Abstract
Aim
Diabetes patients usually have a low activity level and complain about lack of time. Therefore, we investigated the effect of short time, postprandial moderate-intensity exercise on glucose homeostasis in type 2 diabetes patients.
Methods
Eleven patients with type 2 diabetes were recruited. Patients spent the first day of the study without exercise (non-exercise day; NE day). In the second day, they walked at moderate-intensity (40% of the maximum heart rate reserve) for 15 min, 30 min after each meal (exercise day; E day). Glucose homeostasis was estimated by a continuous glucose monitor (CGM). All meals during the study were of standard composition. We compared NE day and E day concerning 24-h glucose homeostasis and 3 h postprandial glucose levels by the incremental area under the curve (iAUC) method. Medications were not changed during the study.
Results
The number of patients under basal supported oral therapy, intensive insulin therapy and oral hypoglycemic agents (OHA) were 5, 4 and 2, respectively. The blood glucose standard deviation over 24 h and the iAUC for the 24-h glycemic variability (NE day vs. E day; 34,765 [21,424–56,014] vs. 23,205 [15,323–39,779]) were smaller in E day than in NE day.
Conclusion
These results suggest that postprandial moderate-intensity walking, easily performable in daily life activities, was effective for improving glucose homeostasis. Further study should be performed to clarify the relationship between postprandial walk and drug therapy (insulin and OHA), including insulin secretory ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise therapy has long been recognized as an important factor in the treatment of diabetes mellitus [1]. The Japan Diabetes Society carried out a nationwide survey using self-recording questionnaires for out-patients with diabetes [2]. According to the results, exercise therapy had poor compliance because of the lack of physical exercise educators. The percentage of exercise therapy adherence was significantly lower than that of diet therapy. The compliance rate was only 52% and numerous patients complained of “lack of time” to practice exercise [3]. Other studies overseas also demonstrated that patients with type 2 diabetes had low physical activity compared with non-diabetes patients [4, 5].
In general, exercise prescription consists of planning frequency, intensity, time and type (FITT) of exercise. Some studies investigated the effect of exercise time [6,7,8,9,10]. Programs such as moderate intensity exercise for 15-min × 3 bouts [6], 15-min × 4 cycles with three 5-min breaks [7] and single 60-min [8] or high-intensity interval training (HIIT) [9] were investigated. Participants were those at risk of impaired glucose tolerance and hyperglycemia without antidiabetic medications [6], type 2 diabetes without insulin [9], and those with type 1 diabetes [7, 10]. Unfortunately, previous studies involved different exercise components and glycemic conditions that are difficult to be adapted to the clinical practice, for the treatment of diabetes.
Therefore, the purpose of this study was to establish an effective exercise prescription by exploring the effect of postprandial, moderate-intensity walking for 15 min on glucose homeostasis in type 2 diabetes mellitus patients.
Research design and methods
Participants
Thirteen patients (9 males and 4 females) with type 2 diabetes were eligible for this study during their educational hospitalization period at the Ichinomiyanishi Hospital, from July 2014 to July 2015. Considering their controlled diabetes condition, all patients were permitted by their primary physicians to be submitted to an exercise program. Inclusion criterion for this study was the ability to walk for at least 15 min, regardless of the use of walking assistance devices or not. Exclusion criteria were decided according to the Japan Diabetes Society [11]: poor blood glucose control (fasting blood glucose > 250 mg/dl, or positive urine ketone bodies), fresh retinal hemorrhage by proliferative retinopathy, kidney disease (serum creatine ≧ 2.5 mg/dl for males, ≧ 2.0 mg/dl for females), ischemic heart disease or heat failure, bone or joint disorder, acute infection, diabetic gangrene, and advanced diabetes autonomic neuropathy.
Intervention
Before the first intervention day (non-exercise day; NE day), we placed a continuous glucose monitoring device (CGM: iPro2 Medtronic, Dublin, Ireland) on the abdomen of the participants. Once inserted, the CGM recorded interstitial fluid glucose concentration every 5 min. To calibrate the CGM, capillary blood samples for glucose measurement were collected 4 times: before breakfast, lunch, supper, and bedtime, according to the manufacturer’s instructions. The patients had standard meals during the NE day and subsequently.
In the first day (non-exercise day; NE day), the patients were allowed to have their usual daily activity, without any specific exercise. In the second day (exercise day; E day), they walked at moderate-intensity for 15 min, 30 min after each meal. Patients were immediately allowed to rest if the subjective symptoms indicated that the walk needed to be discontinued, and those events were reported to their primary physicians or floor nurses. During the study, treatment with oral hypoglycemic agents and injection drugs (i.e., insulin or glucagon-like peptide-1 receptor agonist) was not changed.
Dietary protocol
Standard meals were identical to those recommended by the primary physician in terms of energy intake and protein, fat, and carbohydrate balance (15%, 25% and 60%, respectively) on both the non-exercise day and exercise day. Patients were provided a standard breakfast at 08:00 AM, lunch at 12:00 PM and supper at 18:00 PM.
Exercise protocol
Moderate-intensity exercise was defined as a load at 40% of the maximum heart rate reserve (40% HRmax) calculated using the formula (220 − age) [12]. We applied the treadmill ramp protocol using a precise treadmill (TR-20F: SportsArt, Tainan, Taiwan), which started at 0% inclination and 2 km/h for 3 min, then increased the speed 0.2 km/h per min. When the patients approached their 40% HRmax during the ramp-up test (progressively increased walking speed), the walking speed at that time was defined as the moderate-intensity exercise. For the safety of the participants, the maximum heart rate was limited to 120 bpm for those aged 49 years or less, and 100 bpm for those with 50 years or older [11]. On both NE and E days, we instructed the patients not to exercise excessively and to have their usual daily activity.
Measurements
Blood glucose level was measured by the CGM device. Body composition of all patients was assessed using a HBF-373 Karada scan instrument (OMRON, Kyoto, Japan).
Statistical analysis
All statistical analyses were performed with the EZR program (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More specifically, it is a modified version of the R commander (version 2.4–0) designed to add statistical functions frequently used in biostatistics [13]. Results were expressed as the mean ± standard deviation or median [interquartile range]. The incremental area under curve (iAUC) was calculated using the trapezoid rule [14]. The iAUC was defined as an increase above fasting glucose level during each test day (i.e., NE day and E day). Using the iAUC, we compared the glycemic variability during 24 h and within 3 h after each meal between NE day and E day. The iAUC statistical significance was analyzed by the Wilcoxon signed-rank test. All p-values were two-sided. Statistical differences were denoted by p < 0.05. Bonferroni correction was applied when testing multiple comparisons, applied to iAUC for glycemic variability within 3 h after each meal (i.e., p < 0.017).
Results
Thirteen Japanese diabetes patients (9 males and 4 females) were initially recruited. Given that two patients were excluded due to the lack of CGM data, clinical data of eleven patients (7 males and 4 females) were analyzed. Mean values were: age, 63.9 ± 15.4 years; BMI, 23.2 ± 5.2 kg/m2; 40% HRmax, 110.1 ± 11.6 bpm; walking speed, 3.7 ± 1.3 km/h; HbA1c, 9.6 ± 2.0%. The number of patients under basal supported oral therapy (BOT), intensive insulin therapy and oral hypoglycemic agents (OHA) therapy were 5, 4 and 2, respectively (Table 1). All eleven patients completed the examinations without adverse effects.
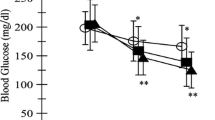
Comparing NE day with E day, fasting glucose level did not change significantly. However, blood glucose standard deviation over 24 h was significantly smaller in the E day than that in the NE day (NE day vs. E day; 27.8 [25.0–41.5] vs. 22.9 [16.0–31.0]; p = 0.014).
The iAUCs for glycemic variability within 3 h after breakfast (p = 0.010) and supper (p = 0.032) in the E day were significantly lower than those in the NE day. However, the iAUCs for glycemic variability within 3 h after lunch (p = 0.067) did not change significantly. The iAUC for the 24-h glycemic variability was also significantly lower in the E day than that in the NE day (34,765 [21,424–56,014] vs. 23,205 [15,323–39,779]; p = 0.014) (Table 2). No patient experienced hypoglycemia, or blood glucose < 70 mg/dl.
As a sub-analysis, NE day and E day were compared in patients with BOT and intensive insulin therapy only. Fasting glucose level (109.3 [102.7–123.9] vs. 114.8 [108.4–117.5] mg/dl, p = 0.426) did not change significantly between NE day with E day. However, the blood glucose standard deviation over 24 h (27.8 [25.8–51.7] vs. 22.9 [17.2–34.8] mg/dl, p = 0.027) was significantly reduced in the E day than that in the NE day. The iAUCs for glycemic variability within 3 h after breakfast (7560 [6598–10,570] vs. 6639 [4228–8260] mg min/dl, p = 0.020), lunch (6511 [4263–14,190] vs. 3500 [2275–4930] mg min/dl, p = 0.039) and supper (7215 [4526–11,234] vs. 3761 [1571–9268] mg min/dl, p = 0.020) in the E day were significantly lower than those in the NE day. The iAUC for the 24-h glycemic variability (37,195 [26,936–65,184] vs. 25,609 [17,056–39,906], p = 0.008) was significantly lower in the E day than that in the NE day.
Discussion
The effect of postprandial walking for 15 min on impaired glucose tolerance has been reported by others [6]. However, the effectiveness and magnitude of glucose lowering in type 2 diabetes mellitus patients were still unclear. Therefore, we investigated the effect of postprandial, moderate-intensity walking for 15 min on glucose homeostasis in type 2 diabetes mellitus patients using continuous glucose monitoring method.
It has been reported that postprandial walking for 15 min, corresponding to approximately 3 METs, significantly lowered postprandial glucose and insulin levels in type 2 diabetes mellitus patients treated by OHA only [15]. However, 24-h glucose level did not change significantly. In our investigation, the mean walking velocity was 3.7 ± 1.3 km/h, which also corresponds to about 3.0 METs [16]. Our patients in sub-analysis underwent different therapies (i.e., basal supported oral therapy (BOT), insulin intensive therapy, and OHA only therapy). The iAUC for the 24-h glycemic variability and the blood glucose standard deviation over 24 h were significantly lower in the E day than in the NE day. These results suggested that 3 METs walking potentially has glucose-lowering effect in patients treated with OHA and with insulin as well. In addition, moderate-intensity walking did not induce hypoglycemia in the E day.
In our study, postprandial moderate-intensity walking resulted in the lowering of iAUC for the 24-h glycemic variability and blood glucose standard deviation over 24 h. Our results are comparable to a previous study that indicated that three 15-min bouts of moderate postmeal walking reduced average 24-h glucose concentration [6]. Another study showed that high-intensity interval training (HIIT) performed in the afternoon was more efficacious than morning HIIT in individuals with type 2 diabetes [9]. Among type 1 diabetes patients under sensor-augmented insulin pump (SAP) therapy, moderate exercise in the morning conferred a lower risk of postexercise hypoglycemia than afternoon exercise [7]. Among active adolescents with type 1 diabetes, 30-min increase in afternoon/evening moderate-to-vigorous intensity physical activity (MVPA) increased the risk of hypoglycemia, although daytime MVPA did not increase that risk [10].
The current study had several limitations. Firstly, it was conducted during educational hospitalization and did not contain a cross-over design. The dietary therapy contributed to lower blood glucose, whereas we provided participants with the same menu with the same energy balance. Secondly, we did not take into account the type and dose of OHA or insulin. The severity of diabetes mellitus may influence exercise effect differently. Finally, the statistical power was small. Furthermore, there was a limitation of CGM. CGM was preferred because it tells us the glucose homeostasis trend, not only the current blood glucose level [17]. On the other hand, CGM measures the interstitial fluid. This compensates the physiological error comparing with one-point blood glucose and reports the difference of mechanical error [18]. Thus, we should discuss the most appropriate frequency of calibration and/or self-monitoring of blood glucose to obtain more accurate results.
In summary, with the aim or establishing a short-time, effective exercise prescription, we investigated the effect of postprandial moderate-intensity walking for 15 min on blood glucose levels in type 2 diabetes mellitus patients. According to our results, postprandial moderate-intensity walking lowers iAUC for the 24-h glycemic variability and blood glucose standard deviation over 24 h. This suggested that postprandial moderate-intensity walking potentially improved whole-day hyperglycemia and elicited good glucose homeostasis without hypoglycemia. However, the relationship between postprandial moderate-intensity walk and drug (insulin and OHA) administration is still unclear. Further study should be performed to investigate the relationship between postprandial walking and insulin secretory ability, insulin injection dose and type of oral hypoglycemic agents.
References
Joslin EP, Kahn CR. Joslin's diabetes mellitus. 14th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
Sato Y, Kondo K, Watanabe T, Sone H, Kobayashi M, Kawamori R, Tamura Y, Atsumi Y, Oshida Y, Tanaka S, Suzuki S, Makita S, Ohsawa I, Imamura S. Present situation of exercise therapy for patients with diabetes mellitus in Japan: a nationwide survey. Diabetol Int. 2012;3:86–91.
Arakawa S, Watanabe T, Sone H, Tamura Y, Kobayashi M, Kawamori R, Atsumi Y, Oshida Y, Tanaka S, Suzuki S, Makita S, Ohsawa I, Sato Y. The factors that affect exercise therapy for patients with type 2 diabetes in Japan: a nationwide survey. Diabetol Int. 2014;6:19–25.
Fagour C, Gonzalez C, Pezzino S, Florenty S, Rosette-Narece M, Gin H, Rigalleau V. Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes Metab. 2013;39:85–7.
van der Berg JD, Stehouwer CD, Bosma H, van der Velde JH, Willems PJ, Savelberg HH, Schram MT, Sep SJ, van der Kallen CJ, Henry RM, Dagnelie PC, Schaper NC, Koster A. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the Maastricht study. Diabetologia. 2016;59:709–18.
DiPietro L, Gribok A, Stevens MS, Hamm LF, Rumpler W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care. 2013;36:3262–8.
Gomez AM, Gomez C, Aschner P, Veloza A, Munoz O, Rubio C, Vallejo S. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J Diabetes Sci Technol. 2015;9:619–24.
Nygaard H, Ronnestad BR, Hammarstrom D, Holmboe-Ottesen G, Hostmark AT. Effects of exercise in the fasted and postprandial state on interstitial glucose in hyperglycemic individuals. J Sports Sci Med. 2017;16:254–63.
Savikj M, Gabriel BM, Alm PS, Smith J, Caidahl K, Bjornholm M, Fritz T, Krook A, Zierath JR, Wallberg-Henriksson H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019;62:233–7.
Metcalf KM, Singhvi A, Tsalikian E, Tansey MJ, Zimmerman MB, Esliger DW, Janz KF. Effects of moderate-to-vigorous intensity physical activity on overnight and next-day hypoglycemia in active adolescents with type 1 diabetes. Diabetes Care. 2014;37:1272–8.
Japan Diabetes Society. Treatment guide for diabetes 2012–2013. Tokyo: Bunkodo; 2013.
Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404–32.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–71.
van Dijk JW, Venema M, van Mechelen W, Stehouwer CD, Hartgens F, van Loon LJ. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care. 2013;36:3448–533.
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–81.
Pickup JC, Ford Holloway M, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38:544–50.
Obermaier K, Schmelzeisen-Redeker G, Schoemaker M, Klotzer HM, Kirchsteiger H, Eikmeier H, del Re L. Performance evaluations of continuous glucose monitoring systems: precision absolute relative deviation is part of the assessment. J Diabetes Sci Technol. 2013;7:824–32.
Acknowledgements
The authors would like to express deep gratitude to the Emeritus Professor Y. Oshida and Professor T. Koike of the Department of Sports Medicine, Graduate School of Medicine, Nagoya University, for the excellent advice. The authors thank all patients who took part in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Human rights statement
This study was approved by the local ethics committee at the Ichinomiyanishi Hospital (approval date: 19 May 2014; approval no. 201516). It was also registered with the UMIN Clinical Trials Registry (UMIN000014567).
Informed consent
All patients provided written informed consent before participating in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iida, Y., Takeishi, S., Fushimi, N. et al. Effect of postprandial moderate-intensity walking for 15-min on glucose homeostasis in type 2 diabetes mellitus patients. Diabetol Int 11, 383–387 (2020). https://doi.org/10.1007/s13340-020-00433-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-020-00433-x