluation of a novel alginate dressing. J Biomed Mater Res. 1999;48:522–7.
Thu H-E, Zulfakar MH, Ng S-F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int J Pharm. 2012;434:375–83.
Wang L, Khor E, Wee A, Lim LY. Chitosan-alginate PEC membrane as a wound dressing: assessment of incisional wound healing. J Biomed Mater Res. 2002;63:610–8.
Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–33.
Abdelrahman T, Newton H. Wound dressings: principles and practice. Surgery (Oxford). 2011;29:491–5.
Laurienzo P. Marine polysaccharides in pharmaceutical applications: an overview. Marine drugs. 2010;8:2435–65.
Eiselt P, Yeh J, Latvala RK, Shea LD, Mooney DJ. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials. 2000;21:1921–7.
Liao J, Wang B, Huang Y, Qu Y, Peng J, Qian Z. Injectable alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS Omega. 2017;2:443–54.
Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–205.
Kessler M, Ubeaud G, Jung L. Anti-and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 2003;55:131–42.
Samadian H, Vaez A, Ehterami A, Salehi M, Farzamfar S, Sahrapeyma H, et al. Sciatic nerve regeneration by using collagen type I hydrogel containing naringin. J Mater Sci Mater Med. 2019;30:107.
Yu J, Wang L, Walzem RL, Miller EG, Pike LM, Patil BS. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. 2005;53:2009–14.
Agus S, Achmadi SS, Mubarik NR. Antibacterial activity of naringenin-rich fraction of pigeon pea leaves toward Salmonella thypi. Asian Pac J Trop Biomed. 2017;7:725–8.
Hsia S-M, Kuo Y-H, Chiang W, Wang PS. Effects of adlay hull extracts on uterine contraction and Ca2+ mobilization in the rat. Am J Physiol Endocrinol Metab. 2008;295:E719–26.
Ng'uni T, Mothlalamme T, Daniels R, Klaasen J, Fielding BC (2015) Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus.
Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2018;24:551–60.
Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21.
Ai A, et al. Sciatic nerve regeneration with collagen type I hydrogel containing chitosan nanoparticle loaded by insulin. Int J Polym Mater Polym Biomater. 2019;68:1133–41.
Raeisi S, Chavoshi H, Mohammadi M, Ghorbani M, Sabzichi M, Ramezani F. Naringenin-loaded nano-structured lipid carrier fortifies oxaliplatin-dependent apoptosis in HT-29 cell line. Process Biochem. 2019;83:168.
Huang L, Xiao Y-H, Xing X-D, Li F, Ma S, Qi L-L, et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch Oral Biol. 2011;56:367–73.
Alizadeh R, et al. Differentiation of human mesenchymal stem cells (MSC) to dopaminergic neurons: a comparison between Wharton’s jelly and olfactory mucosa as sources of MSCs. J Chem Neuroanat. 2019a;96:126–33.
Ghorbani S, Tiraihi T, Soleimani M. Differentiation of mesenchymal stem cells into neuron-like cells using composite 3D scaffold combined with valproic acid induction. J Biomater Appl. 2018;32:702–15.
Salehi M, et al. Porous electrospun poly (ε-caprolactone)/gelatin nanofibrous mat containing cinnamon for wound healing application: in vitro and in vivo study. Biomed Eng Lett. 2019:1–13.
Yang S, Leong K-F, Du Z, Chua C-K. The design of scaffolds for use in tissue engineering. Part I Tradit Factors Tissue Eng. 2001;7:679–89.
Shulman M, et al. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS One. 2011;6:e18033.
Wang M, Yuan J, Huang X, Cai X, Li L, Shen J. Grafting of carboxybetaine brush onto cellulose membranes via surface-initiated ARGET-ATRP for improving blood compatibility. Colloids Surf B: Biointerfaces. 2013;103:52–8.
Kamoun EA, Kenawy E-RS, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8:217–33.
Pereira R, Carvalho A, Vaz DC, Gil M, Mendes A, Bártolo P. Development of novel alginate based hydrogel films for wound healing applications. Int J Biol Macromol. 2013;52:221–30.
Straccia MC, d’Ayala GG, Romano I, Oliva A, Laurienzo P. Alginate hydrogels coated with chitosan for wound dressing. Mar Drugs. 2015;13:2890–908.
Queen D, Orsted H, Sanada H, Sussman G. A dressing history. Int Wound J. 2004;1:59–77.
Thomas A, Harding K, Moore K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-α. Biomaterials. 2000;21:1797–802.
Pinho-Ribeiro FA, et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem. 2016b;33:8–14.
Xu C, Chen J, Zhang J, Hu X, Zhou X, Lu Z, et al. Naringenin inhibits angiotensin II-induced vascular smooth muscle cells proliferation and migration and decreases neointimal hyperplasia in balloon injured rat carotid arteries through suppressing oxidative stress. Biol Pharm Bull. 2013;36:1549–55.
Al-Roujayee AS. Naringenin improves the healing process of thermally-induced skin damage in rats. J Int Med Res. 2017;45:570–82.
Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, et al. Naringenin reduces inflammatory pain in mice. Neuropharmacology. 2016a;105:508–19.
Shan S, Zhang Y, Wu M, Yi B, Wang J, Li Q. Naringenin attenuates fibroblast activation and inflammatory response in a mechanical stretch-induced hypertrophic scar mouse model. Mol Med Rep. 2017;16:4643–9.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:16071.
Yannas I, Lee E, Orgill DP, Skrabut E, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci. 1989;86:933–7.
O’Brien FJ, Harley B, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–41.
Shanmugasundaram N, Ravichandran P, Reddy PN, Ramamurty N, Pal S, Rao KP. Collagen–chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials. 2001;22:1943–51.
Shilpa A, Agrawal S, Ray AR. Controlled delivery of drugs from alginate matrix. J Macromol Sci Polym Rev. 2003;43:187–221.
Venkatesan J, Bhatnagar I, Kim S-K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Marine drugs. 2014;12:300–16.
Prang P, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–9.
Jagetia GC, Ravikiran P. Acceleration of wound repair and regeneration by Nigella sativa in the deep dermal excision wound of mice whole body exposed to different doses of γ-radiation. Am Res J Med Surg. 2015;1:1–17.
Celiz G, Daz M, Audisio MC. Antibacterial activity of naringin derivatives against pathogenic strains. J Appl Microbiol. 2011;111:731–8.
Lee KA, Moon S-H, Lee J-Y, Kim K-T, Park Y-S, Paik H-D. Antibacterial activity of a novel flavonoid, 7-O-butyl naringenin, against methicillin-resistant Staphylococcus aureus (MRSA). Food Sci Biotechnol. 2013;22:1725–8.
Osawa K, Yasuda H, Maruyama T, Morita H, Takeya K, Itokawa H. Isoflavanones from the heartwood of Swartzia polyphylla and their antibacterial activity against cariogenic bacteria. Chem Pharm Bull. 1992;40:2970–4.
Wang L-H, Zeng X-A, Wang M-S, Brennan CS, Gong D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: implications for the antibacterial mechanism of naringenin. Biochim Biophys Acta Biomembranes. 2018;1860:481–90.
Kocyigit A, Koyuncu I, Dikilitas M, Bahadori F, Turkkan B. Cytotoxic, genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines. Asian Pac J Trop Biomed. 2016;6:872–80.
Recourt K, Van Brussel A, Driessen A, Lugtenberg B. Accumulation of a nod gene inducer, the flavonoid naringenin, in the cytoplasmic membrane of Rhizobium leguminosarum biovar viciae is caused by the pH-dependent hydrophobicity of naringenin. J Bacteriol. 1989;171:4370–7.
Funding
This study was funded by the Tehran University of Medical Sciences (Grant No. 96-02-159-35507).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Animal experiments were approved by the ethics committee of the Tehran University of Medical Sciences (ethical code: IR.TUMS.VCR.REC.1396.2876) and were carried out in accordance with the university’s guidelines.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salehi, M., Ehterami, A., Farzamfar, S. et al. Accelerating healing of excisional wound with alginate hydrogel containing naringenin in rat model. Drug Deliv. and Transl. Res. 11, 142–153 (2021). https://doi.org/10.1007/s13346-020-00731-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00731-6