Abstract
A number of viroids can cause serious damage on Citrus spp. ranging from stunting, bark scaling, yellowing and epinasty of leaves over stem pitting and gumming. However, so far, they have never been found in Thailand. In recent years, the import of orange and lime fruits from China and Cambodia, respectively, increased, and holds a substantial risk of viroid introduction and spread in Thailand. Orange and lime fruit samples in 2013 and 2014 were screened for the presence of citrus exocortis viroid (CEVd), hop stunt viroid (HSVd), citrus bent leaf viroid (CBLVd) and citrus dwarfing viroid (CDVd; CVd-IIId) by means of specific RT-PCR methods. Only CBLVd and CDVd were detected, clearly generating the expected amplification bands of around 320 and 300 base pairs, respectively. The presence of CBLVd and CDVd was confirmed by amplicon sequencing and RNA secondary structure analysis. About 34.2 and 19.5% of 41 samples (around 2300 fruits) of the imported lime fruit were infected with CBLVd and CDVd, respectively. CBLVd was detected in 62.3% of the 77 samples (around 2000 fruits) from imported oranges, while CDVd was found in 75.3%. This result indicates that the incidence of both CBLVd and CDVd in the imported citrus fruits is quite high. In addition, both viroid diseases have not been reported in Thailand. However, lack of information on the actual status of both viroids leads to difficulties in determining their impact on the Thai citrus industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Viroids are the smallest known plant pathogens and consist of naked circular single-stranded RNA, ranging in size from 246 to 401 nucleotides. Several viroids, such as the pospiviroid Citrus exocortis viroid (CEVd), the hostuviroid Hop stunt viroid (HSVd), the cocadviroid Citrus bark cracking viroid (CBCVd), and apscaviroid species Citrus bent leaf viroid (CBLVd), Citrus dwarfing viroid (CDVd), Citrus viroid V (CVd-V), and Citrus viroid VI (CVd-VI) have been reported as important citrus diseases causing a various range of symptoms such as bark cracking or scaling, stunting, twigs yellow blotching, gumming and browning of phloem tissues, wood pitting, reduction of fruit size, and tree declining. In addition, CEVd was reported to persist for a long period as an infectious agent on contaminated surfaces, dry infected host tissue and is highly resistant to heat and several viral disinfectants. For these reasons, prevention, control and eradication are hardly successful. To date, only CEVd was found associated with lime (Citrus aurantifolia) in Thailand [2]. On the other hand, four citrus viroids (CBLVd, CDVd, CEVd and HSVd) have been reported from China [5]. Both China and Cambodia are exporting a huge amount of orange and lime fruits, respectively, to Thailand. To be able to conduct a risk assessment and determine management steps, a survey on the presence of the four above-mentioned viroids was set up at the points of entry in Thailand.
From June 2013 and May 2014, imported Chinese orange fruits were sampled from Chiang Khong Plant Quarantine station, Laemchabang Port Plant Quarantine station, Talaad Thai and DOA market. For the Cambodian lime fruits, all samplings were conducted at the markets near the border between two countries (Chanthaburi, Aranyaprathet, Chon Buri, Phetchabun and Sa Kaeo sites). 41 lime samples (around 2300 fruits) and 77 orange samples (around 2000 fruits) were inspected and tested for four citrus viroids and citrus tristeza virus (CTV).
To determine the presence of the four viroids (CBLVd, CDVd, CEVd and HSVd), RNA extraction and reverse transcription polymerase chain reaction (RT-PCR) were performed. Total RNA was extracted from central column of fruits by using CTAB method. Two specific primer pairs for CBLVd and CDVd detection were designed for this work (CBLVd [c-CBLVd: 5′-GTCGACGACGACCAGTCAGCTCC-3′/h-CBLVd: 5′-GAAGGCTCGTCAGCTGCGGAGG-3′], and CDVd [c-CDVd: 5′-GTCGACGACGACAGGTAAGTTCCC-3′/h-CDVd: 5′-GAAGGCAGCTAAGTTGGTGACGCC-3′]), for CEVd and HSVd detection, the described methods of Gross et al. [1] and Shamloul et al. [3], respectively, were used. All the samples were also tested for the internal control of the detection using NAD primer pair [4]. In addition, CTV was tested by the ELISA reagent set from Agdia, Inc. (Elkhart, IN, USA) and preformed according to the manufacturer’s protocol.
One step RT-PCR (SuperScript™ One-Step RT-PCR System with Platinum™ Taq DNA Polymerase (Invitrogen, USA)) was done according to the manufacturer’s instructions in a total reaction volume of 20 µl. All primers were used at a final concentration of 0.2 µM. Cycling conditions were 48 °C for 45 min (cDNA synthesis), 94 °C for 3 min as initial denaturation step, followed by 35 cycles of amplification at 94 °C for 30 s (denaturing), 60 °C for CBLVd or 61 °C for CDVd for 30 s (annealing), 72 °C for 30 s (extension), and completed by a final extension step at 72 °C for 10 min. The PCR products were ligated with pGEM®-T Easy Vector and cloned into E. coli, Library Efficiency DH5α Competent Cells (Invitrogen), according to the manufacturer’s protocol. All recombinant plasmids were purified and sent for sequencing (First BASE Laboratories, Malaysia). The sequence data were analyzed using blastn (http://blast.ncbi.nlm.nih.gov/blast.cgi).
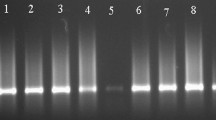
The results revealed that 14/41 lime fruit and 48/77 orange fruit samples were contaminated with CBLVd and 8/41 lime fruit and 58/77 orange fruit samples were contaminated with CDVd. Three of the 41 lime and 36 of the 77 orange samples had mixed CBLVd and CDVd contamination. On the other hand, HSVd and CEVd were not detected in any sample. The genome variants ranged in size from 325 to 328 nucleotides for CBLVd and 293 to 294 nucleotides for CDVd. All individual sequences were submitted to GenBank (Accn. Nos. KM214207.1 through KM214213.1 for CBLVd, and KM214214.1 and KM214215.1 for CDVd). A nucleotide BLAST analysis of the CBLVd and CDVd sequences showed that isolates from this work shared 99–100% and 99% similarity with corresponding CBLVd (KF726093, MF361857) and CDVd (S75465) reference isolates, respectively.
The results show that the contamination rate of these two viroids in both fruit samples is quite high, especially in orange samples, being 62.34% for CBLVd and 75.32% for CDVd. This is the first report of CBLVd and CDVd interception on imported lime and orange fruits from China and Cambodia, respectively. In fact, CBLVd and CDVd have never been found or reported in Thailand. In addition, in this work, we report specific primer sets for two citrus viroids, CBLVd and CDVd. Unfortunately, there is no information about the likelihood of establishment and spread of these diseases, impact and consequence to the orange and lime production in Thailand, or even for other economically important crops. In a next step, all data will be used to update phytosanitary status and measures and will be useful for future risk assessment studies.
References
Gross HJ, Krupp G, Domdey H, Raba M, Jank P, Lossow C, Alberty H, Ramm K, Sänger HL. Nucleotide sequence and secondary structure of Citrus exocortis and Chrysanthemum stunt viroid. Eur J Biochem. 1982;121:249–57.
Reanwarakorn K, Pomma S. Evidence of Citrus exocortis viroid in Thailand. Kasetsart J. 2003;37(4):453–9.
Shamloul AM, Faggioli F, Keith JM, Hadidi A. A novel multiplex RT-PCR probe capture hybridization (RT-PCR-ELISA) for simultaneous detection of six viroids in four genera: Apscaviroid, Hostuviroid, Pelamoviroid, and Pospiviroid. J Virol Methods. 2002;105(1):115–21.
Thompson JR, Wetzel S, Klerks MM, Vaskova D, Schoen CD, Spak J, Jelkmann W. Multiplex RT-PCR detection of four aphid-borne strawberry viruses in Fragaria spp. in combination with a plant mRNA specific internal control. J Virol Methods. 2003;111:85–93.
Wang XF, Zhou CY, Tang KZ, Li ZA. Occurrence of four citrus viroids in Chongqing, China. Plant Dis. 2008;92:978.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tangkanchanapas, P., Juenak, H. & De Jonghe, K. First reported occurrence of citrus bent leaf viroid and citrus dwarfing viroid on imported oranges from China and lime fruits from Cambodia. VirusDis. 29, 416–417 (2018). https://doi.org/10.1007/s13337-018-0466-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-018-0466-0