Abstract
The aims of this study were to investigate various factors associated with protective anti-rabies antibody status (0.5 EU/ml) in vaccinated pet dogs and anti-rabies antibody status in unvaccinated stray dogs. One hundred and seven serum samples were collected from vaccinated pet dogs, out of these 58 (62.36 %) dogs showed antibody titre above 0.5 EU/ml. All the dogs were divided into different groups based on age, sex, breed, vaccine brand and time of vaccination after last vaccine to assess the relationship of these factors with vaccinal immune response. One way analysis of variance was performed in graphpad prism software to check the effect of all these factors. Statistical analysis of ELISA titres of pet dog serum samples suggested that age, sex, breed and vaccine brands have no significant effect on the anti-rabies antibody titres. To check anti-rabies antibody status in stray dogs 53 serum samples were collected and only one out of 53 (1.88 %) stray dogs showed anti-rabies antibody titre above 0.5 EU/ml indicating susceptibility to rabies infection and thereby posing possible threat to surrounding human and animal populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rabies is a highly neglected and under-reported zoonotic disease that is estimated to kill at least 60,000 people worldwide each year, of which, 30,000 human deaths occur in Asia alone and it is largely related to uncontrolled dog rabies in developing countries [12, 22]. The dog has been and is the main reservoir of rabies in India. An estimated 20,000 people die of rabies in India and 17.4 million exposures to animal bites are reported every year in India, which corresponds to an incidence rate of 1.7 % [21]. The majority of rabies deaths occurs from rabid dog bites in developing countries where medical resources are limited and/or a lack of awareness about the risk of rabies virus exposure exists [10].
Majority of the dogs are stray, ownerless and unprotected with rabies vaccine, which live in and around human dwellings [15]. Blue Cross of India found that a steady decrease in human rabies cases was noticed at those places where a proper Animal Birth Control and anti-rabies vaccination programs were carried out [7]. Fatal encephalitis caused by rabies is preventable through timely administration of post-exposure vaccination and sero-therapy [20]. Vaccinating domestic dogs against rabies results in significant reduction in the incidence of bites among the human population from dogs suspected to be rabid, and this control strategy has been shown to be the most cost-effective [13]. Since the days of Pasteur, vaccination has played an important part in the protection of man against rabies. Pre-exposure immunization of both domestic animals and persons at risk is widely practised [4]. It has been estimated that vaccinating more than 70 % dogs would be sufficient to create an immunological barrier that prevents the spread of the rabies virus [17].
The purpose of vaccinating dogs against rabies is to establish pre-exposure immunity and protect animals from contracting rabies, hereby preventing further spread to humans or other domestic animals. Mainly G protein is utilised for ideal anti-rabies vaccine preparation due to its purity and potency [5]. It is imperative to maintain specific level of anti-rabies antibody in animals to confer the effective protection and prevent virus transmission [9]. A serum titre of 0.5 IU/ml and above of rabies virus-specific antibodies is considered adequate protection against rabies. A titre below this level is considered a vaccination failure. Literature reflects very little research effort on monitoring the duration of immunity in dog populations therefore this study conducted to monitor the vaccinal anti-rabies antibodies in dogs nearby Anand region in Gujarat.
Materials and methods
Collection of serum samples for seromonitoring of anti-rabies antibody
Serum samples (n = 107) were collected from pet dogs with details such as age, sex, breed and history of vaccination. Pet dog serum samples were collected from the Teaching Veterinary Clinical Complex (TVCC), College of Veterinary science and Animal Husbandry, A.A.U., Anand, Gujarat and from Veterinary Poly Clinic, Vadodara, Gujarat. Of these, 33 samples from Labrador retriever, 21 from German shepherd, 14 from non-descript breed, 10 from Pomeranian, 10 from Doberman, 6 from Rottweiler, 3 from Pug, 2 from Dalmatian, 2 from Great Dane and 1 each from Boxer, Cocker spaniel, Lhasa Apso, Dachshund, Golden Retriever and Pit bull terrier. Out of 107 pet dogs, 93 dogs were earlier vaccinated by rabies vaccines, while 14 dogs were not vaccinated.
Serum samples (n = 53) were additionally collected from stray dogs with only available information about sex from the dogs brought to Animal Birth control programme (ABC) unit, Ahmedabad, Gujarat. These dogs were not vaccinated against rabies at the time of sample collection. Thus, totally 160 serum samples were collected for sero-monitoring of anti-rabies antibody.
ELISA for sero-monitoring of anti-rabies antibodies
Anti-rabies antibodies were detected by using PLATELIA™ RABIES II ASSAY Ad Veterinarium (Ref: 355-0180) BIORAD ELISA kit. This test is based on the use of a solid phase enzyme immunoassay technique referred to as an indirect ELISA. A microplate is coated with rabies glycoprotein extracted from the inactivated and purified virus membrane. The enzymatic conjugate consists of a protein A from Staphylococcus aureus coupled with peroxidase. Positive controls, calibrated against OIE standard, allow the qualitative or quantitative determination of anti-rabies antibody titre in the serum.
Preparation of quantification standards for the assay
Each quantification assay using PLATELIA™ Rabies II kit includes six quantification standards from S1 to S6. The calibrated R4b Positive control (4 EU/ml) corresponds to the S6 quantification standard. Serial dilutions out of the R4b reagent allowed the preparation of S5–S1 Quantification standards (Table 1). The dilutions were performed using the sample diluent (reagent R6).
Assay protocol
Negative control, positive control and quantification standards were deposited in each plate for the quantification purpose. 100 µl of diluted samples, controls and quantification standards were distributed in the corresponding microplate wells. Microplate was sealed with adhesive film and incubated at 37 °C for 60 min. After first incubation adhesive film was removed. Three wash cycles were performed. Drying was done by inversion on absorbent paper before the next step. 100 µl of the conjugate solution (R7) was distributed into each well. Plates were covered with a new adhesive film and incubated for 60 min at 37 °C. Enzymatic development solution (R8 + R9) was prepared just before use. Adhesive film was removed. Five wash cycles were performed. Microplate was kept away from the direct light, 100 µl of the enzymatic development solution (R8 + R9) was distributed into each well and incubated in darkness at room temperature (+18 to +30 °C) for 30 min. 100 µl of the stop solution (R10) was added to each well according to the same sequence and same distribution rate as for the enzymatic development solution. Bottom of the plate was thoroughly wiped. Optical density was read at 450–620 nm (Dual wavelength mode) within 30 min after stopping the reaction. To draw a standard curve, mean of OD values of the quantification standards (S1–S6) were plotted on the ‘y’ axis and concentrations (EU/ml) of quantification standards on the ‘x’ axis in graphpad prism software. Using the graph, OD values of different serum samples were converted to EU/ml.
Statistical analysis
The optical density (OD) values for all sera samples and standards (S1–S6) obtained by ELISA were corrected as per manufacturer’s correction formula. OD for all the samples and standard samples were subtracted from negative control’s OD and then the ratio of ‘negative control subtracted’ OD to positive control OD was taken as corrected OD. A standard curve was drawn in graphpad Prism software using corrected OD values for all standards (S1–S6) obtained by ELISA and their corresponding EU/ml values as provided by the manufacturer. The standard curve was fitted using 2nd order polynomial (R2: 0.99) in graphpad Prism software. For all sera samples, EU/ml values were extrapolated from standard curve using their corresponding corrected OD as per manufacturer’s instructions (PLATELIA™ Rabies II kit, Bio-Rad, India).
A general linear model was fitted with age, sex, breed, and vaccine type as fixed effects and rabies antibody titre (continuous scores) as outcome. Descriptive analysis for extrapolated EU/ml values for all sera samples obtained from standard curve was carried out using graphpad prism software. Mean, standard deviation, minimum value, maximum value, variance and standard error of EU/ml values were calculated for pet dogs’ serum samples. One way analysis of variance (ANOVA) was performed to detect effect of age, breed, sex vaccine brand and period from last vaccination on the mean EU/ml values in vaccinated pet dogs.
Results
In the present study, ninety-three dog samples were analysed for the anti-rabies antibodies after the vaccination and 14 dogs were analysed for maternal antibody status. We couldn’t find proper history of vaccination for each and every dog but all the vaccinated pet dogs (n = 93) had undergone pre-exposure single or multiple rabies vaccination by following either 0 day—annual booster regimen or 0–28th day—annual booster regimen. Of these, 58 (62.37 %) pet dogs showed antibody titre equal to/above the cut-off value of 0.5 EU/ml.
Persistence of anti-rabies antibodies after vaccination
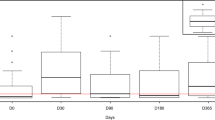
The results indicated that anti-rabies antibody level were found maximum during >1 to ≤3 months after vaccination, which after 3 months from vaccination, was found decreased in different groups. Lower mean antibody titre of 0.8 EU/ml found during >3 to ≤6 months after last vaccination (Table 2).
Effect of various factors on antibody status in vaccinated dogs
In present study, univariable analysis of descriptive data was performed to check the association of various factors with anti-rabies antibody response in dogs vaccinated against rabies. Ninety-three samples were analyzed in this study and their p values in univariable analysis are presented in Table. Statistical analysis suggested that there was no effect of age, sex, breed and vaccine brand on anti-rabies antibody titre because p values for all these factors were more than 0.05 (Table 3).
Anti-rabies antibody status in non-vaccinated pet dogs
Fourteen samples out of 107 were from unvaccinated pet dogs and were analyzed to check the presence/persistence of maternal antibodies against rabies. Out of these 14 samples, 8 samples were from the dogs below 3 months of age, 2 samples were from the dogs between 3 and 6 months of age and rest 4 samples were from dogs between 6 and 12 months of age. Two out of 14 dogs revealed the protective immune titre of 0.5 EU/ml.
Anti-rabies antibody status in stray dogs
Fifty-three stray dog serum samples were analyzed and optical density values obtained during ELISA were converted to EU/ml as described earlier (Section 3.8.1). All these dogs were not vaccinated at the time of sample collection. Of these, only one (1.88 %) stray dog (RS 47) showed protective anti-rabies antibody titre of >0.5 EU/ml.
Discussion
The aim of this study to know the anti-rabies antibody status in dog population. In pet dogs out of 93 vaccinated dogs, only 58 pet dogs showed anti-rabies antibody titre above 0.5 EU/ml. This finding indicates low level of sero-conversion as remaining 35 (37.63 %) vaccinated pet dogs showed antibody titre less than the cut-off value of 0.5 EU/ml, thereby suggesting an increased risk of exposure to rabies. It also indicates lack of consistent vaccination programme and/or possible vaccination failure in some cases. These findings were in agreement with this another study found that only 16 % of household dogs in Chandigarh had protective titre above 0.5 EU/ml. [18]. A study from Japan showed that only 27.7 % pet dogs had protective immune status [16]. These findings also did not attain the World Health Organization (WHO) prescribed 70 % epizootiological base line of herd immunity to keep canine rabies under control [1].
Maximum Mean antibody titre was found during >1 to ≤3 months interval after most recent vaccination while lower mean antibody titre of 0.8 EU/ml found during >3 to ≤6 months after last vaccination than the last two groups was surprising, which could be possibly due to unequal sample size of these groups and various other factors including possible interference of maternal antibodies with vaccinal response. Although mean antibody titre of all the five groups was above the desirable level of 0.5 EU/ml, total 35 (37.63 %) dogs (5, 2, 10, 12 and 6 in each respective groups) showed antibody levels below 0.5 EU/ml, which demands attention in terms of their susceptibility to possible exposure to rabies. An increasing proportion of dogs failing to reach the antibody response cut-off with increasing day from vaccination to sampling [8]. This study confirms the finding of another study that dogs sampled up to 4 months after vaccination had significantly higher chance to reach the antibody response cut-off than the dogs sampled at later time-points independently of once or twice vaccination of dogs [6]. Dog breed had a significant effect on anti-rabies antibody titre. Their results showed that larger breeds failed to reach the cut-off value of anti-rabies antibody titre [8].
Dogs are primarily protected against rabies through anti-rabies antibodies after vaccination. However, protective efficacy of vaccines can be influenced by various associated factors viz, age, sex, breed, vaccine brand [2, 6, 8, 11]. The adjusted model with fixed effects was found to be non-significant with Fisher’s F test probability value of p = 0.68. Age group (p = 0.29), breed group (p = 0.63), sex (p = 0.77), and vaccine type (p = 0.50) had no significant effects on the anti-rabies antibody titre. Another study also found that age, sex, breed and vaccine brand had no significant effect [19]. Other scientists showed higher risk of lower antibody titres with increasing age as well as for dogs <1 year of age compared to adults [8, 11]. The higher risk of lower antibody titres in older dogs could be due to a reduced efficiency of the immune system with increasing age. A study reported that age of the dog has a significant (p < 0.05) effect on the anti-rabies antibody titre. It suggested that dogs vaccinated at an age <6 months or over 5 years of age had a higher failure rate than dogs between 6 months and 5 years, which is in contrast to the present study [2].
In mammals, antibodies are transferred from dam to the offspring through the placenta and via colostrum [14]. Fourteen non-vaccinated pet dog serum samples were analyzed to check the presence/persistence of maternal antibodies in dogs. Only two out of 14 dogs aged 2.5 and 2 months showed presence of protective levels of maternal anti-rabies antibodies. Dams of both the puppies were vaccinated against rabies. Therefore, it implies that antibodies were transferred to the offspring through the colostrum or placenta. Clinical trial was conducted to check the neonatal antibodies against rabies. They collected serum samples from 12 Beagle puppies and their dams were vaccinated against rabies. Ten of these received colostrum immunity from their dams and had antibody titres above the cut-off value of 0.5 EU/ml [3].
To check the anti-rabies antibody status in stray dogs, 53 serum samples were collected from unvaccinated stray dogs and only 1 (1.88 %) dog showed the protective immune titre above the cut off value of 0.5 EU/ml. Rest of the dogs (98.12 %), as expected, showed antibody levels less than the protective level, indicating their vulnerability for getting rabies infection and thus posing threat to surrounding human beings and animals. In agreement with this study, other scientists detected protective anti-rabies antibody titre only in one (1.0 %) stray dog out of 100 [18]. This goes very much against the WHO recommendation to ensure at least 70 % of dog population with protective antibody level so on to keep rabies under control.
The present study concluded that, all the vaccinated pet dogs did not show protective anti-rabies antibody levels, as 62.36 % of vaccinated pet dogs showed antibody levels above the cut-off value of 0.5 EU/ml. Age, sex, breed and vaccine brand did not show significant effect on anti-rabies antibody levels in vaccinated pet dogs. Persistence of anti-rabies antibodies was found more within 1–3 months after anti-rabies vaccination. Protective immune titre declined after 3 months of vaccination. 98.12 % stray dogs showed unprotective anti-rabies antibody levels indicating their susceptibility to rabies infection and thus posing threat to other animals and human beings for rabies infection.
References
Aiyedun JO. Community-based investigation of rabies antibody profile of dogs and control in Ilorin, Kwara state. J Environ Issues Agric Dev Ctries. 2013;5(2):51–5.
Berndtsson LT, Ann-Kristin JN, Esteban R, Berndt K. Factors associated with the success of rabies vaccination of dogs in Sweden. Acta Vet Scand. 2011;53:22.
Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine. 1998;16(14):1468–72.
Crick J. The vaccination of man and other animals against rabies. Postgrad Med J. 1973;49:551–64.
Cox JH, Dietzschold B, Schneider LG. Rabies virus glycoprotein II: biological and serological characterization. Infect Immun. 1977;16(3):754–9.
Jakel V, König M, Cussler K, Hanschmann K, Thiel HJ. Factors influencing the antibody response to vaccination against rabies. Dev Biol. 2008;131:431–6.
Jayakumar R. Elimination of rabies in dogs is the optimal control method for prevention of rabies in humans. Apricon. 2009;16–18.
Kennedy LJ, Lunt M, Barnes A, McElhinney L, Fooks AR, David NB, William ERO. Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine. 2007;25:8500–7.
Kim N, Lee S, Seo M, Kim D, Lee J, Kwak D. Serologic detection of antibodies agains rabies virus in dogs from animal shelters in Seoul, South Korea. J Anim Vet Adv. 2013;12(6):721–5.
Knobel DL, Cleavel S, Coleman PG, Fèvre EM, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin FX. Re-evaluating the burden of rabies in Africa and Asia. World Health Org. 2005;83:60–368.
Mansfield KL, Burr PD, Snodgrass DR, Sayers R, Fooks AR. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet Rec. 2004;154(14):423–6.
Martinez L. Global infectious disease surveillance. Int J Infect Dis. 2000;4:222–8.
Meslin FX, Fishbein DB, Matter HC. Rationale and prospects for rabies elimination in developing countries. In: Rupprecht CE, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin: Springer; 1994. p. 1–26.
Muller TF, Peter S, Vos AC, Thomas SD, Wenzel A, Neubert M. Effect of maternal immunity on the immune response to oral vaccination against rabies in young foxes. AJVR. 2001;62(7):1154–8.
Nagarajan T, Nagendrakumar SB, Mohanasubramanian B, Rajalakshmi S, Hanumantha NR, Ramya R, Thiagarajan D, Srinivasan VA. Phylogenetic analysis of nucleoprotein gene of dog rabies virus isolates from Southern India. Genet Evol. 2009;9:976–82.
Ogawa T, Gamoh K, Kanda K, Suzuki T, Kawashima A, Narushima R, Shimazaki T. Rabies immune status of dogs brought into the Hyogo Prefecture Animal Well-being Center, Japan. J Vet Med Sci. 2009;71:825–6.
Olugasa OO, Aiyedun JO, Emikpe BO. Prevalence of antibody against rabies among confined, free roaming and stray dogs in a transit city of Nigeria. Vet Ital. 2011;47(4):453–60.
Singh MP, Goyal K, Majumdar M, Ratho RK. Prevalence of rabies antibodies in street and household dogs in Chandigarh, India. Trop Anim Health Prod. 2011;43:111–4.
Shyamsundar KA. M.V.Sc. Thesis submitted to College of Karnataka Veterinary science and Fisheries on the topic of “Studies on status of anti-rabies vaccinal antibodies in dogs”; 2014.
Warrell MJ, Warrell DA. Rabies and other lyssaviruses disease. Lancet. 2004;363:959–69.
WHO. Assessing the burden of rabies in India. WHO sponsored national multicentric rabies survey. Association for prevention and control of rabies in India; 2004.
WHO. Animal-bites. Fact sheet. http://www.who.int/mediacentre/factsheets/fs373. Accessed 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of M.V.Sc. thesis submitted by Bhumika F. Savaliya to AAU, Anand.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Savaliya, B.F., Mathakiya, R.A., Bhanderi, B.B. et al. Evaluation of phenotypic factors for anti-rabies antibody in vaccinated pet dogs. VirusDis. 26, 282–287 (2015). https://doi.org/10.1007/s13337-015-0284-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-015-0284-6