Abstract
The purpose of this study was to explore poly(vinylpyrrolidone-co-vinyl acetate) (PVP VA64) as a novel release-modifier to tailor the drug release from ethylcellulose (EC)-based mini-matrices prepared via hot melt extrusion (HME). Quetiapine fumarate (QF) was selected as model drug. QF/EC/PVP VA64 mini-matrices were extruded with 30% drug loading. The physical state of QF in extruded mini-matrices was characterized using differential scanning calorimetry, X-ray powder diffraction, and confocal Raman microscopy. The release-controlled ability of PVP VA64 was investigated and compared with that of xanthan gum, crospovidone, and low-substituted hydroxypropylcellulose. The influences of PVP VA64 content and processing temperature on QF release behavior and mechanism were also studied. The results indicated QF dispersed as the crystalline state in all mini-matrices. The release of QF from EC was very slow as only 4% QF was released in 24 h. PVP VA64 exhibited the best ability to enhance the drug release as compared with other three release-modifiers. The drug release increased to 50–100% in 24 h with the addition of 20–40% PVP VA64. Increasing processing temperature slightly slowed down the drug release by decreasing free volume and pore size. The release kinetics showed good fit with the Ritger-Peppas model. The values of release exponent (n) increased as PVP VA64 is added (0.14 for pure EC, 0.41 for 20% PVP VA64, and 0.61 for 40% PVP VA64), revealing that the addition of PVP VA64 enhanced the erosion mechanism. This work presented a new polymer blend system of EC with PVP VA64 for sustained-release prepared via HME.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hot melt extrusion (HME) has been widely applied in the pharmaceutical industry with different dosage forms, such as granule pellets [1], sustained-release mini-tablets [2], transdermal/transmucosal films [3, 4], and implants [5]. Compared with traditional methods, HME is a simpler process to continuously prepare sustained-release tablets.
Ethylcellulose (EC) was applied for the melt extrusion of sustained-release mini-matrices [6,7,8,9,10] due to its good processability and perfect thermal stability. The glass transition temperature (Tg) of EC is between 131 and 137 °C [11], while its degradation temperature is about 215 °C in air [11], resulting in a very broad temperature window for extrusion. However, poor water solubility of EC and low porosity of extrudates led to incomplete drug release. Therefore, some water-soluble polymers were investigated as release-modifiers to tailor the drug release, such as xanthan gum [9], HPMC [10], and L-HPC [12]. The release-controlled ability of L-HPC was not so good, while the modification of drug release by xanthan gum highly depended on the ionic strength (incomplete drug release under low ionic strength) [9]. HPMC has a high Tg between 170 and 180 °C with poor thermoplasticity [13]. Therefore, it is necessary to develop an excellent release-modifier for extruded EC-based mini-matrices.
Poly(1-vinylpyrrolidone-co-vinyl acetate) (PVP VA64), a water-soluble polymer, was extensively used as the matrix of amorphous solid dispersion prepared via HME [14,15,16], for example, two commercial products, Kaletra® and Norvir®. Glass transition temperature and degradation temperature of PVP VA64 were reported as 101 and 230 °C, respectively [17], indicating a very wide processing window for HME. Quetiapine fumarate (QF), a water-soluble drug for schizophrenia treatment, was used as the model drug. The aim of the present study was to explore PVP VA64 as an effective release-modifier for EC-based mini-matrices prepared via HME.
Materials and methods
Materials
EC (ETHOCEL® Standard 7 FP Premium) was kindly supplied by Dow Chemical Company (Midland, Michigan, USA) with an ethoxyl content of 48.0–49.5% (w/w). PVP VA64 (Kollidon® VA64) was gifted by BASF SE (Ludwigshafen, Germany). QF was purchased from Suzhou No. 4 Pharmaceutical Factory (Suzhou, China). Xanthan gum was purchased from Tianjin Fuchen Chemical Reagents Factory (Tianjin, China). Low-substituted hydroxypropylcellulose (L-HPC) was kindly supplied by Anhui Sunhere Pharmaceutical Excipients Co., Ltd. (Anhui, China). Crospovidone (CPVP) was gifted by Ashland Inc. (Kidderminster, UK).
Hot melt extrusion
Physical mixtures of EC, release-modifiers, and QF were blended for 10 min in a mortar and then manually fed into a conical co-rotating screw hot-melt extruder (HAAKE MiniCTW, Thermo Scientific, Germany). Screw speed and die diameter were fixed at 100 rpm and 3 mm, respectively. The extruded QF/EC/PVP VA64 formulations were slight yellow and opaque cylinders with a smooth surface. The rod-like extrudates were manually cut into mini-matrices with the length of 5 mm. For the formulations containing L-HPC, xanthan gum, and CPVP, the weight ratio of QF/EC/release-modifier was fixed at 30:50:20 and the processing temperature was 130 °C. For PVP VA64-related solid dispersions, the formulations and processing temperatures are listed in Table 1.
Quetiapine fumarate assay
The content of QF and related substances in extrudates were detected using high-performance liquid chromatography (HPLC, UltiMate 3000, Dionex, USA) equipped with a UV detector. The flow rate of the solvent pump was 1.0 ml/min and the injection volume was 20 μl. The mobile phase was composed of methanol, distilled water, and triethylamine (670:330:4, v/v) with a pH value of 6.8 adjusted by phosphoric acid. The milled extrudates were dissolved by the mobile phase and then diluted to 100 ml in a measuring flask. All samples were filtered through a 0.45-μm membrane filter before the test. All analyses were carried out in triplicate.
Thermal behavior
Thermal behavior of individual components, physical mixtures, and extrudates was measured using a thermogravimetric analyzer (NETZSCH STA-409, NETZSCH group, Germany). Samples (5–10 mg) were accurately weighted into aluminum pans and then heated to 200 °C at a heating rate of 10 °C/min with 40 ml/min nitrogen purge. Data were analyzed using Proteus analysis software (NETZSCH Group, Selb, Germany).
X-ray powder diffraction
Physical states of QF in physical mixtures and extrudates were characterized by X-ray powder diffraction (Bruker D2 PHASER X, Germany), operating at 20 mA and 30 kV (CuKα, λ = 0.154). Scanning rate was 0.1 s/step with a scanning step of 0.014° and 2θ range of 5–40°.
Confocal Raman microscopy
Confocal Raman microscopy (Renishaw inVia, England) was employed to evaluate the distribution of QF in mini-matrices with excitation at 785 nm. All spectra were recorded at a resolution of 4 cm−1 and an exposure time of 30 s with a laser power of 400 mW. Then a surface sized 20 μm × 20 μm was scanned in point-by-point mapping mode. The number of mapping points was about 231. The software package WIRE 2.0 (Renishaw) was employed for spectral acquisition and analysis.
Scanning electron microscopy
SEM measurements were performed using a JEOL JSM-6330F microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 15 kV and a 15-μA emission current. Samples were coated with platinum before measurement.
In vitro dissolution
Dissolution behaviors were studied by using USP paddle dissolution apparatus (ZRD-8B dissolution tester, Tianda Tianfa Technology Co., Ltd., Tianjing, China) combined with an automatic sampling collector (RCQ-8C, Tianda Tianfa Technology Co., Ltd., Tianjing, China). The samples, which were equivalent to 60 mg QF, were introduced into the dissolution medium. All dissolution testings were carried out in 900 ml of distilled water at 37 ± 0.5 °C with a stirring rate of 100 rpm. Five milliliters of sample was withdrawn at each sampling point (0.5, 1, 2, 4, 6, 8, 12, 16, 20, and 24 h) with the same volume of fresh medium as replacement. QF concentration was assessed at a wavelength of 289 nm with a double beam spectrophotometer (TU-1901, PGeneral, Beijing, China). Each experiment was performed in triplicate. The dissolution data were fitted using DDSolver (version 1.0) software.
Liquid uptake, swelling, and erosion measurement
Mini-matrices were introduced into 900 ml of distilled water at 37 ± 0.5 °C with a stirring rate of 100 rpm. At each sampling point, the mini-matrices were withdrawn from the dissolution medium and weighed after removing excessive water from the surface (Ww) and drying to a constant weight (Wd), respectively. The liquid uptake was calculated using Eq.1 [18].
where Ww and Wd represent the wet and dry weight of the mini-matrices at time t, respectively. Wi represents the initial weight of the mini-matrices, while DRi is the initial weight of drug in the mini-matrices.
The radial swelling and axial swelling of the mini-matrices during dissolution were determined by measuring the diameter and length with an electric vernier caliper (Guanglu, China). The degree of erosion (%Erosion) was determined based on the weight difference between dried mini-matrices at time t and initial mini-matrices, taking into account the drug released at each time point (Eq. 2).
where the meanings of Wd, Wi, and DRi are the same as mentioned above. DRt represents the amount of drug in the mini-matrices at time t.
Results and discussion
In the present work, drug loading was fixed at 30% since burst effect was obvious at higher drug loading.
Thermal stability of QF during HME
Chemical stability of drug during the extrusion process is considered as the most important issue in pharmaceutical HME. Therefore, the chemical stability of QF at high temperature was firstly investigated. As shown in Fig. 1, crystalline QF melted from 171 °C and degraded from 190 °C. Considering the melt viscosity of EC and the desired crystalline state of QF in final extrudates, extrusion was performed between 110 and 130 °C. As shown in Table 1, the formulation, containing 30% QF, 30% PVP VA, and 40% EC, was extruded at 110 °C (named formulation 4), 120 °C (named formulation 7), and 130 °C (named formulation 8), respectively. The normalized contents of QF and related substances were detected by HPLC as 98.85 and 0.40% for formulation 4, 100.97 and 0.46% for formulation 7, 100.83 and 0.38% for formulation 8, respectively. Based on the results, QF was considered to be chemically stable when extruded between 110 and 130 °C.
Physical state and distribution of QF in mini-matrices
Considering the high solubility of QF, crystalline state is preferred for QF in extruded mini-matrices because it will be beneficial to keep good physical stability. Physical state and distribution of QF in the extruded formulations were investigated by using PXRD, DSC, and Raman spectroscopy. The PXRD patterns are shown in Fig. 2. No diffraction peak was observed for EC and PVP VA64 due to their amorphous natures. QF showed the specific peaks at 2θ = 7.4°, 16.7°, 20.0°, and 23.3° in both physical mixtures and extrudates, suggesting that QF existed in its crystalline form in the mini-matrices. DSC results confirmed this conclusion (Fig. 3). Crystalline QF showed a melting peak around 178 °C, while amorphous EC and PVP VA64 did not exhibit any endothermic peaks. The melting point of QF slightly shifted to 174 °C in the physical mixture of QF/EC/PVP VA64. For HME mini-matrices, the melting peak of QF was dramatically widened and moved to a low-temperature range of 155–174 °C. This might be attributed to the fact that the extrusion process reduced the size of QF crystal and facilitated the contact between QF and polymers. Therefore, the melting point depression of QF was more obvious in extrudates than in physical mixture. This phenomenon suggested the possible intermolecular interactions between QF and polymers, which needs further investigation, but is beyond the scope of this work.
The appearance of melting peak and the absence of exothermic recrystallization peak revealed the crystalline form of QF in extrudates. Raman spectra of raw crystalline QF showed strong Raman bands at 1575 and 1030 cm−1 (Fig. 4). These two peaks also can be observed in the spectra of mini-matrices. The specific Raman band of 1575 cm−1 was then selected to acquire Raman map for investigating the QF distribution in mini-matrices. As shown in Fig. 5, red color meant strong scattering intensity and therefore high QF concentration, while blue color corresponded to weak scattering intensity and low QF concentration. It can be observed that QF distributed in the matrix with the size about 5–10 μm. The Raman spectra and maps confirmed the crystalline state of QF in extruded matrices, which was in accordance with PXRD and DSC results.
Release controlling ability of PVP VA64
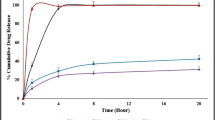
After confirming that QF was chemically stable during extrusion and was kept in crystalline state in extruded mini-matrices, PVP VA64 was investigated for its ability to modify the QF release in comparison of three hydrophilic polymers (L-HPC, xanthan gum, and CPVP). As mentioned above, HPMC was previously reported as the release-modifier for EC-based mini-matrices. But considering its poor thermoplasticity (especially when extruded below the melting point of drug), HPMC was not selected as the release-modifier for comparison in this work. All formulations were extruded at 110 °C (except formulation 1 at 140 °C) with 20% release-modifier and 30% QF. As shown in Fig. 6, EC/QF formulation without release-modifier presented very slow and incomplete release (only 2% in 24 h), attributing to the hydrophobic and non-swellable nature of EC. The addition of 20% L-HPC showed little enhancement in QF release (5% in 24 h), while the formulations containing xanthan gum and CPVP had medium release rate with 25% drug release in 24 h. PVP VA64-related formulations showed the highest drug release of 57% in 24 h. The burst effect was not observed in all formulations. These results indicated that PVP VA64 was the most effective release-modifier for EC-based mini-matrices.
The influence of PVP VA64 content on the QF release was further investigated. As shown in Fig. 7, increasing PVP VA64 content yielded a faster and more complete drug release. The addition of 40% PVP VA64 makes QF completely released from the mini-matrix in 24 h. Raman spectra and SEM images might be helpful to understand the release controlling mechanism of PVP VA64. PVP VA 64 exhibited the specific band at 933 cm−1 in Raman spectra of both raw material and fresh mini-matrices (Fig. 4). The disappearance of this specific band after 24-h dissolution indicated that PVP VA64 firstly dissolved during the dissolution process, which promoted the dissolving of QF distributed in PVP VA64 and created pores within the EC matrix. Those pores further accelerated the release of QF distributed in the EC matrix. SEM images confirmed this hypothesis. Formulation 6 (40% PVP VA64, Fig. 8c) exhibited a smoother surface than formulation 2 (20% PVP VA64, Fig. 8a), benefiting from the low Tg and good processability of PVP VA. After 24-h dissolution, formulation 6 (Fig. 8d) has more pores than formulation 2 (Fig. 8b) because the former has higher content of PVP VA64, which rapidly dissolved and created many pores. Consequently, the release of QF from formulation 6 was faster and more complete than that from formulation 2. Both Raman and SEM results revealed that PVP VA64 favors smooth surface of the extrudates and creates pores during dissolution to accelerate QF release from mini-matrices.
The influence of processing temperature on QF release
The formulations, containing 30% QF, 40% EC, and 30% PVP VA64, were extruded at 110, 120, and 130 °C, respectively. The dissolution results (Fig. 9) indicated that mini-matrices extruded at lower temperature exhibited a faster release profile. For formulation 4 extruded at 110 °C, 83% of QF was released in 24 h. As the processing temperature increased to 130 °C (formulation 8), the accumulative drug release in 24 h decreased to 69%. SEM images indicated that formulation 4 (30% PVP VA64, extruded at 110 °C, Fig. 8e) exhibited a much coarser surface and faster QF release than formulation 8 (Fig. 8f). Therefore, it can be concluded that higher processing temperature tends to give a smoother surface with less pores. It is easy to understand that extrusion near the Tg of EC (131–137 °C) favors more sufficient mixing between components and then lower free volume in comparison with extrusion at lower temperature, resulting in a smaller pore and more tortuous network in the EC matrix during dissolution [19, 20]. Consequently, increasing processing temperature hindered the release of QF (formulation 8 < formulation 7 < formulation 4, shown in Fig. 9).
Swelling study
Water uptake and erosion degree of the mini-matrices were carefully investigated. As shown in Figs. 10 and 11, increasing PVP VA64 contents dramatically enhance the water uptake and the degree of erosion after 24 h dissolution (48 and 28% for 20% PVP VA64, 79 and 63% for 40% PVP VA64, respectively). Increasing processing temperature only had slight enhancement on the water uptake and the degree of erosion after 8 h (67 and 41% for 110 °C, 59 and 40% for 130 °C in 8 h, respectively). The content of release-modifier has more significant influence on the QF release than the processing temperature. Radial swelling rates of some formulations are shown in Fig. 12. Almost all formulations showed low swelling rate due to the non-swellable nature of the EC matrix, except formulation 6. The diameter of formulation 6 remarkably decreased, which should be attributed to the fact that the dissolving of high-content PVP VA64 (40%) created many large pores and resulted in the collapse of the EC matrix.
Drug release mechanism
To explore the release kinetics of QF from mini-matrices, the release data of QF was fitted to three kinetic models, including zero-order release (Eq. 3), first-order release (Eq. 4), and Higuchi model (Eq. 5).
where Qt is the amount of drug released at time t, Q0 is the initial amount of drug, and k0 is the zero-order release constant.
where Q∞ is the total amount of drug in the matrix and k1 is the first-order release constant.
where kH is the release rate constant for the Higuchi model.
The fitting results are listed in Table 2. The best fit was obtained with the Higuchi model, indicating that diffusion was the main kinetics for the drug release.
The transport mechanism was investigated using the semi-empirical Ritger-Peppas model (Eq. 6) [21].
where Qt/Q∞ is the fraction of drug released at time t, k is a constant depending on the structural and geometric characteristics of the tablet, and n is the release exponent to determine the type of release. For a cylinder, n = 0.45 indicates Fickian diffusion, while n = 0.89 reflects zero-order release due to erosion. If n = 0.45–0.89, anomalous transport is suggested [10, 21]. Since the Ritger-Peppas model usually applies to the initial stage of release, the portion of release profile with Qt/Q∞ ≤ 0.6 was used for the determination of n value. The results are listed in Table 2.
Fickian release (diffusion mechanism) only can be seen for formulation 1 (pure EC matrix) and formulation 2 (20% PVP VA64). As the content of PVP VA64 increased, the values of n increased and distributed between 0.41 and 0.61, indicating QF release type tends towards non-Fickian release (a combination of diffusion and erosion mechanisms). Therefore, it is speculated that EC domains diffusion mechanism while PVP VA64 favors erosion mechanism. Therefore, the release mode was determined by the ratio of PVP VA64 to EC.
Conclusion
PVP VA64 was approved to be a novel drug release-modifier for EC-based mini-matrices prepared via hot melt extrusion. QF was selected as the model drug. PVP VA64 has the highest ability to accelerate QF release from EC in comparison with xanthan gum, CPVP, and L-HPC (three extensively used release-modifier). The addition of 40% PVP VA64 dramatically enhanced QF release from 4 to 100% in 24 h. Increasing processing temperature slightly slowed down the QF release due to the improved mixing and the decreased pore size. The release kinetics of QF/EC/PVP VA64 mini-matrices showed good fit with the Ritger-Peppas model. The increase of PVP VA64 in the formulation enhanced the erosion mechanism. EC/PVP VA64 was a novel polymer blend system for sustained release prepared via HME.
References
Yang Y, Shen L, Li J, Shan WG. Preparation and evaluation of metoprolol tartrate sustained-release pellets using hot melt extrusion combined with hot melt coating. Drug Dev Ind Pharm. 2017;43:939–46.
Verstraete G, Van Renterghem J, Van Bockstal PJ, Kasmi S, De Geest BG, De Beer T, et al. Hydrophilic thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Int J Pharm. 2016;506:214–21.
Albarahmieh E, Qi S, Craig DQM. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: the inclusion of hydrophilic additives to develop moisture-activated release systems. Int J Pharm. 2016;514:270–81.
Palem CR, Dudhipala NR, Battu SK, Repka MA, Yamsani MR. Development, optimization and in vivo characterization of domperidone-controlled release hot-melt-extruded films for buccal delivery. Drug Dev Ind Pharm. 2016;42:473–84.
Cosse A, Konig C, Lamprecht A, Wagner KG. Hot melt extrusion for sustained protein release: matrix erosion and in vitro release of PLGA-based implants. AAPS PharmSciTech. 2017;18:15–26.
Islam MT, Maniruzzaman M, Halsey SA, Chowdhry BZ, Douroumis D. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug Deliv Transl Res. 2014;4:377–87.
Verhoeven E, De Beer TR, Schacht E, Van den Mooter G, Remon JP, Vervaet C. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: in vitro and in vivo evaluations. Eur J Pharm Biopharm. 2009;72:463–70.
Verhoeven E, De Beer TR, Van den Mooter G, Remon JP, Vervaet C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. Eur J Pharm Biopharm. 2008;69:312–9.
Verhoeven E, Vervaet C, Remon JP. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2006;63:320–30.
De Brabander C, Vervaet C, Remon JP. Development and evaluation of sustained release mini-matrices prepared via hot melt extrusion. J Control Release. 2003;89:235–47.
Douroumis D. Hot-melt extrusion: pharmaceutical applications. United Kingdom: John Wiley & Sons; 1st edition. 2012.
Quinten T, Gonnissen Y, Adriaens E, De Beer T, Cnudde V, Masschaele B, et al. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur J Pharm Sci. 2009;37:207–16.
Williams RO III, Watts AB, Miller DA. Formulating poorly water soluble drugs. 1st ed. New York: Springer; 2012.
Thiry J, Lebrun P, Vinassa C, Adam M, Netchacovitch L, Ziemons E, et al. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: preformulation, optimization and design space determination. Int J Pharm. 2016;515:114–24.
Ashour EA. Majumdar S, Alsheteli A, Alshehri S, Alsulays B, Feng X, Gryczke Andreas, Kolter, K, Langley N, Repka MA. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. J Pharm Pharmacol. 2016;68:989–98.
Haser A, Huang SY, Listro T, White D, Zhang F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int J Pharm. 2017;524:55–64.
Kolter K, Karl M, Gryczke A. Hot-melt extrusion with BASF pharma polymers: extrusion compendium. BASF company. 2nd revised and enlarged edition. 2012.
Liu JP, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot melt extrusion. Eur J Pharm Biopharm. 2001;52:181–90.
Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004;269:509–22.
Zhang YE, Tchao R, Schwartz JB. Effect of processing methods and heat treatment on the formation of wax matrix tablets for sustained drug release. Pharm Dev Technol. 2001;6:131–44.
Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42.
Funding
The present work was financially supported by the National Sciences Funding of China (No. 51103184), Fundamental Research Funds for the Central Universities (No. 12ykpy08), and Medical Scientific Research Foundation of Guangdong Province (No. A2015169).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, Y., Lu, M. & Wu, C. PVP VA64 as a novel release-modifier for sustained-release mini-matrices prepared via hot melt extrusion. Drug Deliv. and Transl. Res. 8, 1670–1678 (2018). https://doi.org/10.1007/s13346-017-0437-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0437-9