Abstract
Pulmonary arterial hypertension (PAH) is the increase in mean pulmonary arterial pressure (> 25 mmHg). The development of the non-reversible plexiform lesions on the arterial walls of the pulmonary arteries has evolved as the reason to increase the pressure. The current treatments are directed towards the vasodilation of the pulmonary arteries via the endothelin, prostacyclin, and NO pathways which provides symptomatic relief. Deeper understanding of the disease leads to the various pathophysiological targets that play an important role in the development of PAH. Out of these, the angiogenetic mechanism of the pulmonary arterial smooth muscle cells has been proved to play an important role in PAH. Targeted therapies by anti-proliferative drugs may lead to the efficient treatment strategies to the root cause of PAH. Erlotinib, a receptor tyrosine kinase inhibitor, which acts on the epidermal growth factor receptor (EGFR), has shown promising results in clinical trials of PAH. The objective of the work has been the development of liposomal formulation of anti-proliferative drug, erlotinib HCl, via Quality by Design (QbD) approach. The liposomal formulation was developed using thin-film hydration technique and characterised for various physicochemical parameters, like particle size, % entrapment efficiency, DSC, FTIR, pXRD, and TEM. In the drug release study, the formulation showed sustained release of erlotinib over 24 h in simulated lung fluid pH 7.4. This developed formulation was evaluated in zebrafish tail fin regeneration assay for its anti-angiogenetic activity. The liposomal formulation inhibited the tail fin regeneration for 14 days indicating anti-angiogenetic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disorder characterised by the increase in pulmonary vascular resistance leading to an elevated pulmonary arterial pressure (mPAP) higher than 25 mmHg at rest [1]. Due to its silent clinical occurrence, the disease goes undiagnosed leading to right heart failure and eventually death. The incidence of PAH is 1–2 cases per million per year with a prevalence of 12–50 cases per million adults [2, 3]. Common symptoms include fatigue, shortness of breath, and non-productive cough, and may worsen to syncope, angina, fainting, and peripheral oedema in legs and ankles. PAH is diagnosed by a 6-min walk test (6MWT), which has served as the common endpoint in PAH-specific clinical trials [4]. Current research and deeper understanding of the disease revealed that vascular endothelial and smooth muscle cells show abnormal growth in PAH, with excessive cellular proliferation and resistance to apoptosis. These abnormalities along with inflammation, excess vasoconstriction, and thrombosis contribute to physical narrowing of the distal pulmonary arterioles. This narrowing leads to an increase in pulmonary vascular resistance, which causes progressive elevation of mPAP [5]. This arterial proliferation of the smooth muscle cells with myofibroblasts and necrotizing inflammatory mediators contributes to the formation of plexiform lesions. The underlying mechanism for PAH is an idiopathic form or in association with other disease states or exposures and is probably a final common response to environmental or disease-related inciting factors coupled with genetically determined susceptibilities [6, 7]. Current PAH management involves the following four main categories of drugs: prostanoids or prostaglandin analogues, phosphodiesterase-5 (PDE-5) inhibitors, endothelial receptor antagonists (ERAs), and soluble guanylate cyclase (sGC) stimulators. These therapies target the prostacyclin pathway, endothelin (ET)-1 pathway, and nitric oxide (NO) pathway, which have been identified in the pathogenesis of PAH. However, the medial survival rate with the current treatment is only 2.8 years, indicating the need for a newer efficient treatment [8]. Hence, there is a lot of research exploring treatment options acting at the molecular and cellular levels of PAH, including Rho-kinase inhibitors, serotonin inhibitors, vasoactive intestinal peptide, adrenomedullin, apelin, endothelial NO synthase couplers, epidermal growth factor receptor blockers, drugs acting on mitochondria-metabolic dysfunction (dichloroacetate, peroxisome proliferator-activated receptors), epigenetic targets, stem and progenitor cells, and gene therapy [4, 9].
During the last decade, the understanding of cellular and molecular mechanisms involved in the development of PAH has been a matter of intense research. Angiogenesis, the process of formation of new blood vessels from pre-existing ones, plays a critical role in the pathomechanisms of PAH. These findings led to clinical trials involving inhibition of angiogenesis as a key therapeutic target to inhibit, for instance, tumor growth. In this regard, several in vitro and in vivo models have been established to visualise and study migration of endothelial progenitor cells, formation of endothelial tubules, and the generation of new vascular networks, while assessing the conditions and treatments that either promote or inhibit such processes [10, 11]. Considering the similarity to the human system, zebrafish is the only vertebrate model currently used for rapid in vivo compound screening and pharmacological evaluation. Zebrafish model delivers various advantages like rapid development, optical transparency, large number of offsprings, and straightforward strategies for forward and reverse genetic manipulation [12, 13]. Due to its remarkable capacity to regenerate its organs and limbs even in adult stages, zebrafish is a very well-known model for studies on tissue regeneration. The caudal fin of adult zebrafish particularly provides an ideal tissue for studies related to vascular and tissue regeneration due to its simple, thin, and transparent architecture. The caudal fin regeneration model has been extensively used to study the angiogenetic mechanisms involved in tissue regeneration, such as cell differentiation, migration, and patterning, which eventually lead to the restoration of the fin’s original morphology and functionality [14].
Previously, erlotinib liposomes were developed and evaluated for its anti-angiogenic activity in different types of cancers like non-small cell lung cancer (NSCLC) and liver cancer [15, 16]. In the present work, we demonstrate the development of liposomal formulation by Quality by Design (QbD) approach and the evaluation of the anti-angiogenic capability of the developed liposomal formulations of erlotinib HCl in hypoxia-induced zebrafish caudal fin regeneration model. Erlotinib is a potent and selective tyrosine kinase inhibitor, which reversibly binds the ATP binding site of the epidermal growth factor receptor tyrosine kinase domain to inhibit the automatic phosphorylation of tyrosine kinases. This inhibition promotes apoptosis, inhibits angiogenesis, and ultimately prevents excessive cell proliferation [17]. The pharmaceutical QbD is a systematic approach that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management. This strategic control of the formulation parameters using QbD results in the stable and efficient formulation [18, 19]. The formulation was optimized using Box Behnken design, which was further validated using point prediction method. This developed formulation was evaluated for its anti-angiogenic potential in hypoxia-induced tail fin regeneration by measuring the horizontal and vertical length of the fin and the microscopic visualisation of the fin after 7th and 14th days of its amputation.
Materials and methods
Chemicals and reagents
Erlotinib was obtained as a gift sample from Sun Pharma Ltd., Co. (Mumbai, India). The phospholipid DPPC (1,2-dipalmitoyl-n-glycerol-3-phosphocholine) was received as a generous gift sample from VAV Life Sciences Ltd., Mumbai, and was used without further purification as the major lipid. Cholesterol (SD Fine Chemicals, India) was used as the bilayer stabiliser in the formulation. Cobalt chloride, absolute ethanol, methanol, chloroform (99.8%, AR grade), sodium chloride, disodium hydrogen phosphate, and potassium dihydrogen phosphate of AR grade were obtained from SD Fine Chemicals, India. All other salts and reagents were obtained from SD Fine Chemicals, Mumbai, India.
QbD approach
Defining the target product profile and determination of critical quality attributes
The first step in QbD study is the identification of the potential target product profile (TPP) and critical quality attributes (CQAs) of the formulation. CQAs were enlisted based on the literature reports of the formulation and preliminary formulation studies. Accordingly, formulation particle size and entrapment efficiency were categorized as the final CQAs that affect the quality of the product.
Risk assessment
Risk assessment was carried out to identify the critical material and process parameters that affect the formulation quality. In order to identify critical material attributes (CMAs) and critical process parameters (CPPs), an Ishikawa fish bone diagram, also known as cause–effect diagram, was constructed. After the risk analysis using the diagram and preliminary studies, three parameters were pinpointed as the important factors affecting the formulation, i.e., drug to lipid ratio as CMA and sonication time and hydration time as CPPs.
Experimental design
Based on the risk analysis, three variables were selected, namely molar ratio of drug to lipid (X1), sonication time (X2; min), and hydration time (X3; min), for the optimization study, using response surface method. Each of the three factors was tested at two different levels, and four centre points were included. A Box Behnken design was employed to study the influence of these variables on the preparation of liposomes. Design Expert 7.0.2 software was used for the analysis, and two response variables were evaluated, i.e., particle size (nm) (Y1) and entrapment efficiency (%) (Y2).
Erlotinib HCl-loaded liposome development
Multilamellar vesicles (MLVs) were prepared by the thin-film hydration method using drug and phospholipid in the required molar ratio. The phospholipid phase comprised of DPPC and cholesterol in 7:3 M ratio, and erlotinib HCl was dissolved in a mixture of absolute ethanol and chloroform (1:1) in a round-bottom flask. A thin film of drug in lipid was obtained by evaporating the solvents using a rotary vacuum evaporator (Heidolf rotary evaporator, Germany) under reduced pressure at 60 °C for 20 min. The dry thin film containing flask was kept overnight in a vacuum oven at 40 °C to remove residual solvent and then hydrated with 5 mL of Milli-Q water by vigorous hand shaking, followed by gentle rotation at 50 °C for a specified time at 150 rpm in a water bath to produce MLVs. The size of the resultant MLVs was reduced by bath sonication to achieve the reduced vesicle size. Unentrapped drug was separated by centrifugation of the liposomal dispersion at 10,000 rpm and re-dispersing the resultant centrifuged particles into the Milli-Q water. The particle size and zeta potential were measured using Malvern particle size analyser, and the entrapment efficiency of the developed formulation was calculated.
Numerical optimization of the design
The experimental results were analysed, and the process was optimized to control the risk by numerical optimization followed by point prediction technique. The criteria was set to 1:3 for drug:lipid ratio, 30 to 90 min for hydration time, 5 to 15 min for sonication time, and the software suggested 30 runs from which 1 run was selected as the point prediction and the run was carried out in triplicate to validate the design. The expected limit was set from 300 to 600 nm for the particle size and 60 to 80% for the entrapment efficiency. Considering the feed criteria of the software for numerical optimization, the level was selected from the 30 runs where drug:lipid ratio was 1:9.20, hydration time was 54.13 min, and sonication time was 5.71 min. This level gives the predicted particle size of 485.82 nm and entrapment efficiency of 69.10%. The run was carried out in triplicates, and the results were compared with the predicted outcomes. The liposomal particle size and % entrapment efficiency were calculated after the preparation of the liposomes.
Optimization of freeze-drying process based on S f/S i ratio
The developed liposomal formulation was further lyophilized on Labconco freeze dryer (FreeZone 4.5, USA) using 1, 5, and 10% (w/v) of mannitol and trehalose as cryoprotectants. The process includes freezing at − 40 °C for 24 h, primary drying at 0 °C for 5 h, followed by 10 °C for 2.5 h and 15 °C for 2 h, and secondary drying at 25 °C for 2.5 h. The chamber pressure and cold trap temperature were maintained at 20 Pa and 50 °C for the entire process. The freeze-dried liposomal powder was resuspended in 2 ml Milli-Q water, and the particle size was measured to determine Sf/Si ratio, where Sf is the final particle size of the formulation after reconstitution of freeze-drying and Si is the initial particle size of the formulation without freeze-drying. The freeze-dried liposomes were further characterised for powder appearance, particle size, entrapment efficiency, and appearance.
Erlotinib liposomal formulation evaluation
Dynamic light scattering and zeta potential measurements
Liposomal size and polydispersity index (PDI) were measured in triplicate at 25 °C by dynamic light scattering (DLS) method using Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). Prepared samples were diluted with Milli-Q water at least 50 times, and liposomal size and PDI were measured in a 1 ml disposable polystyrene cuvette. The values were reported as Z-average which is the mean hydrodynamic diameter of the sample and PDI as a measure of width of size distribution. Zeta potential was also measured at 25 °C using the Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK) in an applied field of 50 V using a polycarbonate folded capillary cell. It is estimated on the basis of electrophoretic mobility under an electric field, as an average of 30 measurements.
Drug loading and encapsulation efficiency determination
The prepared liposomes (10 μl) were diluted to 1 ml with Milli-Q water (n = 3) in 1.5 ml capacity microcentrifuge tube and centrifuged at 13,000 rpm for 15 min. The supernatant was collected to determine the free-drug concentration (Erlfree). To assess the total drug concentration (Erltotal), 10 μl of the same prepared liposomes was disrupted in methanol (total volume of 1 ml) and vortexed. Both Erlfree and Erltotal were assessed using RP-HPLC system consisting of Agilent 1100 modules (G1310A Isocratic Pump with solvent container, G1314A VW detector with standard flow cell, G1328A manual injector) using acetonitrile and 0.2 mM potassium dihydrogen phosphate buffer (pH 5) (60:40) at a detection wavelength of 245 nm. All the experiments were performed in triplicates. The results were reported as mean ± SD (n = 3). The encapsulation efficiency was calculated as: % Entrapment Efficiency = Erltotal − Erlfree / Erltotal × 100.
Differential scanning calorimetry
Powdered samples were accurately weighed (5 mg) and filled in aluminium pans, sealed, and subjected to DSC using a Perkin-Elmer Pyris 6 DSC coupled with Pyris software and equipped with a thermal analyser (Perkin-Elmer Instruments, Norwalk, USA). Thermograms were recorded by heating samples in nitrogen atmosphere from 35 to 300 °C at a heating rate of 10 °C min−1 using an empty aluminium pan as the reference.
Powder X-ray diffraction analysis
Powder X-ray diffraction (pXRD) analysis was performed using X-ray diffractometer (model, Miniflex, Rigaku Corporation, Japan) with nickel-filtered Cu K α radiation (λ = 1.54 A°). The sample was prepared by spreading the powder samples on specimen holder rings followed by sample angular scan ranging from 10 to 50° (2θ) with scan speed of 1°/min at 30 kV and 15 mA.
Fourier transform infrared spectroscopy
The Fourier transform infrared (FTIR) spectrum of the pure drug and the erlotinib liposomal powder was obtained using FTIR spectrophotometer (Shimadzu model 8033, USA). The samples were crushed to a fine powder, mulled with anhydrous potassium bromide, pressed to form a thin pellet, and subjected to FTIR analysis.
Transmission electron microscopy
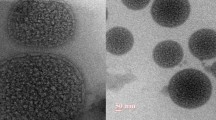
The morphology of erlotinib liposomal formulation was determined by transmission electron microscopy (TEM) (CM 200, Philips Briarcliff Manor, NY, USA). Imaging was performed on TEM at a voltage of 200 kV having magnification of 0.23 nm. Freeze-dried formulation was suspended in Milli-Q water, and a drop of dispersion was placed on Formvar®-coated copper grids (Ted Pella, Redding, CA) followed by the addition of a drop of 2% (w/v) uranyl acetate. After 3 min incubation at room temperature, excess liquid was drained, the grid was air-dried, and the images were captured using TEM.
In vitro release study
In vitro drug release studies were performed by direct dispersion method. Accurately weighed freeze-dried erlotinib liposomes containing 100 μg of erlotinib HCl were placed individually in 50 ml of simulated lung fluid (SLF) of pH 7.4 and stirred at 400 rpm. Sample aliquots of volume 1 ml were withdrawn at predefined time intervals and estimated by the HPLC method for the amount of drug released. The same volume of 1 ml was again replaced with SLF into the release buffer. The released profile was calculated by plotting the graph of the amount of the drug released against time.
Comparative evaluation of anti-angiogenic potential of liposomal erlotinib in adult zebrafish
Zebrafish (wild type) were procured from local aquarist and maintained in internal filtered water tanks at controlled temperature of 25 °C with 14 h light/10 h dark cycle. Fishes were fed with dry fish flakes twice a day. Adult zebra fishes were divided in two groups, group I or control (no treatment), group II (treatment with liposomal erlotinib formulation, dose equivalent to 20 ng of erlotinib), and group III (treatment with erlotinib solution, 20 ng). Each group consisted of eight fishes placed in separate fish tanks containing sufficient water being suitably aerated. Zebrafish were anesthetized using cold anaesthesia, and their caudal fin was partially amputated on day “0.” Amputated fishes were then exposed to 1% cobalt chloride for a day to induce the hypoxic conditions. The tail fins were then allowed to grow, and the sizes of the re-grown tail fins were measured on days 7 and 14 using vernier callipers. The microscopic evaluation was done under inverted microscope having 10× and 40× lens where the bony rays and regenerating cells from the fins were compared.
Results
Quality by Design approach
Defining the TPPs
Various formulation considerations were taken into consideration while deciding the TPP. In this study, we have aimed to develop the liposomal formulation of erlotinib HCl for PAH using the QbD approach. Accordingly, various TPPs were decided which are mentioned in Table 1.
Determination of CQAs
Depending on the listed TPPs in Table 1, liposomal particle size and % entrapment efficiency are considered as CQAs. Based on this knowledge, the aim of our study was to apply QbD concept in order to optimize the liposomal erlotinib formulation in terms of liposomal particle size and % drug entrapment as CQAs. Table 2 enlists all the TPPs and the consideration for CQAs.
Risk assessment
In this study, particle size and encapsulation efficiency were identified as CQAs of the final product. Therefore, it is very important to understand the potential risks affecting these major product qualities. Accordingly, two Ishikawa diagrams (cause and effect diagrams) were constructed to identify the potential causes affecting the critical attributes i.e., particle size and entrapment efficiency, as shown in Fig. 1a,b.
Risk analysis—particle size and entrapment efficiency
From the Ishikawa fish bone diagram, selected CMAs and CPPs were assigned the power from low to high in order of affecting the CQAs. Table 3 highlights that the drug:lipid ratio and sonication time were expected to have a high impact on particle size while the drug:lipid ratio and hydration time have the highest impact on entrapment efficiency.
Box Behnken design
The Box Behnken design was applied, and the response particle size and entrapment efficiency were analysed using the software for effect of various factors. The matrix of the experimental design comprising 17 formulations is presented in Table 4. It was observed that the particle size varies from 358.6 to 3749 nm while entrapment efficiency varies from 23.99 to 79.53% and the rest of the points are distributed evenly across this range.
For particle size, the software analysed four mathematical models i.e., linear model, 2FI model, cubic model, and quadratic model. From these four models, the linear and quadratic models were suggested in order to obtain the highest prediction power while the cubic model was found to be aliased. After application of ANOVA, the quadratic model was shown to be significant and lack of fit (p < 0.05) as not significant. The final equation describing the response surface is particle size = +6272.80765 – 1546.67296 ∗ Drug:Lipid + 46.697 ∗ Hydration time − 187.25214 ∗ Sonication time − 0.36071 ∗ Drug:Lipid ∗ Hydration time + 16.48571 ∗ Drug:Lipid ∗ Sonication time − 0.83633 ∗ Hydration time ∗ Sonication time + 85.82347 ∗ Drug:Lipid2 − 0.30368 ∗ Hydration time2 + 5.56250 ∗ Sonication time2 (Fig. 2, Fig. 3, Fig. 4).
For entrapment efficiency, from the four models, the software suggested the linear model to obtain the highest prediction power while the cubic model was found to be aliased. From the ANOVA, the linear model was significant and lack of fit (p < 0.05) was not significant. The final equation describing the response surface is Entrapment efficiency = +58.74411 + 2.71821 ∗ Drug:Lipid + 0.044917 ∗ Hydration time − 2.99175 ∗ Sonication time (Fig. 5, Fig. 6, Fig. 7).
Optimization of the design using point prediction method
The applied design was validated using the numerical optimization followed by the point prediction method. According to the selected levels, the run was carried out in triplicates. The particle size obtained was 500.86 ± 33 nm, and the entrapment efficiency was 68.14 ± 4.32%. Hence, the applied design was validated as the obtained values were much closer to the predicted values and below the confidence interval of 95%. The zeta potential of the optimized formulations was found to be 4.76 mV for liposomal formulation (Table 5).
Optimization of freeze-drying process based on S f/S i ratio
The Sf/Si ratio indicates difference in particle size before and after freeze-drying. Amongst the screened cryoprotectants, mannitol at 5% displayed Sf/Si ratio of 1.27 and showed promising results in inhibiting particle aggregation and resisting change in particle size and PI after freeze-drying. In comparison, formulation in the presence of trehalose did not produce dry free flowing powder after the freeze-drying process. Hence, the Sf/Si ratio was not calculated for trehalose.
Erlotinib liposomal formulation evaluation
Particle size, zeta potential measurement, and entrapment efficiency
The particle size of the optimized batches obtained by the modified thin-film hydration technique was found to be 479.3 nm with a PDI value of 0.2, while the entrapment efficiency was found to be 66.29%. The zeta potential of the optimized formulations was found to be 4.76 mV for the developed liposomal formulation. The final optimized liposomal formulation was further characterised for different characterisation parameters, like DSC, pXRD, FTIR, and TEM.
Differential scanning colorimetry
DSC chromatogram provides the information regarding the crystal habit and the polymorphic changes that the drug might have undergone after formulation development. Pure erlotinib HCl exhibited single sharp endothermic peak at 235 °C which was in accordance with the previous reports. In the case of freeze-dried liposomal erlotinib formulation, endothermic peak of erlotinib at very low intensity was observed in the melting range of drug. This indicates the drug being molecularly entrapped in the lipid matrix and lower amount of drug compared to the cryoprotectant. The peak at 168 °C is attributed to mannitol used as cryoprotectant in the formulation (Fig. 8).
pXRD studies
The effects of process parameters during the liposomal preparation were studied by XRD to evaluate the physiochemical properties of erlotinib (Fig. 9). The diffraction spectrum of pure erlotinib HCl exhibited intense and distinct peak at 2θ values of 5.6°, 9.5°, 10.87°, 16.58°, 18.45°, 20.76°, 23.11°, 24.96°, and 26.20°. Freeze-dried erlotinib liposomes exhibited characteristic peaks of pure erlotinib with higher intensities, which can be attributed to entrapment of erlotinib in its crystalline form in the lamellar structure of liposomes during freeze-drying.
FTIR
The IR spectra showed the characteristic peaks of pure erlotinib, i.e., CH stretch of alkyne (3271 cm−1), weak C☰C stretch (2120 cm−1), strong C〓C and C〓N stretch of aromatic structure (1581.63, 1620.21 cm−1), and C-O-C stretch (1118.71 cm−1). FTIR spectra of freeze-dried erlotinib liposomal powder retained most of the major characteristic peaks of pure erlotinib (Fig. 10). These results suggested that there was no drug excipient molecular interaction or drug degradation. The retention of the characteristic bands indicates that the processing parameters do not have any effects on the drug entrapped within the system.
TEM
Figure 11 represents the negative-staining TEM image of the liposomal erlotinib formulation which confirms round-shaped liposomes with smooth surface, and the particle size of the liposomes is in close agreement with the values obtained using the DLS method.
In vitro release study
Figure 12 exhibits the release profile of the optimized liposomal erlotinib formulation in SLF having pH 7.4 as the release medium. In vitro release study demonstrated biphasic release of the drug from the formulation with an initial rapid release in the first 8 h, followed by a slower rate of drug release for up to 24 h.
Comparative evaluation of anti-angiogenic potential of liposomal erlotinib in adult zebrafish
In our study, we have compared the anti-angiogenetic activity of the developed liposomal formulation in adult zebrafish. Zebrafish caudal fins were partially amputated at day 0 and allowed to recover under (1%) CoCl2 treated condition. Tail fin length under hypoxia was recorded at 7th and 14th days, and % inhibition was calculated. Microscopic changes during the events were captured using the inverted microscope. The liposomal formulation shows 51.44 ± 10.21% growth of the vertical length and 57.92 ± 12.84% growth of the horizontal length at day 7 and 63.06 ± 6.21% growth of the vertical length and 69.21 ± 7.95% growth of the horizontal length at day 14. While free erlotinib solution shows 41.83 ± 7.11% growth of the vertical length and 63.82 ± 12.13% growth of the horizontal length at day 7 and 50.30 ± 13.45% growth of the vertical length and 86.26 ± 5.97% growth of the horizontal length at day 14. After 14 days, the microscopic evaluation of the caudal fin (Fig. 13) depicts that the fin regeneration was inhibited as compared to the control one. Zebrafish treated with 1% CoCl2 exhibited increase in tail fin length when measured from the site of amputation.
Discussion
The basic rationale for utilization of the QbD approach is to develop a formulation using pre-determined quality control strategies thereby achieving the desired quality product. According to the definition of ICH Q8 (R2) (2009), the TPP is defined as “a prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy of the drug product” [20]. For our formulation development, various TPP elements were selected like dosage form, route of administration, drug product attributes involving particle size, PDI, entrapment efficiency, content uniformity, assay, residual solvents, microbial limit, and container, and closure systems. These TPPs were set to specified targets and justified for their inclusion into the QbD product development. Dry free flowing powder to be filled in capsule was the target for developing dry powder inhaler (DPI). The route of administration for DPI was pulmonary to deliver the drug directly at the site of action. The TPP elements chosen are said to play a role in product efficiency via the pulmonary route. From drug product quality attributes for pulmonary delivery, final formulation would be the dry powder having particle size in the range of 300 to 500 nm with entrapment efficiency of 60 to 80%. The microbial limit as per compendial limit will ensure that the product is microbe free and safe to be delivered. The container closure system is necessary to keep the dosage form protected from the environmental conditions and during patient handling. This may be selected as per the product suitability.
According to ICH Q8 (R2) definition, a CQA is “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality” (ICH 2009). Considering the delivery of formulation for PAH, submicron particle size is desired. This will help the drug to reach the target effectively via the pulmonary route. The acceptable particle size of the formulation to reach the deep lung tissues is 300 to 500 nm [20, 21]. Hence, liposomal particle size was assigned as the CQA considering the pulmonary delivery of formulation. Considering the amphiphilic nature of erlotinib HCl, the encapsulation of the drug into a vesicular carrier is difficult. Hence, the maximum entrapment of the erlotinib will ensure the development of clinically efficacious formulation to deliver the necessary high drug dose to the site of action. Hence, % EE was assigned as CQA considering the therapeutic efficacy of the formulation.
After identification of CQAs, the risk assessment was done using the Ishikawa fish bone diagram. According to the ICH guideline Q9, risk is defined as “the combination of the probability of occurrence of harm and the severity of that harm” [22]. Risk assessment identifies the hazards and leads to the analysis and evaluation of risks which are associated with those hazards. Risk assessment helps to identify the critical factors affecting the final product quality and improve the quality of the process. From the literature, various factors affecting liposomal particle size and EE% were collated and analysed. They were divided into the following 3 categories: formulation, process, and environment, as illustrated in Fig. 1. No factor was selected from the environment factor, as these factors can be controlled in the house. Amongst formulation factors, drug to lipid ratio was found to play an important role in determining the liposomal vesicle size. Phospholipids are the main constituent of liposomal membrane; thus, they can have a direct effect on liposome particle size. These phospholipids and cholesterol are necessary to entrap the molecule into their structures. In the lipid, the ratio of the DPPC:Cholesterol was optimized and fixed to 7:3, which produces a stable liposomal formulation. Higher cholesterol content leads to leaky liposomes and may tend to destabilise the system. Also, the entrapment efficiency is dependent on the drug:lipid ratio where higher ratio is required to entrap an amphiphilic molecule like erlotinib. Hence, drug:lipid ratio was considered as the Critical Material Attribute affecting the particle size and entrapment efficiency [23]. Of the various process factors, hydration time and sonication time have direct impact on the liposomal particle size as higher values of these decrease the particle size. Sonication process disrupts the MLVs and tends to form small unilamellar vesicles which leads to decrease in particle size of the formulation. Also, this may result in the drug leaching out of the system and subsequent lowering of the entrapment efficiency. Higher hydration time provides a good contact time for the vesicles to entrap the drug and gives higher entrapment efficiency. After risk assessment of these factors on CQAs, the next step was to reduce the risk using control strategy to develop the liposomal formulation having good quality and stability [24, 25].
Following the risk assessment, to optimize the particle size and entrapment efficiency, three factors, drug:lipid ratio, hydration time, and sonication time, were evaluated using the response surface design, i.e., Box-Behnken design. From the models for particle size, model summary statistics suggested linear and quadratic models whereas the cubic model was aliased. The normal probability plot indicates whether the residuals follow a normal distribution, in which case, the points will follow a straight line. The data points were distributed around the straight line in the model diagnostic plot (Fig. 2). Figure 3 shows that the model was fitting the Box Cox plot of transformation and no transformation was recommended. From the equation, it can be depicted that the factor drug:lipid ratio is the major factor affecting the particle size and has negative impact on the response. From the 3D contour plot (Fig. 4), it can be observed that when drug:lipid ratio is increased, the particle size is decreased. This can be attributed to the effect of hydrogen bonding between the neighbouring phospholipid molecules. At neutral pH, the repulsive forces between DPPC molecules predominate over hydrogen bonding, reducing the vesicular size. This may lead to formation of small lamellar structures instead of MLVs [23].
The model evaluation for entrapment efficiency suggests linear model. This model was applied for the evaluation and analysed by ANOVA for the response surface analysis. Values of “Prob > F” less than 0.0500 indicate that model terms are significant. In this case, drug:lipid ratio and sonication time were the significant factors affecting the entrapment efficiency. Figure 5 indicates the normal residuals plot validating the model where the data points were distributed around the line. It can be observed that all the points were very close to their predicted values indicating the model was robust and accurate. Box Cox plot of power transformation further recommended no power transformation as the lambda value is 1 which is close to 0.97 and is in between the 95% confidence interval (Fig. 6). From the equation, it can be depicted that the factors drug:lipid ratio and sonication time are major in affecting the entrapment efficiency. The factor drug:lipid ratio has positive impact on the entrapment efficiency where an increase in the lipid concentration causes a proportional increase in the encapsulation efficiency. As the drug is amphiphilic, higher lipid concentration is necessary to entrap the molecule. The contour plots support the same observation wherein the entrapment efficiency was found to have increased when the drug:lipid ratio is increased. The factor sonication time has negative effect on the entrapment efficiency where an increasing sonication time decreases the encapsulation efficiency. This can be attributed to the disruption of liposomal structure when sonicated for longer time thus expelling the drug out of the system. This observation was concluded from the contour plots obtained for the effect of sonication time on entrapment efficiency.
The model was further validated using numerical optimization by point prediction technique. The level was run in triplicate where the particle size and entrapment efficiency were close to the predicted values. This suggests that the design was validated and the control strategy was successful in reducing the risk. Hence, the control strategy was applied during the production of liposomal formulation for better quality product. The formulation was reproduced which has particle size of 500.86 ± 33 nm with a PDI of 0.2 and entrapment efficiency of 68.14 ± 4.32%. As seen in Fig. 11, the liposomal particle size matches with the TEM image particle size. TEM image shows that liposomes are spherical in shape with smooth surfaces.
The formulation characterisation involving DSC, FTIR, and pXRD confirms the stability of the erlotinib after the processes involved in the liposomal formulation. FTIR of formulation in Fig. 10 indicates that most of the characteristic bands of pure erlotinib HCl were retained. In pXRD (Fig. 9), the crystalline nature of the drug was evidenced by the sharp peaks. Nonetheless, significant pXRD peaks were still present in the diffraction pattern of the liposomal formulation, thus indicating the process has no effect on the crystalline nature of the drug and the physicochemical stability of the API is retained. The same results were interpreted from the DSC analysis (Fig. 8) where the minor endothermic peak was retained in the formulation thermogram. Moreover, sharp peak at 165 °C represents crystalline nature of mannitol which was used as cryoprotectant.
The drug release from the liposomal formulation indicates the amount of drug released at different time points in respective media. From the in vitro release study (Fig. 12), it was observed that the liposomal erlotinib formulation has sustained drug release profile. The initial faster release from the formulation is attributed to the release of drug from the liposome surface and to the outer lamellae, while the later sustained release is due to the sustained release of the drug from the inner lamella. After 24 h, 65% of the drug was released from the freeze-dried liposomal formulation.
Angiogenesis is the process of formation of new blood vessels from the pre-existing ones and plays an important role in several conditions, including tissue repair, embryonic development, and several diseases. Excessive vascular growth or abnormal remodelling or angiogenesis is prevalent in cancer, inflammatory disorders, and retinopathies [26]. Current research into this area has proved that PAH shares similar pathological abnormalities with cancer, i.e., excessive cell proliferation and remodelling. Hence, the newer therapies are targeted to the inhibition of the processes involved in the cell proliferation, angiogenesis, and remodelling. The zebrafish emerges as a convenient alternative for the other in vivo mammalian models due to the increase in restrictions on the experimental use of mammalian models for research. Since angiogenesis is one of the main focuses of vascular regeneration research, caudal fin regeneration in zebrafish models has been developed for this type of vascular development research [27, 28]. The microscopic images (Fig. 13) show that the fin regeneration is at blastema formation stage where proliferating blood cells disseminate to the tip of the regenerating caudal fin and the bony rays of the fin are still in the development stage. This leads to the inference that liposomal erlotinib successfully inhibits hypoxia-induced angiogenesis. It has been reported that CoCl2 mimics hypoxic microenvironment via the hypoxia inducing factor-1α (HIF-1 α), both in vivo and in vitro. This HIF-1 α mechanism activation is the pathophysiological response of the cells undergoing deprived oxygen supply. This in turn activates VEGF signalling to maintain vascular homeostasis for endothelial cells thereby promoting wound healing and angiogenesis in regenerated tissue [29, 30]. Erlotinib, an epidermal growth factor receptor inhibitor, indirectly inhibits VEGF signalling through tyrosine kinase inhibition and successfully halts the angiogenesis process. The erlotinib solution treatment group shows lesser inhibition of the tail fin regeneration as compared to the erlotinib liposomal formulation treatment group. This signifies that anti-angiogenetic activity is exhibited for sustained duration when the erlotinib is encapsulated in liposomes. No treatment group shows complete regeneration of the zebrafish tail fin. This inhibitory action of liposomal erlotinib against CoCl2-induced caudal fin regeneration compared to control fin strikes the significance of this treatment in PAH.
Conclusion
As PAH shares the angiogenetic phenotype similar to cancer, the treatment needs to be directed against newer targets that play important role in the process. Erlotinib, an ERB receptor inhibitor, has been proposed to play the role of angiogenetic inhibitor in PAH. To achieve sustained drug release at the diseased site, i.e., lung, the liposomal erlotinib HCl formulation was developed according to the QbD approach. The QbD approach helps to develop the product with improved quality and safety profile in a systematic manner. The Box Behnken design was applied to evaluate the effects of drug:lipid ratio, hydration, and sonication time on particle size and entrapment efficiency. The design was validated using numerical optimization by point prediction technique. The particle size obtained was 500.86 ± 33 nm, and the entrapment efficiency was 68.14 ± 4.32%. The solid-state characterisation studies involving FTIR, DSC, and XRD revealed that there is no change in the physical form of erlotinib after the formulation processing. TEM imaging of the formulation depicted that the liposomes were round shaped and had particle size correlating with the DLS results. This formulation was evaluated for its anti-angiogenetic potential in zebrafish tail fin regeneration under hypoxic condition where the growth of the amputated tail fin was measured. The growth of the tail fin was inhibited in the zebrafish treated with the liposomal formulation as compared to the control fin. Thus, this study concluded that the liposomal formulation of erlotinib was successfully developed by the QbD approach and showed anti-angiogenetic potential in PAH.
References
Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19(4):558–73.
George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance United States, 2001 to 2010. Chest. 2014;146(2):476–807.
Duarte JD, Hanson RL, Machado RF. Pharmacologic treatments for pulmonary hypertension: exploring pharmacogenomics. Futur Cardiol. 2013;9(3):335–49.
Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51(16):1527–38.
McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65(18):1976–97.
McLaughlin VVMD, McGoon MDMD. Pulmonary arterial hypertension. Circ. 2006;114:1417–31.
Smythe W, Mcilleron H, Merle C, Horton J, Smith P, Simonsson, USH. A semi-mechanistic pharmacokinetic auto-induction model for the characterisation of rifampicin pharmacokinetics in African pulmonary tuberculosis infected adults. Poster Abstracts page 2010, 39.
Frumkin LR. The pharmacological treatment of pulmonary arterial hypertension. Pharmacol Rev. 2012;64(3):583–620.
Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43(6):629–34.
Vaidya B, Gupta V. Novel therapeutic approaches for pulmonary arterial hypertension: unique molecular targets to site-specific drug delivery. J Control Release. 2015;211:118–33.
Galie N, Ghofrani A-H. New horizons in pulmonary arterial hypertension therapies. Eur Respir Rev. 2013;22(130):503–14.
Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10(2):116–24.
Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Di De. 2003;6:218–23.
Chávez MN, Aedo G, Fierro FA, Allende ML, Egaña JT. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front Physiol. 2016;7(MAR):1–15.
Xu H, He C, Liu Y, Jiang J, Ma T. Novel therapeutic modalities and drug delivery—erlotinib liposomes modified with galactosylated lipid: in vitro and in vivo investigations. Artif Cell Nanomed B. 2018;46(8):1902–7.
Nimmano N, Somavarapu S, Taylor KM. Aerosol characterisation of nebulised liposomes co-loaded with erlotinib and genistein using an abbreviated cascade impactor method. Int J Pharm. 2018;542(1–2):8–17.
Pitsiou G, Zarogoulidis P, Petridis D, Kioumis I, Lampaki S, Organtzis J, et al. Inhaled tyrosine kinase inhibitors for pulmonary hypertension: a possible future treatment. Drug Des Dev Ther. 2014;8:1753–63.
Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. Int J Pharm. 2012;423(2):543–53.
Sylvester B, Porfire A, Muntean DM, Vlase L, Lupuţ L, Licarete E, et al. Optimization of prednisolone-loaded long-circulating liposomes via application of quality by design (QbD) approach. J Lipos Res. 2018;28(1):49–61.
ICH. Pharmaceutical development Q8. ICH Harmon Tripart Guidel. 2009;8(August):1–28.
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–99.
ICH Expert Working Group. Quality risk management Q9. ICH Harmon Tripart Guidel. 2005;(November):1–23.
Roy B, Guha P, Bhattarai R, Nahak P, Karmakar G, Chettri P, et al. Influence of lipid composition, pH, and temperature on physicochemical properties of liposomes with curcumin as model drug. J Oleo Sci. 2016;65(5):399–411.
Mahmoud M, Labiba N, Khalil EK, Khalafallah N. Effect of various formulation variables on the encapsulation and stability of dibucaine base in multilamellar vesicles. Acta Pol Pharm Drug Res. 2005;62(5):369–79.
Kulkarni SB, Betageri GV, Singh M. Factors affecting microencapsulation of drugs in liposomes. J Microencapsul. 1995;12(3):229–46.
Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: an overview. Eur J Intern Med. 2009;20(7):663–71.
Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dynam. 2003;226(2):202–10.
Schuermann A, Helker CSM, Herzog W. Angiogenesis in zebrafish. Semin Cell Dev Biol. 2014;31:106–14.
Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103(4):432–40.
Rathinasamy VS, Paneerselvan N, Jagadeeshan S, Malathi R. Hypoxia induced angiogenesis and upregulation of VEGF: an in vivo study using zebrafish model. Int J Sci Eng Res. 2015;6(6):831–9.
Ethical approval and animal care and use
All institutional and national guidelines for care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Dhoble, S., Patravale, V. Development of anti-angiogenic erlotinib liposomal formulation for pulmonary hypertension: a QbD approach. Drug Deliv. and Transl. Res. 9, 980–996 (2019). https://doi.org/10.1007/s13346-019-00641-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00641-2