Abstract
Objective
To examine long-term efficacy/safety of ipragliflozin, a sodium–glucose cotransporter 2 inhibitor, added to ongoing insulin therapy in Japanese patients with type 2 diabetes.
Methods
We conducted a 36-week, open-label extension of ipragliflozin therapy following a 16-week, randomized, placebo-controlled, double-blind period (treatment periods II and I, respectively). Prior to the open-label period, patients taking insulin with/without a dipeptidyl peptidase-4 (DPP-4) inhibitor were randomized to receive placebo or 50 mg once-daily ipragliflozin. Oral antidiabetic drugs other than DPP-4 inhibitors were discontinued 4 weeks before screening. Following treatment period I, all patients received open-label ipragliflozin 50 mg, with the possibility of a dose increase to 100 mg at week 24 if HbA1c was ≥ 7.0% at week 20. Efficacy endpoints were changes in HbA1c, fasting plasma glucose (FPG), self-monitored blood glucose, bodyweight, and metabolic hormones. Drug-related treatment-emergent adverse events (TEAEs) were monitored for safety.
Results
Of 175 patients randomized to ipragliflozin, 168 entered treatment period II, 121 (69%) of whom completed this period. The mean ± standard deviation changes in HbA1c, FPG, and bodyweight from baseline (start of treatment period I) to the end of treatment were − 0.83 ± 0.72%, − 31.5 ± 41.2 mg/dL, and − 1.34 ± 1.80 kg, respectively. Between weeks 8 and 32, HbA1c was lower in patients taking a DPP-4 inhibitor than in those without. The most common drug-related TEAE was hypoglycemia; no drug-related TEAEs not already reported for ipragliflozin were observed.
Conclusions
Ipragliflozin was well tolerated, effective, and reduced bodyweight over a period of 52 weeks in patients treated with insulin with/without a DPP-4 inhibitor.
Clinicaltrials.gov identifier
NCT02175784
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Basal, prandial, and premixed insulins are effective treatment options for the management of patients with type 2 diabetes mellitus with inadequate glycemic control despite the use of oral antidiabetic drugs [1,2,3]. Although the doses of insulin can be increased according to blood glucose levels, this approach is limited by the risks of hypoglycemia and weight gain, or by the patient’s lifestyle. Therefore, some patients do not achieve glycemic targets with insulin alone [4]. Indeed, a recent study suggested that only 26.5% of basal insulin-treated patients achieved therapeutic success, similar to patients treated with dipeptidyl peptidase-4 (DPP-4) inhibitors (24.8%) [5]. Therefore, patients may require alternative treatment strategies, such as the addition of another oral antidiabetic drug to ongoing insulin therapy.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors have recently been approved in many countries for the treatment of type 2 diabetes. Owing to their insulin-independent mechanism of action and low risk of hypoglycemia, SGLT2 inhibitors represent potential candidate drugs for combined use with insulin. Ipragliflozin is a selective SGLT2 inhibitor that is effective and well tolerated when used as monotherapy or in combination with other oral antidiabetic drugs [6,7,8].
We recently reported the results of a 16-week, randomized, placebo-controlled, double-blind study of ipragliflozin in Japanese patients in the early stages of insulin therapy (defined as the use of long-acting, intermediate-acting, or premixed insulin) with or without a concomitant DPP-4 inhibitor [9]. As a continuation of this 16-week, randomized, placebo-controlled study, an open-label extension study was conducted in which all participants received ipragliflozin for 36 weeks at the end of the randomized trial, regardless of whether they had been in the placebo or treatment group. Here, we report the results of this open-label extension period in terms of the effects of ipragliflozin on glycemic variables as well as its safety, with an analysis of results over the entire 52-week study period.
Materials and methods
Study design
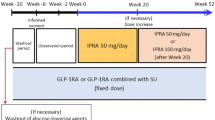
The design of the 16-week, randomized, placebo-controlled study is described in more detail in our prior publication [9]. Briefly, it comprised a 4-week washout period (only for patients using oral antidiabetic drugs other than DPP-4 inhibitors); a 4-week screening period; a 2-week single-blind placebo run-in period; and a 16-week, randomized, double-blind, placebo-controlled period (designated as treatment period I). The present 36-week, open-label extension period comprised an initial 8-week period of administration of ipragliflozin at a fixed dose of 50 mg once daily, followed by 28 weeks of ipragliflozin at either 50 mg or 100 mg once daily (treatment period II). The overall design of the current open-label extension period as a continuation of the previous study is illustrated in Supplementary Fig. 1.
The study was conducted at 43 sites in Japan and it was approved by the institutional review board at each site (Supplementary Information). The study complied with all relevant laws and regulations, including Good Clinical Practice and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines.
This study was registered at ClinicalTrials.gov (identifier: NCT02175784). All patients provided written informed consent.
Study eligibility and withdrawal
Eligibility for the study is described in more detail in our prior report [9]. Briefly, patients aged ≥ 20 years who were diagnosed with type 2 diabetes ≥ 12 weeks before enrollment were eligible provided they had been prescribed a stable dose/regimen of insulin (8–40 units/day) for ≥ 6 weeks, alone or in combination with other oral antidiabetic drugs; their HbA1c was ≥ 7.5% to ≤ 10.5% with a maximum change in HbA1c of 1% during the 4-week screening period; and their body mass index was 20.0–45.0 kg/m2.
Fixed doses of mixed insulin (provided that the rapid-acting or ultra-rapid-acting insulin component was not > 30% of the total daily dose), intermediate-acting insulin, or long-acting insulin alone were allowed, as well as a DPP-4 inhibitor if taken at a fixed dose for ≥ 6 weeks before enrollment and the dose was continued throughout the study.
Patients could be withdrawn at any time during the study for a number of reasons, including safety concerns and severe hypoglycemia (hypoglycemic coma, convulsions, or other conditions requiring infusion or injection of glucose or glucagon). In treatment period II, patients were withdrawn if their HbA1c was > 8.5% on two consecutive visits from week 24 onwards.
Treatments
At the beginning of the 16-week period (treatment period I), patients were randomized at a 2:1 ratio to receive 50 mg of ipragliflozin once daily or placebo. After completion of treatment period I, all patients received 50 mg of ipragliflozin once daily. The ipragliflozin dose could be increased to 100 mg at week 24 in patients whose HbA1c was ≥ 7.0% at week 20 and who wished to increase the dose, provided that the investigator had no safety concerns. Thus, the dose groups of ipragliflozin were 50/50/50 and 50/50/100 mg (i.e., the first 50 mg refers to the dose given in the double-blind period, the second 50 mg refers to the start of the open-label period, and the last dose of either 50 or 100 mg refers to week 24 and beyond, when the dose could be maintained at 50 mg or increased to 100 mg). Treatment was discontinued in patients whose HbA1c was > 8.5% at two consecutive visits from visit 10 (week 24) onwards. The type of insulin used could not be changed during the treatment period. The dose of insulin was to continue unchanged during the treatment period, unless dose changes were required for safety reasons as follows. The insulin dose could be increased from week 28 onwards if the patient’s self-monitored fasting blood glucose (SMBG) was ≥ 180 mg/dL twice in a row and if the investigator considered it was necessary to increase the dose. The insulin dose could also be decreased during treatment period II in the event of (1) hypoglycemic symptoms and the investigator deemed it was necessary to reduce the insulin dose for safety reasons or (2) the SMBG was < 70 mg/dL twice in a row and the patient was suspected of experiencing hypoglycemic symptoms or considered to have a high risk of hypoglycemia.
The insulin dose could be adjusted by a maximum of 4 units throughout the study, regardless of the number of dose adjustments. If the insulin dose was changed by > 4 units, the patient discontinued the study and was prescribed an appropriate treatment regimen. During treatment period II, if glycemic control was inadequate with the present doses of insulin and ipragliflozin, it was preferable to increase the dose of ipragliflozin rather than that of insulin, provided that the patients met the ipragliflozin dose increase criteria described above. Antidiabetic drugs (including metformin) other than insulin and DPP-4 inhibitors at enrollment were prohibited. Unlike in Europe and the US, the Japanese clinical guidelines do not recommend any specific class of antidiabetic drug (including metformin) as first-line therapy. DPP-4 inhibitors could not be started or stopped after starting the study. Corticosteroids, immunosuppressants, glucagon, and glucose could be administered temporarily; however, continuous use of these agents was prohibited. Patients continued their diet and exercise therapies unchanged throughout the study.
Patients performed self-monitoring of fasting blood glucose every morning or if they felt symptoms of hypoglycemia, and recorded the values in their diary.
Endpoints and assessments
The changes in HbA1c, fasting plasma glucose (FPG), glycoalbumin, C-peptide, SMBG values recorded by patient diary, bodyweight, waist circumference, glucagon, leptin, and adiponectin from baseline (start of treatment period I) to the end of treatment period II were examined.
Safety was assessed in terms of vital signs, treatment-emergent adverse events (TEAEs), and laboratory tests. TEAEs were classified according to system organ class and preferred term (MedDRA version 16.1), and their relationship to the study drug, seriousness, and severity were evaluated. We examined several TEAEs of special interest, including those related to hypoglycemia, urinary tract infection, genital infection, and body fluid volume and electrolytes. TEAEs related to body fluid volume and electrolytes included cerebral infarction, dehydration, hemorrhagic cerebral infarction, and thirst.
Statistical analysis
The rationale underlying the sample size is described in the supporting information in our prior publication [9]. Data were analyzed for all patients randomized to ipragliflozin in treatment period I. Data were also analyzed by the dose of ipragliflozin received in treatment periods I and II (50/50/50 and 50/50/100 mg), which did not include patients who discontinued the study before the visit when the dose was increased. Analysis of the subgroups based on DPP-4 inhibitor use was also performed.
Baseline characteristics are presented descriptively as mean ± standard deviation (SD) or n (%) of patients for continuous and categorical variables, respectively.
Efficacy analyses were carried out using the full analysis set, which comprised all patients who received at least one dose of ipragliflozin and in whom at least one efficacy variable was measured after administration of ipragliflozin. For efficacy analyses, data obtained after changing the insulin dose were excluded from analyses. Efficacy data were analyzed descriptively in terms of the mean ± SD values at each timepoint together with the mean changes from baseline to each timepoint. Missing data were not imputed. The mean values and changes in efficacy variables from baseline to the end of treatment using the last observation carried forward were also calculated.
Safety analyses were performed using the safety analysis set, which comprised all patients who received at least one dose of ipragliflozin. TEAEs are presented as the number and percentage of patients in each treatment group.
Results
Patients
Baseline characteristics of patients are shown in Table 1. As shown in the patient flow diagram (Fig. 1), 262 patients were randomized and treated with placebo (n = 87) or ipragliflozin (n = 175), 245 (placebo, n = 76; ipragliflozin, n = 169) completed treatment period I, 243 received ipragliflozin in treatment period II (placebo, n = 75; ipragliflozin, n = 168), and 178 completed treatment period II (placebo, n = 57; ipragliflozin, n = 121). The ipragliflozin dose was increased to 100 mg at week 24 in 94 of 161 patients in the ipragliflozin group.
Patient disposition. The boxes indicate the treatment and dose received for treatment period I/from weeks 16 to 24/from weeks 24 to 52. *Some patients discontinued for more than one reason. †Ipragliflozin dose was maintained at 50 mg once daily after visit 10 (week 24). Note: the first 50 in 50/50/50 or 50/50/100 refers to the dose administered in treatment period I. ‡Ipragliflozin dose was increased to 100 mg once daily from visit 10 (week 24). §Discontinued before the decision was made whether to increase the ipragliflozin dose at week 24 (visit 10). FAS full analysis set, SAS safety analysis set
HbA1c, FPG, and SMBG
As indicated in Fig. 2a, HbA1c decreased progressively between weeks 0 and 12, then decreased slightly from weeks 28 to 40, corresponding to the opportunity to increase the ipragliflozin dose (week 24). The mean change in HbA1c from baseline to end of treatment was − 0.83 ± 0.72% in all ipragliflozin-treated patients (n = 168). The mean changes from baseline to week 24 and from baseline to end of treatment were − 1.08 ± 0.73 and − 1.10 ± 0.76%, respectively, in the 50/50/50 mg group and − 0.76 ± 0.66 and − 0.90 ± 0.73%, respectively, in the 50/50/100 mg group (Table 2). The reduction in HbA1c was more pronounced and stable in patients using ipragliflozin with insulin and a DPP-4 inhibitor than in patients using ipragliflozin with insulin without a DPP-4 inhibitor (Fig. 2b). As indicated in Fig. 2c, there was minimal change in HbA1c in the 50/50/50 mg group after week 24, whereas HbA1c decreased from weeks 24 to 36 in the 50/50/100 mg group and remained approximately the same level thereafter. When examined according to concomitant DPP-4 inhibitor use, HbA1c continued to decline after week 24 in the 50/50/50 mg group without a DPP-4 inhibitor, while HbA1c remained at approximately the same level in the same group of patients taking a DPP-4 inhibitor (Fig. 2d). Within the 50/50/100 mg group, HbA1c was lower in DPP-4 inhibitor-treated patients (Fig. 2e).
Time-course of HbA1c from baseline to the end of treatment (with last observation carried forward) in all patients (a), all patients with/without DPP-4i (b), stratified by ipragliflozin dose (50/50/50 and 50/50/100 mg) used in treatment period II (c) and by ipragliflozin dose and DPP-4i use (d and e). Data are shown as the mean ± standard deviation (full analysis set). The number of patients at each timepoint is shown below the x-axis. DPP-4i dipeptidyl peptidase-4 inhibitor, EOT end of treatment, LOCF last observation carried forward
FPG decreased rapidly within 4 weeks of starting treatment with ipragliflozin (Fig. 3a). In the 50/50/100 mg group, FPG declined in the 12-week period after week 24 and then stabilized, whereas FPG declined slightly in the 50/50/50 mg group (Fig. 3b). Unlike for HbA1c, the use or non-use of a DPP-4 inhibitor did not profoundly affect FPG (Supplementary Fig. 2a–c). SMBG decreased at all timepoints at a magnitude consistent with that seen for FPG (Table 2). Furthermore, when SMBG data (1 h after breakfast, lunch, and dinner) were stratified by the presence or the absence of concomitant DPP-4 inhibitor, the postprandial glucose-lowering effect of the ipragliflozin + DPP-4 inhibitor combination at week 24 was suggested (Table 3).
Time-course of FPG from baseline to the end of treatment (with last observation carried forward) in all patients (a) and in patients stratified by ipragliflozin dose (50/50/50 and 50/50/100 mg) used in treatment period II (b). Data are shown as the mean ± standard deviation (full analysis set) for all patients who received ipragliflozin in both treatment periods. The number of patients at each timepoint is shown below the x-axis. EOT end of treatment, FPG fasting plasma glucose, LOCF last observation carried forward
Consistent with the changes in glycemic variables, glycoalbumin decreased from baseline to end of treatment by − 3.36 ± 2.40% in all patients, − 3.88 ± 2.59% in the 50/50/50 mg group, and − 3.38 ± 2.55% in the 50/50/100 mg group (Table 2).
Metabolic parameters
Table 2 shows the changes in bodyweight, waist circumference, and metabolic parameters from baseline to end of treatment in all ipragliflozin-treated patients and according to the dose of ipragliflozin used in treatment period II. Bodyweight decreased during the 52-week study (Fig. 4a). A gradual decline in bodyweight was observed in the 50/50/50 mg group after week 24, while bodyweight decrease was minimal after week 24 in the 50/50/100 mg group (Fig. 4b). The change in waist circumference at the end of ipragliflozin treatment compared with baseline was − 1.27 ± 3.22 cm in the 50/50/50 mg group and − 1.39 ± 3.66 cm in the 50/50/100 mg group (Table 2).
Change in bodyweight from baseline to the end of treatment (with last observation carried forward) in all patients (a) and in patients stratified by ipragliflozin dose (50/50/50 and 50/50/100 mg) used in treatment period II (b). Data are shown as the mean ± standard deviation (full analysis set) for all patients who received ipragliflozin in both treatment periods. The number of patients at each timepoint is shown below the x-axis. EOT end of treatment, LOCF last observation carried forward
The change in C-peptide was small in all patients and in each ipragliflozin dose group. Glucagon decreased between baseline and end of treatment by − 3.4 ± 13.6 pg/mL in the 50/50/50 mg group and − 1.4 ± 26.6 pg/mL in the 50/50/100 mg group. Leptin and adiponectin were increased in both dose groups (Table 2).
Safety
TEAEs occurring in the placebo or ipragliflozin groups in treatment period I and in all patients who received ipragliflozin in treatment periods I or II combined are shown in Table 4. TEAEs occurred in 89.7% (157/175) of patients treated with ipragliflozin. Serious TEAEs occurred in 5.1% (9/175) and TEAEs led to permanent discontinuation of the study drug in 5.7% (10/175) of patients. Overall, 58.9% (103/175) of patients experienced drug-related TEAEs, which were classified as serious in 2.9% (5/175) of patients and led to permanent discontinuation of the study drug in 4.6% (8/175) of patients. Hypoglycemia-related events were the most common TEAEs of special interest, occurring in approximately one-third of patients. Although efficacy and other safety data for patients who received placebo in treatment period I and ipragliflozin in treatment period II are not described here, the incidence of hypoglycemia was 38.7% (29/75) in these patients. No notable difference was observed in the incidence of hypoglycemia between patients treated with or without a DPP-4 inhibitor (data not shown).
TEAEs associated with urinary tract infection, genital infection, and body fluid volume and electrolytes occurred in ≤ 5.7% of patients. By preferred term, the most common drug-related TEAEs were hypoglycemia, pollakiuria, and thirst.
Administration of ipragliflozin was not associated with any clinically significant changes in laboratory variables or vital signs in treatment period II, and there were no apparent effects of ipragliflozin dose on these variables (data not shown).
Discussion
Here, we report the results of a 36-week extension of a 16-week randomized study in which all patients were switched from placebo or 50 mg ipragliflozin to open-label ipragliflozin. The ipragliflozin dose could be increased from 50 to 100 mg at week 24 in patients with HbA1c ≥ 7.0% at week 20.
This study demonstrated that the improvement in glycemic control at week 16 in the ipragliflozin group was maintained through to week 52 (i.e., end of treatment period II). Between weeks 8 and 32, the HbA1c-lowering effect of ipragliflozin tended to be greater in patients using a DPP-4 inhibitor than in patients not using a DPP-4 inhibitor, but changes in FPG were similar in both subgroups. This effect of DPP-4 inhibitor coadministration, although no longer apparent after week 32, might reflect its impact on postprandial glucose levels rather than on fasting glucose levels, suggesting that coadministration of insulin, ipragliflozin, and a DPP-4 inhibitor might be beneficial for improving glycemic control. The effects on HbA1c were comparable between patients with and without a coadministered DPP-4 inhibitor after 52 weeks, presumably because of better self-management in general among patients who completed the 52-week study period. Nevertheless, a decrease in HbA1c was observed in the end of treatment data, which included patients with HbA1c levels > 8.5% at weeks 28 and 32 who had discontinued treatment according to the protocol discontinuation criterion.
In treatment period II, when the dose of ipragliflozin could be increased at week 24 in patients who had insufficient efficacy, the dose increase of ipragliflozin from 50 mg once daily to 100 mg once daily led to a gradual decline in HbA1c over a period of 12 weeks before plateauing. Similarly, FPG decreased after the dose increase of ipragliflozin to 100 mg daily. These results suggest that an increase in the daily dose of ipragliflozin from 50 to 100 mg could improve glycemic control in patients who do not experience sufficient efficacy. HbA1c and FPG levels decreased between weeks 0 and 12 (i.e., after starting ipragliflozin) and between weeks 24 and 36 (i.e., after the dose increase) in the 50/50/100 mg group. Therefore, in patients newly prescribed ipragliflozin, the ipragliflozin dose could be increased from 50 to 100 mg after about 12 weeks of treatment, providing that there are no safety concerns.
Accompanying the improvement in glycemic control, we also observed a gradual reduction in bodyweight over the 52-week period, a relevant clinical finding, because weight gain is commonly associated with insulin therapy. In a meta-analysis of long-term randomized controlled trials of SGLT2 inhibitors, weight reduction at 1 and 2 years was 2.48 and 2.99 kg, respectively [10]. Our study supports the observation that SGLT2 inhibitors have a beneficial effect on bodyweight in addition to glycemic control.
In the present study, adiponectin levels increased in both dose groups, but leptin levels did not change. Because decreased adiponectin levels were shown to have a causal role in insulin resistance [11], our observation suggests that ipragliflozin contributes to improvement of glycemic control by lowering insulin resistance.
In terms of safety, hypoglycemia was the most common drug-related TEAE in ipragliflozin-treated patients. In treatment period I, hypoglycemia occurred in about double the proportion of patients in the ipragliflozin group than in the placebo group (29.1 vs. 14.9%). However, the incidence of hypoglycemia in ipragliflozin-treated patients did not increase markedly in both treatment periods combined (36.0%) relative to its incidence in treatment period I (29.1%). Other drug-related TEAEs occurred with low incidence (≤ 8.6% in treatment periods I and II combined), and the TEAEs that occurred have also been reported in other clinical trials of ipragliflozin. Therefore, no previously unreported TEAEs were found when ipragliflozin was used in combination with insulin. In terms of laboratory variables, we observed no changes in markers of liver function or lipids, which were measured as safety variables, in treatment period II. These findings are consistent with those previously reported for treatment period I. Although ipragliflozin was associated with a slight reduction in the estimated glomerular filtration rate in treatment period I compared with placebo, no further changes occurred in treatment period II.
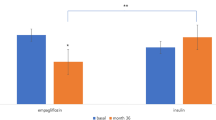
Several other studies have examined the efficacy and safety of adding an SGLT2 inhibitor to ongoing insulin. For example, the EMGA-REG BASAL study [12] showed that 10 and 20 mg empagliflozin added to basal insulin was associated with significant reductions in HbA1c and bodyweight compared with placebo at weeks 18 (10 mg, 25 mg, placebo: − 0.6, − 0.7, 0.0%) and 78 (− 0.5, − 0.6, 0.0%), while hypoglycemia occurred in about one-third of patients. Another long-term study examined the efficacy of dapagliflozin vs. placebo in combination with high doses of insulin with or without up to two oral antidiabetic drugs for up to 2 years [13]. The placebo-adjusted mean changes in HbA1c from baseline to week 104 were − 0.4% in the 5 mg/10 mg group and − 0.4% in the 10 mg group. The placebo-adjusted mean changes in HbA1c between patients taking no oral antidiabetic drugs vs. patients taking one/two oral antidiabetic drugs were − 0.3 and − 0.4%, respectively. However, caution should be exercised when comparing the results of these studies with our findings, owing to differences in patient populations and study designs. In particular, the previous studies included predominantly obese Western patients (body mass index ~ 31 kg/m2) with long-standing diabetes, who were using high doses of insulin (~ 48 U/day in [12] and ~ 75 U/day in [13]), whereas our patients had a much lower body mass index (~ 25 kg/m2) and insulin dose of < 40 U/day.
Studies of SGLT2 inhibitors in Japanese patients have examined the efficacy and safety of adding 100 mg canagliflozin [14] (16 weeks) or tofogliflozin [15] (24 weeks) to insulin with outcomes consistent with ours in terms of the improvements in glycemic control and incidence of hypoglycemia at 16–24 weeks. In a 16-week placebo-controlled trial of 5 mg dapagliflozin added on to insulin in Japanese patients, a mean HbA1c change of − 0.55% relative to baseline was observed [16]. Furthermore, the 36-week open-label extension period of this long-term study revealed a change in HbA1c of − 0.74% over 52 weeks in patients receiving 5 or 10 mg dapagliflozin [17]. The most common adverse event was hypoglycemia, which ranged from 35 to 41.7% in the dapagliflozin-treated groups [17].
Our study had some limitations that should be mentioned. First, our results may not be generalizable to patients in other countries or of other ethnicities. Second, a large number of patients discontinued the study in treatment period II owing to the prespecified discontinuation criteria, especially for HbA1c > 8.5% on two consecutive visits from week 24 onwards. This likely introduced significant bias into the analysis of baseline to end of treatment changes, in which the last observation was carried forward. In addition, data collected after the change in insulin dose were excluded from the full analysis set, which may have affected the results. Finally, all analyses reported here were performed in an exploratory manner, without formal statistical tests.
In conclusion, ipragliflozin as add-on therapy to insulin for 52 weeks was associated with clinically relevant decreases in HbA1c, FPG, and SMBG that were sustained throughout the treatment periods. It is also notable that administration of ipragliflozin in combination with insulin was associated with ongoing reductions in bodyweight. Coadministration of a DPP-4 inhibitor was associated with greater improvements in HbA1c, particularly in treatment period I. Increasing the ipragliflozin dose from 50 to 100 mg might be valuable in patients whose HbA1c remains > 7.0%. As might be expected, hypoglycemia was the most common drug-related TEAE, and other drug-related TEAEs that occurred in this study have already been described for ipragliflozin.
References
Lovre D, Fonseca V. Benefits of timely basal insulin control in patients with type 2 diabetes. J Diabetes Complications. 2015;29:295–301.
Pettus J, Santos Cavaiola T, Tamborlane WV, et al. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2016;32:478–96.
Wu T, Betty B, Downie M, et al. Practical guidance on the use of premix insulin analogs in initiating, intensifying, or switching insulin regimens in type 2 diabetes. Diabetes Ther. 2015;6:273–87.
Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27(Suppl 3):13–20.
Monami M, Ragghianti B, Zannoni S, et al. Identification of predictors of response to basal insulin and DPP-4 inhibitors in patients with type 2 diabetes failing to other therapies. Acta Diabetol. 2016;53:35–40.
Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17:304–8.
Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–91.
Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–60.
Ishihara H, Yamaguchi S, Nakao I, et al. Efficacy and safety of ipragliflozin as add-on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi-centre, randomized, placebo-controlled, double-blind study. Diabetes Obes Metab. 2016;18:1207–16.
Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295–303.
Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32(Suppl 7):S13–8.
Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48.
Wilding JP, Woo V, Rohwedder K, et al. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–36.
Inagaki N, Harashima S, Maruyama N, et al. Efficacy and safety of canagliflozin in combination with insulin: a double-blind, randomized, placebo-controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89.
Suzuki K, Mitsuma Y, Sato T, et al. Comparison of combined tofogliflozin and glargine, tofogliflozin added to insulin, and insulin dose-increase therapy in uncontrolled type 2 diabetes. J Clin Med Res. 2016;8:805–14.
Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: results of the interim analysis of 16-week double-blind treatment period. J Diabetes Investig. 2016;7:555–64.
Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin over 1 year as add-on to insulin therapy in Japanese patients with type 2 diabetes: the DAISY (Dapagliflozin Added to patients under InSulin therapY) trial. Diabetes Obes Metab. 2016;19:562–70.
Acknowledgements
The authors wish to thank all of the investigators involved in this trial, as well as Nicholas Smith, PhD, and William Ng, MB, BS, PhD, of Edanz Medical Writing for providing medical writing services.
Funding
This study was sponsored by Astellas Pharma Inc. Medical writing and editorial support was funded by Astellas Pharma Inc. and provided by Dr. Nicholas D. Smith and Dr. William Ng (Edanz Medical Writing) and Elsevier/ELMCOM™.
Author information
Authors and Affiliations
Contributions
HI, SA, and TS contributed to study design, data analysis, and writing of the manuscript; SY and IN contributed to study design, study conduct, data collection and analysis, and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
HI has served on the scientific advisory board of Astellas Pharma Inc.; received lecture or consulting fees from Astellas Pharma Inc., MSD, Sanofi, Mitsubishi Tanabe Pharma, Boehringer Ingelheim Japan, and Novartis Pharma; and received grants/research support from Astellas Pharma Inc., Ono Pharmaceutical, Boehringer Ingelheim Japan, AstraZeneca, Sanofi, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Daiichi-Sankyo, Novo Nordisk Pharma, Kyowa Hakko Kirin, and MSD. SY, IN, SA, and TS are employees of Astellas Pharma Inc., Japan.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study. The study was approved by the institutional review board at each participating site.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ishihara, H., Yamaguchi, S., Nakao, I. et al. Efficacy and safety of ipragliflozin as add-on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a 36-week, open-label extension of a 16-week, randomized, placebo-controlled, double-blind study. Diabetol Int 10, 37–50 (2019). https://doi.org/10.1007/s13340-018-0359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-018-0359-x