Abstract
The Japanese Ministry of Health, Labour and Welfare issued the basic principles for bioequivalence evaluations of generic dry powder inhaler (DPI) drug products in 2016. This document presents the recommendations of the methodology for the effective development of generic DPI drug products. Based on this document, the Pharmaceuticals and Medical Devices Agency (PMDA) advises the efficient development in the consultation meeting with generic companies. The PMDA generally requires the data of in vitro tests, pharmacokinetics studies, and clinical endpoint studies for generic development. In vitro tests play a critical role in the development of the generic versions because these tests are used to predict the efficacy and safety of other populations on whom clinical endpoint studies have not been conducted. We are aware that some points need further discussion, such as the recommendations for at least four groups of stages (group 1: the induction port and pre-separator, group 2: greater than 5 μm, group 3: ranging from 3 to 5 μm, group 4: ranging from 0.8 to 3 μm) for in vitro tests of the generic DPI products. This article shows the current understanding and recommendations with respect to in vitro tests, particularly for at least four groups of stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The PMDA requires evaluation of the delivered dose, fine particle mass, and at least four groups of stages by the generic applicants as in vitro tests |
In vitro tests play a crucial role in the evaluation of the bioequivalence for generic dry powder inhaler drug products |
1 Introduction
Aerodynamic particle size distribution (APSD) is widely accepted in the evaluation of the formulation characteristics of the delivery phase of inhalation products in the research and development of new inhalation products. Moreover, this is used for the equivalence evaluation of generic inhalation products by various regulatory agencies [1,2,3,4,5]. The Japanese Ministry of Health, Labour and Welfare (MHLW) issued the basic principles of bioequivalence evaluations for generic dry powder inhaler (DPI) drug products in 2016 [5]. This document shows that the Pharmaceuticals and Medical Devices Agency (PMDA) generally requires in vitro studies, pharmacokinetics studies, and clinical endpoint studies to develop generic DPI drug products [6]. We believe it is difficult for generic drug applicants to conduct clinical endpoint studies for all originator’s indications, such as asthma and/or chronic obstructive pulmonary disease (COPD). In addition, there are various populations with mild, moderate, and severe asthma and COPD as well as pediatric asthma patients. Therefore, the PMDA believes that in vitro tests can supplement scientific evidence for other populations in whom clinical endpoint studies have not been conducted. However, further discussions are necessary with respect to some points. One such point is that there are at least four groups of stages in the APSD test. In this article, we discuss the current scientific views regarding the in vitro tests, especially in at least four groups of stages.
1.1 Assessment of In Vitro Tests
The PMDA requires evaluation of the delivered dose (DD), fine particle mass (FPM), and at least four groups of stages at three flow rates (10th, 50th, and 90th percentiles; e.g., 30 L/min, 60 L/min, and 90 L/min) as the in vitro tests by the generic applicants for DPI products based on the basic principles [5]. We discuss the objective of each test from the viewpoint of equivalence between the generic and originator DPI products.
1.2 DD Test
The DD test is conducted using the dosage unit sampling apparatus specified by the Japanese Pharmacopoeia 17th edition, to evaluate the amount available to patients. Generic drugs are defined as drugs with the same active pharmaceutical ingredient (API), dosage form, and strength as the original drugs. The same strength generally ensures that the same amounts are administered. Therefore, generic inhalation products should emit equivalent amounts from the device container as the originator’s inhalation products. For example, generic applicants should show the equivalent DD as the original ones at three flow rates, such as 30 L/min, 60 L/min, and 90 L/min. These flow rates are based on the peak inspiratory flow of the patient population [7,8,9].
1.3 APSD Tests
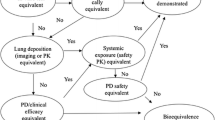
APSD tests are currently conducted using the Andersen cascade impactor or the next generation impactor specified in the Japanese Pharmacopoeia 17th edition. These impactors are used to evaluate not only the aerodynamic particle size, but also the API amounts in the aerodynamic condition. Generally, the aerodynamic particle size for lung deposition is considered to be ≤ 5 μm, and the mass of this size is defined by the FPM. Figure 1 shows the current recommendations for at least four groups of stages. Group 1, the induction port and pre-separator, is the fraction due to the collection of a significantly large particle and the removal of the coarse particle, corresponding to the oral cavity. Group 2, with size > 5 μm is the fraction for safety concern because this particle size does not reach pulmonary deposition. Most particles of this size are deposited approximately in the larynx and pharynx [10, 11]. Group 3, with size ranging from 3 to 5 μm, is the main targeted region for central airways, corresponding approximately to the trachea and the bronchus. Group 4, with size ranging from 0.8 to 3 μm, is a critical region for reaching the peripheral airway, corresponding approximately to the terminal bronchioles, respiratory bronchioles, and alveoli. In contrast, those < 0.8 μm are exhaled [11]. If the original products, including inhaled corticosteroids, have the indication for COPD, we recommend more detailed grouping evaluation because the inflammation in COPD patients is in a deeper region (i.e., respiratory bronchioles and alveoli) than that in asthma patients [12,13,14]. More detailed groups are group 5, with size ranging from 2 to 3 μm, and group 6, with size ranging from 0.8 to 2 μm.
2 Conclusion
In this article, we present the current understanding regarding in vitro tests, such as DD and FPM, and at least four groups of stages for generic DPI drug products. In vitro tests play a crucial role in the evaluation of the bioequivalence for generic DPI drug products because it is difficult for generic applicants to conduct clinical studies in all patients, such as those with adult asthma, pediatric asthma, and COPD. The PMDA recommends the evaluation of representative patients with indications for original products and the use of in vitro tests for the equivalence evaluation in other populations. This is the first article to discuss in vitro tests for generic DPI drug products in Japan.
References
US FDA. Draft guidance on albuterol sulfate. 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM619932.pdf Accessed 19 Dec 2018.
US FDA. Draft guidance on mometasone furoate. 2016. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495387.pdf Accessed 19 Dec 2018.
US FDA. Draft guidance on budesonide. 2012. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM319977.pdf Accessed 19 Dec 2018.
EMA. Guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003504.pdf#search=‘EMA%2C?guideline?on?the?requirements?for?clinical%2C2009 Accessed 19 Dec 2018.
MHLW. Basic principles on the bioequivalence evaluation for the generic DPI drug products. 2016. https://www.pmda.go.jp/files/000210452.pdf#search=%27%E5%90%B8%E5%85%A5%E7%B2%89%E6%9C%AB%E5%89%A4?%E5%BE%8C%E7%99%BA%27 Accessed 21 Dec 2018 (In Japanese).
Kuribayashi R, Yamaguchi T, Sako H, Takishita T, Takagi K. Bioequivalence evaluations of generic dry powder inhaler drug products: similarities and differences between Japan, USA, and the European Union. Clin Pharmacokinet. 2017;56(3):225–33.
Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–24.
Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–7.
Hira D, Koide H, Nakamura S, Okada T, Ishizeki K, Yamaguchi M, Koshiyama S, Oguma T, Ito K, Funayama S, Komase Y, Morita SY, Nishiguchi K, Nakano Y, Terada T. Assessment of inhalation flow patterns of soft mist inhaler co-prescribed with dry powder inhaler using inspiratory flow meter for multi inhalation devices. PLoS One. 2018;13(2):e0193082.
Dunbar C, Mitchell J. Analysis of cascade impactor mass distributions. J Aerosol Med. 2005;18(4):439–51.
Aerosol consensus statement. Consensus conference on aerosol delivery. Chest. 1991;100(4):1106–9.
Tillie-Leblond I, Gosset P, Tonnel AB. Inflammatory events in severe acute asthma. Allergy. 2005;60(1):23–9.
Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–21.
Cukic V, Lovre V, Dragisic D, Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD)—differences and similarities. Mater Sociomed. 2012;24(2):100–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Ryosuke Kuribayashi, Aya Myoenzono, Kazunori Takagi, and Mitsue Hirota declare that they have no conflict of interest that might be relevant to the contents of this article.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official views of the Pharmaceuticals and Medical Devices Agency.
Rights and permissions
About this article
Cite this article
Kuribayashi, R., Myoenzono, A., Takagi, K. et al. Current Understanding of the Equivalence Evaluations for In Vitro Tests on Generic Dry Powder Inhaler Drug Products in Japan. Eur J Drug Metab Pharmacokinet 44, 743–745 (2019). https://doi.org/10.1007/s13318-019-00561-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-019-00561-z