Abstract
The Canary Island date palm (Phoenix canariensis) is one of the most representative tree species of the urban landscape of México City. However, since the last decade, severe foliar damage and decay has been observed, causing the death of hundreds of individuals in different boroughs of the city’s north zone. The symptoms observed in these affected palms were indicative of Texas Phoenix palm decline (TPPD), a serious disease associated with phytoplasmas of the 16SrIV-D subgroup. In this study, the use of nested-PCR and real-time PCR detected the presence of phytoplasmas of group 16SrIV in 21 out of 25 Canary Island date palms located in the Miguel Hidalgo, Benito Juárez, and Cuauhtémoc boroughs of México City. Sequencing the F2nR2 fragment of the 16S rRNA gene generated from the phytoplasma DNA samples of six positive palms, and subsequent in silico analysis, revealed that these phytoplasmas belonged to the 16SrIV-D subgroup. The presence of this phytoplasma strain in México City extends the range of known climates in which this pathogen and its vectors are capable of subsisting, in addition to evidencing an increase in the geographical distribution of this pathogen in recent years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ornamental palms are an essential element of the urban landscape in tropical, subtropical and Mediterranean climates around the world (Broschat et al. 2014). One of the most used species for this purpose is the Canary Island date palm (Phoenix canariensis Chabaud), which was introduced to Europe and the Americas in the nineteenth century, from the Canary Islands, where it is endemic (Zona 2008). In the case of México City, México, this palm species was introduced in 1919 to decorate streets, squares and public gardens (Cervantes et al. 2008).
In 2001 in Texas (USA), mature P. canariensis palms with a disease showing declining symptoms of inflorescence necrosis, progressive discoloration (browning instead of yellowing), and desiccation of successively younger leaves, with eventual foliar discoloration in all leaves in the crown, as reported by Harrison et al. (2002c), who named the disease as Texas Phoenix palm decline (TPPD). These symptoms are similar to those of lethal yellowing (LY) in coconut palms, a disease associated with phytoplasmas of group 16SrIV, subgroup A (Harrison et al. 1994; Lee et al. 1998). Therefore, the presence of phytoplasmas was evaluated in P. canariensis palms affected by TPPD. A phytoplasma was found and identified as corresponding to 16SrIV group, subgroup D (Harrison et al. 2002c). This phytoplasma can be transmitted by the planthopper Haplaxius crudus Van Duzee, as evidenced in México (Dzido et al. 2020) and the USA (Mou et al. 2022). Phoenix canariensis is considered one of the most susceptible species to TPPD (Harrison and Elliott 2016) and in recent years, other significant TPPD outbreaks have been reported in the USA in the states of Florida (Bahder et al. 2019) and Louisiana (Ferguson et al. 2020). In México, there have been reports associating 16SrIV-A, -B, -E, and -D phytoplasma strains with different palm species displaying LY-type symptoms (Oropeza et al. 2020). In countries in the Caribbean region, similar reports have associated strains 16SrIV-A, -D, and -E with LY-type diseases (Oropeza et al. 2020).
More recently, in Mexico there have been two cases with TPPD symptomatology, including leaf browning, in P. canariensis. One in Torreón, Coahuila State (Fig. 1A), that started in 2015 and has affected over a thousand palms, and analysis of the palms showed the presence of 16SrIV-D phytoplasmas, confirming that this was a case of TPPD (Palma-Cancino et al. 2020).
The second and more recent case, becoming noticeable about five years ago, and the subject of the present study, is an outbreak in México City (about 1000 km away from Torreón, Fig. 1A), affecting already hundreds of P. canariensis palms displaying TPPD symptoms, including: fruit fall, necrosis of inflorescences, and browning of the foliage, starting in the basal or older leaves and advancing upwards until reaching the upper leaves, prior to the total collapse of the bud and the death of the palm. Thus, the objective of the present study was to determine if group 16SrIV phytoplasmas were associated to P. canariensis palms in México City with symptoms suggestive of TPPD, and their subsequent molecular identification at the subgroup level.
Materials and methods
Sampling and health assessment of palms
Stem samples from 25 P. canariensis were taken during February (n = 12) and April (n = 13) 2022, in four sites located in Paseo de las Palmas, Miguel Hidalgo borough (n = 8), Papalote Museo del Niño, Miguel Hidalgo borough (n = 7), Diagonal San Antonio, Benito Juarez borough (n = 4) and Glorieta de la Palma, Cuauhtémoc boroughs (n = 6) in México City, México (Fig. 1B, C), where the presence of palms with TPPD-like symptoms was previously determined. This procedure was carried out with the help of an electric drill, following the sample collection and transport techniques described by Oropeza et al. (2010).
The apparent health state of all the palms included in this study was evaluated at the time of sampling, based on a four-category scale with emphasis on the crown (de Saavedra-Romero et al. 2023), as indicated below: apparently healthy (palm without visible symptoms of leaf damage; Fig. 2A); initial damage (palm with brown basal leaves; Fig. 2B); intermediate damage (palm with up to 50% brown foliage; Fig. 2C) and advanced damage (palm with brown leaves above 50%, including palms with the entire crown collapsed; Fig. 2D). Samples from multiple individuals were taken for each of the four established categories (Table 1).
DNA extraction
Total DNA was extracted from 1 g of stem tissue following a CTAB protocol (Doyle and Doyle 1990). The DNA pellet was resuspended in 30 μL of TE buffer (Tris 10 mM, EDTA 1 mM, pH 8).
Group 16SrIV phytoplasma detection protocols
Phytoplasma detection in the 12 palms sampled in February was carried out by a nested-PCR assay designed for the specific detection of the 16SrIV group (Harrison et al. 2002a). Each DNA sample was diluted 1:10 with sterile deionized water prior to analysis. Then, 2 μL of the diluted samples were used as the template for the first amplification with phytoplasma universal primers P1 (Deng and Hiruki 1991) and P7 (Schneider et al. 1995). Subsequently, the P1/P7 product was diluted 1:40 and 5 μL of the dilution was used as the template for the second amplification with group 16SrIV specific primers LY16Sf (Harrison et al. 2002c) and LY16-23Sr (Harrison et al. 2002b). In addition to template DNA, each reaction mix - final volume of 25 μL - contained 0.5 μL of 10 mM dNTP solution mix (Invitrogen), 2 μL of 10 μM solution of each primer, 1.5 U of MangoTaq™ DNA polymerase (Meridian Bioscience), as well as PCR buffer and MgCl2 at a final concentration of 3 mM. PCR reactions were carried out in a C1000™ thermal cycler (Bio-Rad). The same amplification parameters specified by Harrison et al. (2002a) were used for both rounds of amplification. PCR products were visualized in 1.5% agarose gels stained with ethidium bromide, using a UVP transilluminator (Analytik Jena). Positive and negative controls consisted of DNA from a group 16SrIV phytoplasma-positive palm and DNA from a healthy palm, respectively.
Additionally, the 13 palms sampled in April were analyzed by the real-time TaqMan PCR assay of Córdova et al. (2014), designed for the specific detection of group 16SrIV phytoplasmas. A Rotor-Gene® Q (QIAGEN) thermal cycler was used with the same reagents and amplification parameters reported by the authors. The Ct values of each sample were assigned with the Rotor-Gene Q® - Pure Detection software version 2.0.2 (QIAGEN). In this study, samples with Ct values greater than 30 were considered negative for the presence of group 16SrIV phytoplasmas. Positive and negative controls were employed as described above.
Cloning and sequencing
Molecular characterization of the phytoplasmas was carried out by amplifying and sequencing the F2nR2 fragment of the 16S rRNA gene. For this, the P1/P7 products were re-amplified with the R16F2n/R16R2 primer pair and the conditions reported by Gundersen and Lee (1996). Products were purified with the QIAquick® Gel Extraction kit (QIAGEN) and cloned into the pGEM®-T Easy Vector System I (Promega), following the manufacturer’s instructions. Recombinant plasmids were purified with the QIAprep® Spin Miniprep kit (QIAGEN). Lastly, cloned inserts were fully sequenced at the UCDNA Sequencing Facility (University of California, Davis).
Sequence analysis
Sequences obtained in this study were edited and assembled with BioEdit software (Hall 1999) version 7.2.5. Subsequently, they were compared with other phytoplasma sequences using the BLAST® tool from the National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The F2nR2 fragment of the sequences was analyzed with iPhyClassifier (Zhao et al. 2009) for further characterization of the phytoplasmas.
Lastly, a phylogenetic tree was constructed with the sequences obtained in this study and other 16S rRNA phytoplasma sequences downloaded from the GenBank® database. This analysis was performed with MEGA software version X (Kumar et al. 2018), applying the maximum likelihood method and the Hasegawa-Kishino-Yano substitution model. Statistical support for internal nods was estimated with a bootstrap test of 1000 replicates. A sequence from Acholeplasma palmae (GenBank Accession: L33734) was used as an external group to root the tree.
Results
Detection of phytoplasma and health status of palms
The results of both group 16SrIV phytoplasma detection protocols by nested-PCR and real-time PCR and the health assessment of the 25 P. canariensis included in this study are shown in Table 1. In the case of the nested-PCR protocol, amplification with the LY16Sf/LY16-23Sr primer pair produced fragments of the expected size around 1750 bp in the samples diagnosed as positive. Similarly, samples diagnosed as positive with the real-time PCR protocol showed Ct values between 13 and 23. Based on this, it was determined that 21 of the total 25 sampled palms were infected with group 16SrIV phytoplasmas. These include 16 positive palms of 17 showing symptoms, and 5 positive palms of 8 that were symptomless. No amplification was detected in the negative controls used in both detection assays.
Molecular characterization of phytoplasma sequences
Samples corresponding to six positive palms - at least one per collection site - were selected for sequencing and subsequent characterization of the F2nR2 fragment of the 16S rRNA gene of the detected phytoplasmas (see Table 1). 1249 bp sequences were obtained and submitted to GenBank® with accession numbers OP222480, OP231792, OP231793, OP231794, OP231795 and OP231797. BLAST® analysis revealed a 100% identity of the sequences with each other, and 99.92% - the highest identity in the database - with sequences from three phytoplasma isolates from Coahuila, México (Accession numbers MN384667, MN389510 and MN607700), which were previously characterized by Palma-Cancino et al. (2020).
To confirm characterization at the subgroup level, the virtual RFLP pattern derived from the F2nR2 fragment was compared to the reference pattern of the 16SrIV-D subgroup using iPhyClassifier (Fig. 3), resulting with a similarity coefficient of 0.98 with the reference sequence AF237615 for the 16SrIV-D subgroup. Thus, the phytoplasma detected in this study is a member of the 16SrIV-D subgroup. Likewise, a direct comparison between the virtual RFLP patterns of the phytoplasmas from México City and from Coahuila (Accession numbers MN384667, MN384668, MN384670, MN389510 and MN607700) was made, and it was observed that the phytoplasma sequences from both locations share identical profiles (see Fig. 3C), that is, a similarity coefficient of 1.00.
Virtual RFLP patterns obtained from iPhyClassifier, corresponding to the F2nR2 fragment of the 16S rRNA gene of the phytoplasmas from Mexico City and other phytoplasmas of the 16SrIV group: A and B digestions with AluI and MseI, respectively, C complete digestion profile (17 enzymes) of the phytoplasma isolates from México City (deviation = 5)
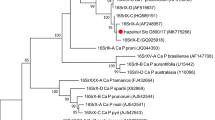
A phylogenetic analysis based on the 16S rRNA gene was also performed to infer relationships between the phytoplasmas from México City and other closely related phytoplasmas. According to the resulting tree (Fig. 4), it is observed that the six sequences obtained in this study are enclosed within a monophyletic clade, together with other phytoplasmas from the 16SrIV-B, D and E subgroups; this clade is in turn separated from the clade in which the 16SrIV-A subgroup is included, that is, the causal agent of LY (Bertaccini and Duduk 2009). In addition, the close relationship between the phytoplasmas from México City and those detected in P. canariensis palms affected by TPPD in the city of Torreón, Coahuila (Palma-Cancino et al. 2020) is highlighted.
Phylogenetic tree of phytoplasma 16S rRNA gene sequences inferred by maximum likelihood. For the analysis, 30 representative sequences deposited in GenBank® and a total of 1235 characters were used. The sequences of phytoplasma isolates from México City are denoted with ▲. The trust level of the internal nodes is displayed next to the branches
Discussion
The 16SrIV-D phytoplasma is the causal agent of the disease known as TPPD (Harrison and Elliott 2016) which was originally described more than 40 years ago in Phoenix palms in the south of Texas, USA (McCoy et al. 1980). Within the past ten years in urban green areas of México City, hundreds of individuals of P. canariensis palms began to show symptoms similar to those of TPPD. In this study, the death of these P. canariensis palms is associated for the first time with the 16SrIV-D subgroup phytoplasma.
Samples from these palms in México City were analyzed by nested-PCR and real-time PCR. In all sites, positive detection of 16SrIV group phytoplasmas was obtained in palms showing TPPD-type symptoms, but also in symptomless palms that eventually developed TPPD-type symptoms (see Fig. 5), as previously reported for symptomless Pritchardia pacifica Seem. & H. Wendl palms with positive detection of 16SrIV phytoplasmas (Narváez et al. 2017). Computer simulated analyses of the sequences of the phytoplasmas in 6 of the positive palms, revealed that these sequences correspond to members of the 16SrIV-D subgroup. Therefore, P. canariensis palms sampled in México City (Fig. 1) were affected by TPPD.
P. canariensis palms affected by TPPD in México City (left), including the monumental specimen of the “Palm roundabout” (25 in our study), between 80–100 years old (center). (Right) Appearance of palm 6 with a positive diagnosis for 16SrIV-D phytoplasma 112 days after sampling and having been evaluated as apparently healthy (see Fig. 2A)
Regarding the possible origin of the TPPD phytoplasma in México City, a comparison with phytoplasma in Torreón (Palma-Cancino et al. 2020), based on phylogenetic (forming a monophyletic clade) and RFLP profile analyses (similarity coefficient of 1.00) within the present study, revealed that the phytoplasmas are identical. So dispersal of the phytoplasma from Mexico City to Torreón, or the other way around could be possible, but dispersal from another source or sources, cannot be ruled out. The phytoplasma could have been spread within host plants. There are several palm species, including Adonidia merrillii (Becc.) Becc., Syagrus romanzoffiana (Cham.) Glassman, P. pacifica, etc., reported as infected with the 16SrIV-D phytoplasma (Oropeza et al. 2020), that are commonly used as ornamental plants in México and transported between different regions within the country. This activity could be contributing to the pathogen’s dispersal.
In regards to vector insects, as mentioned before, reports support that subgroup 16SrIV-D phytoplasmas can be transmitted by H. crudus (Dzido et al. 2020), which has been reported in Torreón (Hernández-Rodríguez et al. 2019), but not yet in México City. However, other species of the same genus or other Auchenorrhyncha insects could be involved in transmission. For instance, Haplaxius skarphion Kramer, already reported in México City (Kramer 1979), and recently reported as infected with group 16SrIV phytoplasmas in Tabasco, México (Ramos-Hernández et al. 2020b).
The geographic distribution of 16SrIV-D phytoplasma in México (Fig. 1A) was initially reported in Campeche state in Carludovica palmata Ruiz & Pav. (not an Arecaceae species) (Córdova et al. 2000). Then in locations in different states and palm species. In Yucatan, in P. pacifica (Narvaez et al. 2017); in Oaxaca and Guerrero, in Cocos nucifera L. (Harrison et al. 2002b); in Tabasco, in Attalea butyracea (Mutis ex L. f.) Wess. Boer, A. merrillii, and C. nucifera (Ramos-Hernández et al. 2020a); in Baja California Sur, in Brahea brandegeei (Purpus) H.E. Moore (Poghosyan et al. 2019); in Guanajuato in Phoenix dactylifera L. and Sabal mexicana Mart. (Aviña-Padilla et al. 2011); and in Torreón in P. canariensis (Palma-Cancino et al. 2020), before appearing in México City (this report), also in P. canariensis. Hence, the 16SrIV-D phytoplasma seems to be widely distributed in México, within an increasing diversity of climates (Fig. 1A), and also an increasing diversity of host plants, and probably insect vectors. Despite the climate and location differences between Torreón and Mexico City, there were no differences when phytoplasma sequences were compared. But certainly, this is a subject that requires further attention comparing sequences from different sites in a study similar to that reported by Pilet et al. (2019) using Multilocus Sequence Analysis.
Most of the reports mentioned above relate to cases in urban areas affecting ornamental palms, mostly P. canariensis. Two of these cases, Torreón and México City, involve damage to several hundred palms (and the amounts are increasing). In México City, there are about 15,000 palms, mostly P. canariensis (SEDEMA 2022), several of which are between 50–100 years old, and they are iconic components of the urban landscape (Fig. 5). Unfortunately, similar cases are appearing in several other cities in México.
In light of the above, it is necessary to think of approaches to deal with TPPD and subgroup 16SrIV-D phytoplasmas in México City, in other locations, and prevent future outbreaks. For immediate results, we might consider the use of antibiotics as reported for palms infected with 16SrIV-D phytoplasmas (Soto et al. 2020). However, it is not recommended because of the potential risk of generating antibiotic resistance in these microorganisms and others that could be pathogenic to plants or animals (Verhaegen et al. 2023). Alternatively, it will be useful to identify sites of production and distribution networks of P. canariensis and other plants used for ornamental purposes, and establish rules and measures to reduce the risk of dispersal of phytoplasmas and any other agents affecting plant health.
In addition, in the long term, it is very important to continue researching to learn more about this pathosystem, including determining which is the vector or vectors of 16SrIV-D phytoplasmas in México City, the search for other host plant species of this phytoplasma that could be contributing to the inoculum production. And finally, the search for resistance, an approach that has worked well for coconut (Zizumbo-Villarreal et al. 2008), should be considered as a priority. Although field trials take very long, usually more than five years, a novel, faster, and more effective approach could be to develop molecular markers (Garavito-Guyot et al. 2022). Any approach to managing outbreaks in México could be useful also for similar cases of such iconic palms in several cities of countries in all continents (Pasiecznik 2006).
References
Aviña-Padilla K, Rodríguez-Páez LA, Nava-Castrejón ÁI, Ochoa-Sánchez JC, Rivera-Bustamante R, Martínez-Soriano JP (2011) Epidemic of lethal yellowing disease affecting Phoenix dactilyfera and Sabal mexicana in Central Mexico. Bull Insectology 64(Supplement):S221–S222
Bahder BW, Soto N, Helmick EE, Dey KK, Komondy L, Humphries AR, Mou D, Bailey R, Ascunce MS, Goss EM (2019) A survey of declining palms (Arecaceae) with 16SrIV-D phytoplasma to evaluate the distribution and host range in Florida. Plant Dis 103:2512–2519. https://doi.org/10.1094/PDIS-03-19-0633-RE
Bertaccini A, Duduk B (2009) Phytoplasma and phytoplasma diseases: A review of recent research. Phytopathol Mediterr 48:355–378. https://doi.org/10.14601/Phytopathol_Mediterr-3300
Broschat TK, Elliott ML, Hodel DR (2014) Ornamental palms: biology and horticulture. In: Janick J (ed) Horticultural reviews, vol 42. Wiley Blackwell, Hoboken, pp 1–120. https://doi.org/10.1002/9781118916827.ch01
Cervantes V, Carabias J, Arriaga V (2008) Evolución de las políticas públicas de restauración ambiental. In: Carabias J, Mohar A, Anta S, de la Manza J (eds) Capital natural de México volumen III: políticas y perspectivas de sustentabilidad. CONABIO, Ciudad de México, pp 155–226
Córdova I, Oropeza C, Puch-Hau C, Harrison N, Collí-Rodríguez A, Narvaez M, Nic-Matos G, Reyes C, Sáenz L (2014) A real-time PCR assay for detection of coconut lethal yellowing phytoplasmas of group 16SrIV subgroups A, D and E found in the Americas. J Plant Pathol 96:343–352
Córdova I, Oropeza C, Almeyda H, Harrison NA (2000) First report of a phytoplasma-associated leaf yellowing syndrome of palma jipi plants in southern México. Plant Dis 84:807. https://doi.org/10.1094/PDIS.2000.84.7.807A
Deng S, Hiruki C (1991) Amplification of 16S rRNA genes from culturable and nonculturable Mollicutes. J Microbiol Methods 14:53–61. https://doi.org/10.1016/0167-7012(91)90007-D
de Saavedra-Romero LL, Alvarado-Rosales D, Almaraz-Sánchez A, Quezada-Salinas A, García-Díaz SE, Aranda-Ocampo S, Ortiz-García CF, Equihua-Martínez A, López-Buenfil JA (2023) Health condition of palm trees of Mexico City, with an emphasis on “crowns.” Open J Agric Res 3:12–27. https://doi.org/10.31586/ojar.2023.690
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dzido J, Sánchez R, Dollet M, Julia J, Narvaez M, Fabre S, Oropeza C (2020) Haplaxius crudus (Hemiptera: Cixiidae) transmits the lethal yellowing phytoplasmas, 16SrIV, to Pritchardia pacifica Seem. & H. Wendl (Arecaceae) in Yucatan. Mexico Neotrop Entomol 49:795–805. https://doi.org/10.1007/s13744-020-00799-2
Ferguson MH, Singh R, Cook M, Burks T, Ong K (2020) Geographic distribution and host range of lethal bronzing associated with phytoplasma subgroup 16SrIV-D on palms in southern Louisiana. Plant Health Prog 21:350–355. https://doi.org/10.1094/PHP-06-20-0046-S
Garavito-Guyot A, Rivallan R, Bocs S, Baudouin L, Yankey EN (2022) Genomic screening for tolerance of coconut populations differentially exposed to coconut’s lethal yellowing in Ghana using genotyping by sequencing. Phytopathogenic Mollicutes 12:70. https://doi.org/10.5958/2249-4677.2022.00035.4
Gundersen DE, Lee I-M (1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol Mediterr 35:144–151
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Harrison NA, Elliott ML (2016) Phytoplasmas associated with date palm in the continental USA: three 16SrIV subgroups. Emir J Food Agric 28:17–23. https://doi.org/10.9755/ejfa.2015-09-736
Harrison NA, Myrie W, Jones P, Carpio ML, Castillo M, Doyle MM, Oropeza C (2002a) 16S rRNA interoperon sequence heterogeneity distinguishes strain populations of palm lethal yellowing phytoplasma in the Caribbean region. Ann Appl Biol 141:183–193. https://doi.org/10.1111/j.1744-7348.2002.tb00211.x
Harrison NA, Narváez M, Almeyda H, Córdova I, Carpio ML, Oropeza C (2002b) First report of group 16SrIV phytoplasmas infecting coconut palms with leaf yellowing symptoms on the Pacific coast of Mexico. Plant Pathol 51:808. https://doi.org/10.1046/j.1365-3059.2002.00778.x
Harrison NA, Richardson PA, Jones P, Tymon AM, Eden-Green SJ, Mpunami AA (1994) Comparative investigation of MLOs associated with Caribbean and African coconut lethal decline diseases by DNA hybridization and PCR assays. Plant Disease 78:507–511. https://doi.org/10.1094/PD-78-0507
Harrison NA, Womack M, Carpio ML (2002c) Detection and characterization of a lethal yellowing (16SrIV) group phytoplasma in Canary Island date palms affected by lethal decline in Texas. Plant Dis 86:676–681. https://doi.org/10.1094/PDIS.2002.86.6.676
Hernández-Rodríguez S, Valdés-Perezgasga MT, López-Hernández J, García Espinoza F, Hernández Hernández V, Obrador-Sánchez JA (2019) Cixiidos (Hemiptera: Cixiidae) asociados a palmas con síntomas del amarillamiento letal del cocotero (ALC) en el área urbana de Torreón, Coahuila, México. Entomol Mex 6:526–529
Kramer JP (1979) Taxonomic study of the planthopper genus Myndus in the Americas (Homoptera: Fulgoroidea: Cixiidae). Trans Am Entomol Soc 105:301–389
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lee IM, Gundersen-Rindal DE, Bertaccini A (1998) Phytoplasma: ecology and genomic diversity. Phytopathology 88:1359–1366. https://doi.org/10.1094/PHYTO.1998.88.12.1359
McCoy RE, Miller ME, Thomas DL, Amador J (1980) Lethal decline of Phoenix palms in Texas associated with mycoplasmalike organisms. Plant Dis 64:1038–1040. https://doi.org/10.1094/PD-64-1038
Mou D-F, Di Lella B, Halbert SE, Bextine B, Helmick EE, Bahder BW (2022) Acquisition and transmission of the lethal bronzing phytoplasma by Haplaxius crudus using infected palm spear leaves and artificial feeding media. Phytopathology 112:2052–2061. https://doi.org/10.1094/PHYTO-03-22-0079-R
Narváez M, Ortíz E, Silverio C, Santamaría JM, Espadas F, Oropeza C (2017) Changes observed in Pritchardia pacifica palms affected by a lethal yellowing-type disease in Mexico. Afr J Biotechnol 16:2331–2340. https://doi.org/10.5897/AJB2017.16218
Oropeza C, Córdova I, Narváez M, Sáenz L, Ortíz CF, Harrison N (2010) Manual para el muestreo y el diagnóstico de amarillamiento letal del cocotero. In: Oropeza Salín C, Narváez M, Echegoyén Ramos PE, Rodas R (eds) Plan de contigencia ante un brote de amarillamiento letal del cocotero (ALC) en un país de la región del OIRSA. OIRSA, San Salvador, pp 91–112
Oropeza C, Sáenz L, Narvaez M, Nic-Matos G, Córdova I, Myrie W, Ortíz CF, Ramos E (2020) Dealing with lethal yellowing and related diseases in coconut. In: Adkins S, Foale M, Bourdeix R, Nguyen Q, Biddle J (eds) Coconut biotechnology: towards the sustainability of the ‘tree of life.’ Springer, Cham, pp 169–197. https://doi.org/10.1007/978-3-030-44988-9_9
Palma-Cancino PJ, Samaniego-Gaxiola JA, Narváez M, Nic-Matos G, Chew-Madinaveitia Y, Pedroza-Sandoval A, Gaytán-Mascorro A, Oropeza C (2020) First report of mortality in Phoenix canariensis associated with subgroup 16SrIV-D phytoplasmas in Coahuila, Mexico. Afr J Biotechnol 19:846–857. https://doi.org/10.5897/AJB2020.17185
Pasiecznik N (2006) Phoenix canariensis (Canary Island date palm). CABI (Phoenix canariensis (Canary Island date palm)). CABI Compendium. cabidigitallibrary.org
Pilet F, Nketsia Quaicoe R, Jesuorobo Osagie I, Freire M, Foissac X (2019) Multilocus sequence analysis reveals three distinct populations of “Candidatus Phytoplasma palmicola” with a specific geographical distribution on the African Continent. Appl Environ Microbiol 85:2–16. https://doi.org/10.1128/AEM.02716-18
Poghosyan A, Hernandez-Gonzalez J, Lebsky V, Oropeza C, Narvaez M, Leon de la Luz JL (2019) First report of 16SrIV palm lethal yellowing group phytoplasma (‘Candidatus Phytoplasma palmae’) in palmilla de taco (Brahea brandegeei) and palma colorada (Washingtonia robusta) in the state of Baja California Sur. Mexico Plant Dis 103:2122. https://doi.org/10.1094/PDIS-02-19-0247-PDN
Ramos-Hernández E, Lesher Gordillo JM, Oropeza Salín C, Ortiz García CF, Magaña Alejandro MA, Sánchez Soto S, García Estrada Y (2020a) Detection and identification of phytoplasmas in the 16SrIV-A, -B, and -D subgroups in palms in Tabasco, Mexico. Plant Dis 104:2606–2612. https://doi.org/10.1094/PDIS-09-18-1488-RE
Ramos-Hernández E, Lesher-Gordillo JM, Ortiz-García CF, Oropeza-Salín C, Sánchez-Soto S, Magaña-Alejandro MA, del Narváez-Cab M, S, (2020b) Cíxidos y dérbidos (Hemiptera) vectores putativos de fitoplasma del grupo 16Sr-IV. Rev Colomb Entomol 46:e7065. https://doi.org/10.25100/socolen.v46i2.7065
Schneider B, Seemueller E, Smart CD, Kirkpatrick BC (1995) Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In: Razin S, Tully JG (eds) Molecular and diagnostic procedures in Mycoplasmology, vol I. Academic Press, San Diego, pp 369–380. https://doi.org/10.1016/b978-012583805-4/50040-6
SEDEMA (2022) Informa SEDEMA Sobre Atención a Palmeras de la Ciudad de México. Secretaría del Medio Ambiente de la Ciudad de México. https://www.sedema.cdmx.gob.mx/comunicacion/nota/informa-sedema-sobre-atencion-palmeras-de-la-ciudad-de-mexico. Accessed 25 May 2023
Soto N, Humphries AR, Mou D, Helmick EE, Glover JP, Bahder BW (2020) Effect of oxytetracycline-hydrochloride on phytoplasma titer and symptom progression of the 16SrIV-D phytoplasma in cabbage palms from Florida. Plant Dis 104:2330–2337. https://doi.org/10.1094/PDIS-01-20-0029-RE
Verhaegen M, Bergot T, Liebana E, Stancanelli G, Streiss F, Mingeot-Leclercq M-P, Mahillon J, Bragard C (2023) On the use of antibiotics to control plant pathogenic bacteria: a genetic and genomic perspective. Front Microbiol 14:1–17. https://doi.org/10.3389/fmicb.2023.1221478
Zhao Y, Wei W, Lee I-M, Shao J, Suo X, Davis RE (2009) Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). Int J Syst Evol Microbiol 59:2582–2593. https://doi.org/10.1099/ijs.0.010249-0
Zizumbo-Villarreal D, Colunga-GarcíaMarín P, Fernández-Barrera M, Torres-Hernández N, Oropeza C (2008) Mortality of Mexican coconut germplasm due to lethal yellowing. Plant Genet Resour Newsl 156:22–32
Zona S (2008) The horticultural history of the Canary Island date palm (Phoenix canariensis). Gard Hist 36:301–309
Funding
The authors would like to thank the Secretary of Education, Science, Technology and Innovation of México City (SECTEI), for the funding of the project: (SECTEI/212/2021) "Agents associated with the decline and death of palms in México City". PJPC thanks the National Council of Humanities, Sciences and Technologies (CONAHCYT) of México for the scholarship number 2008511 granted for the completion of a postdoctoral stay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortiz-García, C.F., Alvarado-Rosales, D., Oropeza, C. et al. The decline and death of Canary Island date palms in México City is associated with subgroup 16SrIV-D phytoplasmas. Australasian Plant Pathol. 53, 175–184 (2024). https://doi.org/10.1007/s13313-024-00970-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-024-00970-y