Abstract
Context
Abdominal obesity (AO) is a definitive link between cardiometabolic complications and metabolic syndrome (MetS). Many traditional and novel anthropometric indices have been identified to determine AO, and their relationship to MetS has been investigated. However, whether these indices are useful in a clinical setting is unknown. Moreover, the cut-off points for these indices to determine MetS have yet to be defined among Southern-Indian adults.
Aims
We aimed to evaluate the cut-off values and clinical efficacy of novel anthropometric indices in identifying MetS and its components.
Materials and methods
Subjects (n = 202) were recruited and then grouped into cases (MetS = 106) and controls (healthy = 96). We measured anthropometric data and assayed glycemic and lipid profiles. Using these, we computed a-body shape index (ABSI), abdominal volume index (AVI), body adiposity index (BAI), body roundness index (BRI), conicity index (CI), lipid-accumulation product (LAP), visceral adiposity index (VAI) and waist-triglyceride index (WTI) from published equations.
Results
Compared to the control group, all the novel anthropometric indices were noticeably higher in both male and female subjects of the MetS group. The area under the curve values (AUCs) demonstrated that BRI, CI, AVI, and WTI had superior detection power in identifying MetS, and the AUCs varied upon stratification by gender. BRI was strongly associated with the highest odds of having MetS (OR 66.03).
Conclusions
The optimal cut-off and AUC values attained for BRI, CI, AVI, and WTI have a clinical approach in identifying MetS and its components. The efficacy of these indices to identify MetS differed by gender.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While cardiovascular disease (CVD) consequences have substantially improved with medical advancements, CVD contributes to most deaths in recent decades [1]. Metabolic syndrome (MetS) is an emerging polymorphic entity of life-threatening risk factors that double the occurrence of developing CVD and type 2 diabetes (T2DM) [2]. The individual components of MetS include hyperglycemia, hypertension, dyslipidemia, and central obesity, interrelated and have common mechanisms and pathways [3, 4]. MetS is a significant community health concern, and it is worrying that both developed and developing countries are experiencing a high and increasing prevalence of MetS [5, 6]. According to the evidence, approximately one-fourth of the world’s population can be classified as having MetS [7]. The prevalence of MetS in developed countries in the world was estimated to be 7.9–39%, while in India, it was ranged from 9.2 to 43.2%, as reported in these studies [8,9,10]. In South India, a study conducted on the rural adult population of Puducherry showed the prevalence of MetS had been reported to be 39.7% [11].
Abdominal (visceral) obesity (AO) is the most predominant component of MetS and serves as a dysfunctional adipose tissue marker. AO is connected with systemic inflammation, dyslipidemia, insulin resistance, and CVD [12]. The MRI-measured abdominal visceral fat region is still believed to be the best index for assessing the degree of abdominal obesity. However, abdominal fat screening is not feasible in the clinical setting due to cost, safety, and methodological complexity [13]. Thus, attention has been given to body mass index (BMI) to measure abdominal obesity [14]. It has been stated that a unit rise in BMI increases the odds of having CVD risk [15]. However, BMI's clinical utility was challenged by its inability to discriminate between fat and lean mass and failure to report the fat distribution [16]. Later on, studies focused on waist circumference (WC) to overcome the BMI limitations. WC's critical drawback is that the subjects' weight and height are not considered, and obesity status in the short or tall individuals may be underestimated or overestimated [17]. Thus, the focus has been shifted toward the ratios involving waist, hip, and height measurements to resolve the WC and BMI limitations. Still, evaluating hip circumference (HC) and height besides waist circumference (WC) has no added value. Therefore, it should be beneficial to consider body fat and lipid parameters as an add-on to height, weight, WC, and HC in formulating novel anthropometric indices to overcome the above-discussed limitations.
Several efforts have been made to find more non-invasive and straightforward novel anthropometric indices that can greatly assist in the early diagnosis of MetS. A body-shape index (ABSI), body roundness index (BRI), visceral adiposity index (VAI), body adiposity index (BAI), conicity index (CI), lipid-accumulation product (LAP), abdominal volume index (AVI), and waist-triglyceride (TG) index (WTI) were computed using anthropometric and lipid profiles [18,19,20,21]. A higher value of these novel anthropometric indices would indicate abdominal adipose tissue dysfunction. However, the study findings evaluating the diagnostic accuracy of novel anthropometric measures in identifying MetS are not precise. Furthermore, the cut-off values for these novel anthropometric measures are not unified across different populations. For example, Asians with shorter height may be more predisposed to visceral fat than Caucasians, and thus they have a greater chance of cardiometabolic risk at lower BMI [21]. Therefore, this study investigated the cut-off values and clinical efficacy of novel anthropometric indices in identifying MetS among Southern-Indians.
Materials and methods
Study design and grouping of participants
This cross-sectional study was conducted in the Department of Physiology in collaboration with Endocrinology and Biochemistry departments at a tertiary care teaching hospital, Puducherry, India. Two hundred and two subjects with an age range between 18 and 65 took part in this study and signed written informed consent to express their willingness. The Institutional Ethics Committee (Human studies) allowed this research. After initial screening, 106 subjects with MetS meeting the inclusion criteria were identified and recruited from the Endocrine outpatient department by the approved protocol and guidelines. MetS was identified with any three of the following five conditions based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines [3, 4]. Abdominal obesity (raised waistline); hyperglycemia; elevated serum triglyceride; reduced HDL-cholesterol; elevated blood pressure ≥ 130/85 mmHg. Ninety-six healthy subjects volunteered in the study as a control group after matching for age and gender.
Sample size calculation
Using OpenEpi, version 3, an online calculator, the sample size has been estimated to observe the difference of (1.2) between two independent means of body roundness index (BRI) (3.5 ± 1.0 vs. 4.7 ± 1.3) with a power of 80% and an alpha error of 0.05 [22]. Therefore, a sample size of 40, with 20 subjects in each group, was recommended. To obtain additional information and reliable results, we have recruited 202 participants, with 106 subjects in the MetS group and 96 subjects in the control group.
Brief procedures
Subjects recruited were instructed to abstain from food for 8–12 h before their assigned date of visit to the obesity research lab.
Assessment of traditional kinanthropometric profile, blood pressure, and body composition
According to a standardized protocol described by the World Health Organization (WHO) [23], the subject’s height (in centimeters) and weight (in kilograms) were assessed with a height scale and weight machine. The body mass index (BMI) was computed using the formula weight in kg divided by height in m2. We used a non-stretchable measuring tape to measure waist circumference (WC) at the narrowest point around the abdomen between the lower part of the last rib and the top of the hip at the end of expiration. Hip circumference (HC) was taken at the widest part of the hips. The ratio of waist-to-hip (WHR) and waist-to-height (WHtR) were calculated. Using Omron automated apparatus, blood pressure (BP) was measured three times on both the arms of the subjects after 5–10 min of rest in a sitting position with a 1-min interval between the recordings. Body fat and the lean percentage were determined via a body composition analyzer (QuadScan 4000).
Assessment of biochemical profile
We assayed glucose and lipid profiles (total cholesterol, triglycerides, high-density lipoprotein) using commercially available kits and an auto-analyzer. Friedewald’s equation was used for the indirect estimation of other lipoproteins [24]. Atherogenic index (AI) was calculated using the formula AI = log10(triglyceride (TG)/high-density lipoprotein (HDL)). Insulin levels were quantified using a commercially available Calbiotech enzyme-linked immunosorbent assay (ELISA) kit. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting glucose (mMol) × insulin (μIU/L)/22.5.
Novel anthropometric measures
Based on WC, BMI, TG, and HDL-C, we calculated the following novel anthropometric indices. VAI and LAP are gender-specific [25, 26].
In the VAI equation, TG and HDL-C are expressed in mmol/L.
LAP in male = (WC (cm) − 65) × TG (mmol/L).
LAP in female = (WC (cm) − 58) × TG (mmol/L).
WTI [27] = WC (cm) × TG (mmol/L).
Based on height, weight, WC, and HC, we calculated the following novel anthropometric indices.
In the ABSI equation [28], WC and height are expressed in meters.
In the BRI equation [21], WC and height are expressed in meters.
\( {\text{AVI }}\, = \,\frac{{2{ } \times { }\left( {{\text{WC}}} \right)^{2} { } + { }0.7{ } \times { }\left( {{\text{WC}}{-}{\text{ HC}}} \right)^{2} }}{1000}.\)
In the AVI equation [29], WC and HC are expressed in centimeters.
CI [30] = \(\large \frac{{{\text{WC }}\left( {\text{m}} \right)}}{{\left( {0.109{ } \times \sqrt {\frac{{\text{Weight in kgs}}}{{\text{Height in m}}}} } \right)}}\)
BAI [31] = \(\large \left( {\frac{{{\text{HC }}\left( {{\text{cm}}} \right)}}{{{\text{ height }}\left( {\text{m}} \right)^{1.5} }}} \right) - 18\)
Statistical analysis
Statistical software Statistical Package of Social Science (SPSS) Windows version 20.00 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. Kolmogorov–Smirnov test was used to assess the normality of the data. We used mean ± standard deviation (SD) for normal distribution and median with an interquartile range for non-normal distribution for continuous data. The Student’s independent t test (two-tailed) (parametric) and Mann–Whitney U test (non-parametric) were used to compare the data between the groups. We assessed the dichotomous data (categorical) among the groups with the chi-square test (χ2). The area under the curve (AUC) in a receiver operating characteristic (ROC) curve analysis was performed using MedCalc 11.4.2.0 (Ostende, Belgium) to investigate the accuracy of novel anthropometric measurements in identifying MetS [32, 33]. DeLong-Clarke-Pearson’s nonparametric approach was used to compare the AUCs of indices. Logistic regression models adjusted for age, sex, BMI, smoking, and alcohol history were applied to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for novel anthropometric indices for having MetS and its components. The lowest quartiles were set as reference. A p value of less than 0.05 was assumed to indicate a statistically significant difference.
Results
Table 1 shows the basal demographic and traditional anthropometric data in healthy and MetS subjects stratified by gender. There were no statistically significant differences in age, smoking, alcohol intake, and familial history of metabolic disorders (HTN, T2D, and CVD) between the control and MetS groups in both male and female subjects (Table 1). Men smoked and consumed alcohol more often than women in both the control and MetS groups. Except for increased waist, the prevalence of other MetS components (hyperglycemia, high TG, low HDL-C, and raised BP) were significantly higher in male MetS subjects than controls. In contrast, female MetS subjects showed a higher prevalence of all the MetS components. In the MetS group, hyperglycemia has predominant distribution in both male and female subjects. However, the incidence of high TG, low HDL-C, and raised BP were higher in male MetS subjects than in females. All the traditional anthropometric parameters except height, including weight, BMI, WC, HC, WHR, and WHtR, were significantly higher (p < 0.05) in the MetS group than the control group in both genders. Body fat percentage values attained are significantly higher, while the lean fat percentage was pointedly lesser among the male and female subjects in the MetS group.
We perceived that significantly higher values of FPG, insulin and HOMA-IR in both male and female subjects of the MetS group might indicate an impaired glycemic state. All the lipid profile parameters (TC, TG, LDL-C, VLDL), including lipid ratios (LDL/HDL, TG/HDL, TC/HDL, Non-HDL/HDL, AIP), were significantly higher. In contrast, HDL-C levels were significantly lower in both male and female subjects of the MetS group compared to the control group (Table 2). Based on the estimated cardiovascular parameters, both male and female subjects in the MetS group exhibited pre-hypertension status in the form of a significantly increased basal heart rate (BHR), systolic blood pressure (SBP), and mean arterial pressure (MAP) (Table 2). Compared to the control group, all the novel anthropometric indices (ABSI, AVI, BAI, BRI, CI, LAP, VAI, and WTI) were significantly higher (p < 0.05) in both men and women of the MetS group (Table 3). Men and women in MetS are also differed with respect to mean ABSI (men 0.0846 vs. women 0.0858), mean AVI (men 18.80 vs. women 19.90), mean BAI (men 28.47 vs. women 38.07), mean BRI (men 5.07 vs. women 6.19), mean LAP (me: 47.42 vs. women 45.39), mean VAI (men 5.40 vs. women 4.10) and mean WTI (men 160.26 vs. women 148.58) (Table 3). We conducted the main analyses stratified by gender due to these disparities in values between men and women.
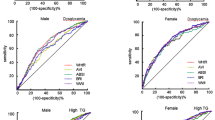
According to the ROC analyses conducted to identify MetS, we derived AUC, optimal cut-off points (using Youden Index), sensitivity, and specificity for MetS risk factors and novel anthropometric indices (Table4). We considered MetS risk factors and novel anthropometric indices with an AUC between 0.7 and 1.0 as foremost predictors for detecting MetS. Among the MetS risk factors, WHtR (AUC 0.816; 95% CI 0.756–0.867), FPG (AUC 0.943; 95% CI 0.902–0.971), TG/HDL (AUC 0.738; 95% CI 0.672–0.797), and AIP (AUC 0.715; 95% CI 0.647–0.776) showed better diagnostic accuracy in identifying MetS. The optimal cut-offs for identifying MetS was determined for WHtR (> 0.58), FPG (> 105), TG/HDL (> 3.2), and AIP (> 0.5). We observed BMI, MAP, and body fat% be the least significant predictors with the lowest AUC in ROC analysis (Table 4). Of the eight novel anthropometric indices examined, BRI (AUC 0.805; 95% CI 0.744–0.857), CI (AUC 0.782; 95% CI 0.718–0.837), AVI (AUC 0.771; 95% CI 0.707–0.827), and WTI (AUC 0.732; 95% CI 0.664–0.792) showed better diagnostic accuracy for detecting MetS. The optimal cut-offs for identifying MetS was determined for BRI (> 4.76), CI (> 1.06), AVI (> 17.3), and WTI (> 129.65). We observed ABSI, BAI, LAP, and VAI as the least significant predictors with the lowest AUC in ROC analysis. Compared with the AUC of WHtR, non-significant differences were found with TG/HDL, AVI, BRI, and CI. In comparison with the AUC of BRI, non-significant differences were found with TG/HDL, AVI, and CI. Thus, WHtR as a traditional anthropometric measure performs well in screening MetS. BRI, AVI, and CI were performed similarly to WHtR in identifying MetS (p > 0.05 vs. WHtR).
Table 5 shows the AUC, optimal cut-off points (using Youden Index) for MetS risk factors, and novel anthropometric indices in ROC analysis to identify MetS stratified by gender. Among men, FPG, WHtR, WHR, TG/HDL, AIP, and fat percent exhibited stronger predictive ability for MetS than other metabolic risk factors, and their AUCs were 0.921, 0.804, 0.753, 0.751, 0.723, and 0.711, respectively. Similarly, BRI, CI, VAI, AVI, and WTI displayed the best predictive ability for MetS in men, with AUCs of 0.801, 0.770, 0.760, 0.721, and 0.701, respectively. Among women, FPG, WHtR, HOMA-IR, TG/HDL, fat percent, AIP, and WC had a better predictive ability for MetS than other metabolic risk factors, and their AUCs were 0.961, 0.837, 0.765, 0.718, 0.707, 0.701, and 0.700, respectively. Similarly, BRI, AVI, BAI, CI, and WTI, displayed the best predictive ability for MetS in women, with AUCs of 0.850, 0.813, 0.803, 0.797, and 0.768, respectively. Thus, whether men or women, BRI was the strongest predictor of MetS with a cut-off value varied between > 4.13 in men and > 5.21 in women. Apart from BRI, AVI, CI, and WTI, VAI in men and BAI in women displayed a better predictive ability for MetS when stratified by gender. However, the lowest AUC in both men and women belonged to BMI and ABSI. Therefore, the cutoff points identified for BMI and ABSI in men and women did not have an acceptable AUC to detect MetS.
Table 6 shows the AUCs (95% CI) for novel anthropometric indices to identify MetS components stratified by gender. In both men and women, the AUCs of all the novel anthropometric indices significantly identified central obesity. AVI, CI, and BRI exhibited the highest AUC for incident central obesity in both genders (0.955, 0.897, 0.875 in men, and 0.952, 0.948, 0.926 in women, respectively). Although none of the indices had an AUC greater than 0.7, the BRI, CI, VAI, and WTI showed significant AUCs for detecting hyperglycemia in men. However, all the indices showed significant AUCs for detecting hyperglycemia in women. Moreover, all of the indices had AUCs greater than 0.7. WTI, VAI, and LAP had the highest AUCs for identifying high TG in both genders, while only WTI and VAI had shown an excellent predicting ability for low TG. None of the novel anthropometric indices could discriminate among men with raised BP. However, in women, the AUCs of WTI, VAI, and LAP were significant in identifying raised BP. ABSI and BAI were revealed to be the least significant predictors for hyperglycemia, high TG, low HDL-C, and raised BP. Moreover, none of the indices have identified all MetS components in men, whereas WTI has identified all the MetS components in women.
Binary logistic regression analysis was performed for MetS, abdominal obesity, hyperglycemia, high TG, low HDL-C, and raised BP as dependent variables. The quartiles of novel anthropometric indices served as independent variables, with the first quartile serving as the reference group (Table 7). The odds ratios for MetS and its components grew along with quartiles for all the novel anthropometric indices. The highest quartile subjects showed the highest risk for developing MetS and its components compared to those in the lower quartile. Among the novel anthropometric indices studied, the subjects in the fourth quartile of BRI showed the highest odds ratios for the occurrence of MetS (OR 66.03; 95% CI 18.01–242.1), central obesity (OR 86.60; 95% CI 24.30–323.8), hyperglycemia (OR 9.31; 95% CI 3.69–23.48) and raised BP (OR 4.13; 95% CI 1.77–9.6). Besides BRI, the subjects in the fourth quartile of CI, AVI, and WTI showed increased odds of developing MetS, central obesity, and hyperglycemia. Moreover, the odds of developing high TG, low HDL-C, and raised BP were observed for the subjects in the fourth quartile of WTI, VAI, and LAP.
Discussion
The current study findings represent the predictive ability (AUCs) of novel anthropometric indices in identifying MetS. Despite being good adiposity predictors to overcome BMI limitations, this study found that BRI, CI, AVI, and WTI have a higher predictive capacity to discriminate MetS cases from controls. Besides BRI, AVI, CI, and WTI, VAI in men and BAI in women displayed a better predictive ability for MetS. In addition, we attained various cut-off values ideal for the anthropometric indices to detect MetS that were inconsistent across studies. These differences could be due to the study population, sample size, and diagnostic criteria for MetS.
Krakauer et al. introduced ABSI in 2007 based on the normalization of WC to BMI and height to measure the health of body shape independently of body size [28]. ABSI's major strength is that it incorporates data from WC, height, and weight. However, a higher value of ABSI would indicate that WC is greater than usual for a specified height and weight, with more deposition of abdominal fat contributing to abdominal obesity, which results in inflammation, insulin resistance, and skeletal muscle loss. In addition, ABSI predicts incident CVD and mortality independently from BMI [34]. In contrast, our study results reveal that ABSI has low predictive power (AUC 0.629; 95% CI 0.559–0.696) with a cut-off value of > 0.08. Our study also showed the AUC, and Youden J-index values for ABSI were the lowest among the novel indices in both men and women to detect MetS. Therefore, based on our results, ABSI could not be a good and reliable indicator for MetS. Moreover, we observed higher ABSI values than those in Krakauer’s investigation (0.0846 ± 0.0070 in males and 0.0858 ± 0.0071 in female MetS subjects vs. 0.0808 ± 0.0053) indicates higher visceral adipose tissue and lower muscular tissue among Southern-Indian adults. We presume that ethnic, racial, and lifestyle variances and the subject's anthropometric criteria were the primary reasons for the differences in the study findings. Maessen et al. reported similar results in 2014 in Nijmegen, the Netherlands. They found that ABSI could not determine the CVD presence compared to BRI, BMI, and WC [35].
The AVI and CI will be a valuable criterion for detecting metabolic abnormalities and representing the visceral adipose tissue. AVI measures the overall volume and is closely associated with impaired glucose metabolism [29]. In comparison, CI is a health indicator similar to the WHR and has advantages over WHR. It has an in-built adjustment of WC for height and weight and does not require HC to measure fat distribution [30]. In this study, both AVI and CI showed a better predictive power (AUC 0.782; 95% CI 0.718–0.837 and AUC 0.771; 95% CI 0.707–0.827 respectively) to identify MetS. Upon stratification, we observed better performance of AVI and CI to detect MetS among female subjects. These findings further highlight the differences in the waist and hip circumferences among the male and female subjects and the resultant effect of the differences in the general body fat distribution attributable to the differences in the relative performances of AVI and CI between men and women. Despite showing a better predictive power, both AVI and CI were not standing superior to BRI in our study. While the height is used in the CI formula, the higher height of men will be minimized by the higher weight, explaining CI's inability to predict MetS better than BRI in men. Also, higher AVI values were observed, particularly in women with a larger HC than WC [20]. Both AVI and CI have superior predictive ability to identify central obesity (Table 6). Moreover, the subjects in the fourth quartile of AVI and CI have a considerable odds ratio (OR) for MetS (OR 18.20 and 39.01), central obesity (OR 24.77 and 58.30), hyperglycemia (OR 5.95 and 8.07), high TG (OR 5.27 and 4.41) and low HDL-C (OR 3.78 and 3.07). Wang et al. published similar findings in 2017 and observed superior predictive ability of BMI and AVI to classify MetS in Chinese adults than other anthropometric indices [36]. However, in the same study, Wang et al. found that CI presented a prognostic function only for newly diagnosed hypertensive patients, suggesting its inferior predictive potential for MetS components. These variations in the results are due to the differences in diagnostic guidelines and anthropometric attributes among the study population.
Thomas et al. [21] introduced BRI in 2013 to estimate body fat and visceral adipose tissue (VAT) using WC to height, allowing the human body's shape to be measured as an ellipse or oval. BRI values range from 1 to 16, so there are higher values for rounder individuals, whereas lean individuals have values close to one. Maessen et al. [35] found that CVD and CVD risk factors could be identified well by BRI and have a higher adjusted odds ratio (OR). In agreement with Maessen et al., our data revealed that BRI could recognize MetS and showed superior predictive power over the other novel anthropometric indices (AUC 0.805; 95% CI 0.744 to 0.857) with a cut-off value of > 4.76. Moreover, we found non-significant differences between BRI and WHtR, implying that BRI could identify MetS similarly to WHtR. In addition, women had notably higher AUC and cut-off value of BRI to identify MetS (AUC 0.850; 95% CI 0.765–0.913; cut-off > 5.21) than men (AUC 0.801; 95% CI 0.710–0.873; cut-off > 4.13). BRI showed the superior predictive ability to detect hyperglycemia in both genders. The BRI’s ability to detect other MetS components, such as central obesity, high TG, low HDL-C, and raised BP, was equivalent to but not superior to those of the other novel anthropometric indices. In this study, we found that the median BRI values were higher than those of Thomas et al. [21] (5.07 (4.65–6.15) in men and 6.19 (5.40–7.54) in women with MetS vs. 4.6 ± 1.3). According to Thomas et al. [21], BRI values can reflect VAT and body fat. Thus, the higher values for BRI in our study would indicate an elevated VAT and body fat in our study participants. Furthermore, among the studied novel anthropometric indices, the subjects in the fourth quartile of BRI had the highest odds ratios for MetS (OR 66.03; 95% CI 18.01–242.1), central obesity (OR 86.60; 95% CI 24.30–323.8), hyperglycemia (OR 9.31; 95% CI 3.69–23.48) and raised BP (OR 4.13; 95% CI 1.77–9.6). Thus, BRI can be considered the best novel anthropometric measure for identifying MetS and its components among Southern-Indians.
In 2011, Bergman et al. proposed BAI as an alternative approach to measure adiposity and resolve BMI deficiencies [31]. They conclude that BAI can be an obesity predictor and represents the body fat percentage (BF%). Dual-energy X-ray absorption (DEXA) measured body fat (r = 0.85, p 0.001) showed a significantly higher association with BAI than BMI. The application of HC in BAI formulae captures gender differences in adiposity better than the BMI, which may be a critical conceptual benefit of the BAI over BMI. However, Lopez et al. reported that BAI is not meeting the BMI constraints since the only advantage of using BAI over BMI is that the formulae do not require weight [37]. In another study, Shin et al. reported that BAI in Korean adults showed less utility in the diagnosis of MetS than BMI and WHtR [38]. Similarly, we found that BAI has lower predictive power (AUC 0.669; 95% CI 0.600–0.733) in identifying MetS. However, in females, BAI exhibited good predictive ability and stood close to BRI and AVI. This is expected because males tend to have lower adiposity compared to females. This is also reflected in a lower value of mean BAI for men (28.47 (25.84–30.39) in men vs. 38.07 (34.79–41.17) in women). Thus, gender differences, adiposity levels, and weight status could be the reasons for BAI's weak predictive ability, as females are shorter than males and have more BF%. LAP is an index created based on WC and TG, first proposed in 2005 by Kahn et al. to quantify lipid over-accumulation and differentiate between visceral and subcutaneous adiposity. Several studies reported LAP’s accurate predictive value in identifying MetS, T2DM, and CVD risk [18, 39]. In T2DM patients, LAP was correlated with glucose intolerance, lipid peroxidation, and systemic inflammation [40]. The higher LAP values would demonstrate a greater degree of lipid deposition in the body. Conversely, in our study, despite a substantial difference between the men and women in the MetS and control groups, LAP had lower predictive accuracy than BRI, AVI, CI, and WTI in detecting MetS (AUC 0.670; 95% CI 0.601–0.735). In particular, LAP exhibited a markedly lower performance among women and men. However, LAP’s performance in detecting high TG was not affected among both genders. This was further reflected as an association of LAP with an increased odds of developing high TG. The primary reason for LAP’s weak predictive power might be the subject's criteria toward MetS, as many of them have regular TG and HDL-C levels. Hence, multiple metabolic abnormalities are needed to produce dyslipidemia in these subjects.
Another recently introduced index for assessing visceral fat and adipose tissue dysfunction is the VAI, dependent on WC, BMI, TG, and HDL-C [41]. Previous studies have suggested that VAI could help assess visceral fat dysfunction linked to impaired glucose metabolism and diabetes. VAI has also been reported to have significant advantages over WC for identifying MetS [42, 43]. Our study results were not concordant with the above studies [42, 43] and observed that VAI has the lower predictive accuracy in detecting MetS (AUC 0.683; 95% CI 0.614–0.747). Even though VAI is calculated using gender-specific equations, our data showed that the cut-offs for both genders (> 4.6 in men vs. > 4.5 in women) were similar, with men having a slightly higher predictive capacity. Among the MetS components, high TG was better identified by VAI in both genders. Moreover, the subjects in the fourth quartile of VAI had increased odds ratios for high TG and low HDL-C. The subject's demographic and anthropometric profiles, especially the recruitment of middle-aged subjects, may be responsible for these findings. Bermudez et al. [44] have also reported considering the age factor when using VAI, as its predictive value seems higher among younger persons. Borruel et al. have published consistent observations and revealed that VAI did not deliver a detailed analysis of visceral adiposity status other than WC and BMI [45]. However, Baveicy et al. reported that VAI has a superior predictive ability than BRI in identifying MetS among adults. Perhaps this is probably the result of more MetS variables in the VAI formula and a high degree association of visceral adiposity with TG and HDL-C [46]. Raised TG and low HDL-C with excessive WC act as an independent risk factor for CVD risk. The WC can roughly estimate the visceral fat accumulation, while TG levels can indirectly reflect LDL-C. This forms the basis for developing WTI, which can predict the outcome of coronary artery disease (CAD) [27]. To support these results, in our study, we found that WTI showed a better predictive power (AUC 0.732; 95% CI 0.664–0.792) to identify MetS than ABSI, BAI, VAI, and LAP, but not demonstrated predictive power as superior as BRI, AVI, and CI. Compared to other novel indices, WTI has shown the highest predictive ability to identify high TG, low HDL-C, and raised BP in women, but only high TG and low HDL-C in men. Furthermore, the subjects in the fourth quartile of WTI showed the highest odds ratios for high TG and low HDL. Similarly, Yang et al. observed a significant positive correlation of WTI with the CAD score and considered WTI a strong predictor of CAD [27].
Our study's primary strength is the detailed interpretation of several novel anthropometric indices, and performing AUC-ROC analysis for diagnostic accuracy is extremely valuable. Our study also proposed optimal cut-off points for these anthropometric indices. There was an apparent difference between the two genders in all of the novel anthropometric indices, implying that gender-specific reference values should be employed in clinical practice. Despite its strengths, this work has a few limitations that must be taken into account. The cross-sectional research strategy used is not ideal for establishing a cause-and-effect relationship. Though the sample size was relatively modest, the numbers were adequate to achieve a reasonable statistical difference. Most of the research on anthropometric indices in metabolic diseases had a similar or even smaller sample size. Thus, a better approach may be conceived for future studies with a larger sample size from a multi-centric population.
In conclusion, we found that novel anthropometric measures, especially BRI, CI, AVI, and WTI, have significantly higher predictive capacity to identify MetS and its components than others. WHtR, a traditional anthropometric measure, offers the best performance in screening MetS among the MetS risk factors. BRI, AVI, and CI performed similarly to WHtR in identifying MetS. Upon stratification by gender, VAI in men and BAI in women displayed a better predictive ability for MetS in addition to BRI, AVI, CI, and WTI. Among traditional and novel anthropometric indices, BMI and ABSI exhibited the weakest predictive power to identify MetS in both genders. We also emphasized the clinical efficacy of the novel anthropometric indices in determining MetS by employing specific cut-off values. The optimal cut-off points for the novel anthropometric indices obtained in the current study were comparable to international cut-offs. Amongst the indices studied, BRI has the highest odds ratios for the occurrence of MetS, central obesity, hyperglycemia, and raised BP. WTI, on the other hand, has the highest odds ratios for the occurrence of high TG and low HDL-C. Therefore, this study suggests that considering BRI, CI, AVI, and WTI besides WHtR could help identify MetS and its components among Southern-Indian adults.
References
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177-232.
Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–78.
Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–3.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–36.
Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones. 2018;17(3):299–313.
Moreira GC, Cipullo JP, Ciorlia LA, Cesarino CB, Vilela-Martin JF. Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0105056.
Saklayen MG. The global epidemic of metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
Bhowmik B, Afsana F, Siddiquee T, Munir SB, Sheikh F, Wright E, et al. Comparison of the prevalence of metabolic syndrome and its association with diabetes and cardiovascular disease in the rural population of Bangladesh using the modified National Cholesterol Education Program Expert Panel Adult Treatment Panel III and International Diabetes Federation definitions. J Diabetes Investig. 2015;6(3):280–8.
Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATP III and IDF definitions in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-34). Diabetes Metab Res Rev. 2007;23(2):127–34.
Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: a community study from urban Eastern India. J Cardiovasc Dis Res. 2012;3(3):204.
Venugopal V, Dongre AR, Saravanan S. Prevalence and determinants of metabolic syndrome among the rural adult population of Puducherry. Indian J Community Med. 2019;44(1):21–5. https://doi.org/10.4103/ijcm.IJCM_132_18.
Shin J-A, Lee J-H, Lim S-Y, Ha H-S, Kwon H-S, Park Y-M, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4(4):334–43.
Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28(6):1039–49.
Cai L, Lubitz J, Flegal KM, Pamuk ER. The predicted effects of chronic obesity in middle age on medicare costs and mortality. Med Care. 2010;1:510–7.
World Health Organization. Obesity: preventing and managing the global epidemic. World Health Org Tech Rep Ser. 2000;894:1–253.
Kang SM, Yoon JW, Ahn HY, Kim SY, Lee KH, Shin H, Choi SH, Park KS, Jang HC, Lim S. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One. 2011. https://doi.org/10.1371/journal.pone.0027694.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–86.
Ma CM, Lu N, Wang R, Liu XL, Lu Q, Yin FZ. Three novel obese indicators perform better in monitoring the management of metabolic syndrome in type 2 diabetes. Sci Rep. 2017;7:9843.
Costa EC, Sa JC, Soares EM, Lemos TM, Maranhao TM, Azevedo GD. Anthropometric indices of central obesity how discriminators of metabolic syndrome in Brazilian women with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28:12–5.
Motamed N, Sohrabi M, Poustchi H, Maadi M, Malek M, Keyvani H, et al. The six obesity indices, which one is more compatible with metabolic syndrome? A population-based study. Diabetes Metab Syndr. 2017;11(3):173–7.
Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–71.
Liu B, Liu B, Wu G, Yin F. Relationship between body-roundness index and metabolic syndrome in type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther. 2019;12:931–5.
WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H, Tsuji C, Ishiwata K, Eguchi Y, Nakazawa H, Tanaka E. Validation of the friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2008;43(1):1–5.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151–3.
Yang RF, Liu XY, Lin Z, Zhang G. Correlation study on waist circumference-triglyceride (WT) index and coronary artery scores in patients with coronary heart disease. Eur Rev Med Pharmacol Sci. 2015;19(1):113–8.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7):e39504.
Guerrero-Romero F, Rodrı́guez-Morán F. Abdominal volume index. An anthropometry-based index for the estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res. 2003;34(5):428–32.
Farzad S, Fatemeh A, Salehi M, Nojomi M. Association of waist circumference, body mass index and conicity index with cardiovascular risk factors in postmenopausal women. Cardiovasc J Afr. 2012;23(8):442.
Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, Xiang AH, Watanabe RM. A better index of body adiposity. Obesity. 2011;19(5):1083–9.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a non-parametric approach. Biometrics. 1988;1:837–45.
Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50(3):419–30.
Krakauer NY, Krakauer JC. Dynamic association of mortality hazard with body shape. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0088793.
Maessen MF, Eijsvogels TM, Verheggen RJ, Hopman MT, Verbeek AL, de Vegt F. Entering a new era of body indices: the feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0107212.
Wang H, Liu A, Zhao T, Gong X, Pang T, Zhou Y, Xiao Y, Yan Y, Fan C, Teng W, Lai Y. Comparison of anthropometric indices for predicting the risk of metabolic syndrome and its components in Chinese adults: a prospective, longitudinal study. BMJ Open. 2017. https://doi.org/10.1136/bmjopen-2017-016062.
López AA, Cespedes ML, Vicente T, Tomas M, Bennasar-Veny M, Tauler P, Aguilo A. Body adiposity index utilization in a Spanish Mediterranean population: comparison with the body mass index. PLoS One. 2012;7(4):e35281.
Shin KA, Hong SB, Shin KS. Body adiposity index and metabolic syndrome risk factors in Korean adults: a comparison with bodymass index and other parameters. Biomed Sci Lett. 2017;23:57–63.
Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5(1):26.
Mirmiran P, Bahadoran Z, Azizi F. Lipid accumulation product is associated with insulin resistance, lipid peroxidation, and systemic inflammation in type 2 diabetic patients. Endocrinol Metab. 2014;29(4):443–9.
Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Wang Y, He S, He J, Wang S, Liu K, Chen X. Predictive value of visceral adiposity index for type 2 diabetes mellitus. Herz. 2015;40(3):277–81.
Chen HY, Chiu YL, Chuang YF, Hsu SP, Pai MF, Yang JY, Peng YS. Visceral adiposity index and risks of cardiovascular events and mortality in prevalent hemodialysis patients. Cardiovasc Diabetol. 2014;13(1):136.
Bermúdez VJ, Salazar J, Añez R, Rivas-Ríos JR, Chávez-Castillo M, Torres W, Núñez V, Mejías J, Wilches-Durán S, Cerda M, Graterol M. Optimal cut-off for visceral adiposity index in a Venezuelan population: results from the Maracaibo City Metabolic Syndrome Prevalence Study. Rev Argent Endocrinol Metab. 2017;54(4):176–83.
Borruel S, Molto JF, Alpanes M, Fernandez-Duran E, Alvarez-Blasco F, Luque-Ramirez M, Escobar-Morreale HF. Surrogate markers of visceral adiposity in young adults: waist circumference and body mass index are more accurate than waist-hip ratio, a model of adipose distribution and visceral adiposity index. PLoS One. 2014. https://doi.org/10.1371/journal.pone.0114112.
Baveicy K, Mostafaei S, Darbandi M, Hamzeh B, Najafi F, Pasdar Y. Predicting metabolic syndrome by visceral adiposity index, body roundness index and a body shape index in adults: a cross-sectional study from the Iranian RaNCD cohort data. Diabetes Metab Syndr Obes Targets Ther. 2020;13:879.
Acknowledgements
We sincerely acknowledge the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) for providing us financial assistance in the form of an intramural Ph.D. research grant. Furthermore, we are thankful to all the subjects who participated in this study.
Funding
Funded by Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. Grant ID: JIP/Res/Intramural/Phs-1/2018-19/98.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This study involved 202 human subjects after seeking approval from the JIPMER Institutional Ethics Committee (Human studies). This approval is valid for 3 years, and the project reference is JIP/IEC/2018/0301.
Informed consent
Prior to participation, all participants were given written and oral informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Endukuru, C.K., Gaur, G.S., Dhanalakshmi, Y. et al. Cut-off values and clinical efficacy of body roundness index and other novel anthropometric indices in identifying metabolic syndrome and its components among Southern-Indian adults. Diabetol Int 13, 188–200 (2022). https://doi.org/10.1007/s13340-021-00522-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-021-00522-5